Abstract
OBJECTIVE
β-Cells have demonstrated altered proinsulin processing after islet transplantation. We compare β-cell metabolic responses and proinsulin processing in pancreas and islet transplant recipients with respect to healthy control subjects.
RESEARCH DESIGN AND METHODS
We studied 15 islet and 32 pancreas transplant recipients. Islet subjects were subdivided into insulin-requiring (IR-ISL, n = 6) and insulin-independent (II-ISL, n = 9) groups. Ten healthy subjects served as control subjects. Subjects were administered an intravenous arginine stimulation test, and insulin, C-peptide, total proinsulin, intact proinsulin, and proinsulin fragment levels were determined from serum samples. Acute insulin response (AIR) and proinsulin processing rates were calculated.
RESULTS
We found that basal insulin and C-peptide levels were higher in the pancreas group than in all other groups. II-ISL patients had basal insulin and C-peptide levels similar to healthy control subjects. The IR-ISL group had significantly lower AIRs than all other groups. Basal processing rates were higher in the pancreas and II-ISL groups than in healthy control subjects and the IR-ISL group. After arginine stimulation, all groups had elevated processing rates, with the exception of the IR-ISL group.
CONCLUSIONS
Our data suggest that II-ISL transplant recipients can maintain basal metabolic parameters similar to healthy control subjects at the cost of a higher rate of proinsulin processing. IR-ISL transplant recipients, on the other hand, demonstrate both lower insulin response and lower basal rates of proinsulin processing even after arginine stimulation.
Although the treatment of choice for type 1 diabetes is maintenance of blood glycemia through regular administration of exogenous insulin, this has proven to be troublesome in a small proportion of patients. About 10% of type 1 diabetic patients will face problems due to poor glycemic control and/or hypoglycemic unawareness (1). Whole pancreas transplantation has been accepted and established as a suitable treatment option to induce insulin-independence in such patients, but remains a major surgical procedure with high morbidity and mortality. This fact combined with the enormous potential of cell therapy has led to increased interest in islet of Langerhans transplantation as an alternative to whole pancreas transplantation.
Islet of Langerhans transplantation involves implantation of β-cells directly into the liver via portal infusion. This process can achieve insulin independence but, thus far, with moderate long-term results (2). A majority of patients will eventually return to insulin therapy (3–5). The gradual decline in graft function is likely due to both allo- and autoimmune mechanisms as well as nonimmune mechanisms (6,7). Initial β-cell mass may be insufficient, and many of the transplanted cells may fail to engraft effectively into the liver owing to poor revascularization (8,9). Evidence also suggests that immunosuppressive drugs may play an additional role in β-cell malfunction (10).
Insulin is synthesized by pancreatic β-cells as preproinsulin, which is subsequently converted to proinsulin intracellularly. Proinsulin is then cleaved to produce insulin, C-peptide, and proinsulin fragments—all of which are secreted into the portal circulation. Under normal conditions, insulin and C-peptide make up >90% of the peptides secreted by β-cells (11). This process is normally very efficient; however, it has been shown that subjects with reduced glucose tolerance as well as those with type 2 diabetes demonstrate impaired processing, resulting in increased secretion of immature proinsulin (12–14). This phenomenon is strongly associated with β-cell dysfunction, as both relative and absolute hyperproinsulinemia have also been observed in islet grafts demonstrating decreased function (15–17). A better understanding of this phenomenon may allow us to explain how and why islet grafts tend to lose function over time.
With this goal in mind and in order to better understand endocrine reserve and hormonal processing in islet transplants compared with whole pancreas transplants and healthy control subjects, we assessed and compared insulin, C-peptide, and proinsulin responses to arginine stimulation in islet transplant recipients, whole pancreas transplant recipients, and healthy control subjects.
RESEARCH DESIGN AND METHODS
The protocol for this cross-sectional study was reviewed and approved by the Institutional Ethical Committee for Clinical Research at the Geneva University Hospitals. All patients who had received a whole pancreas or an islet of Langerhans transplant at the Geneva University Hospitals and who had regular follow-ups at our institution were asked to participate in the study. Patients were required to have at least partial graft function (as determined by positive C-peptide levels). All patients who met the criteria were included in the study.
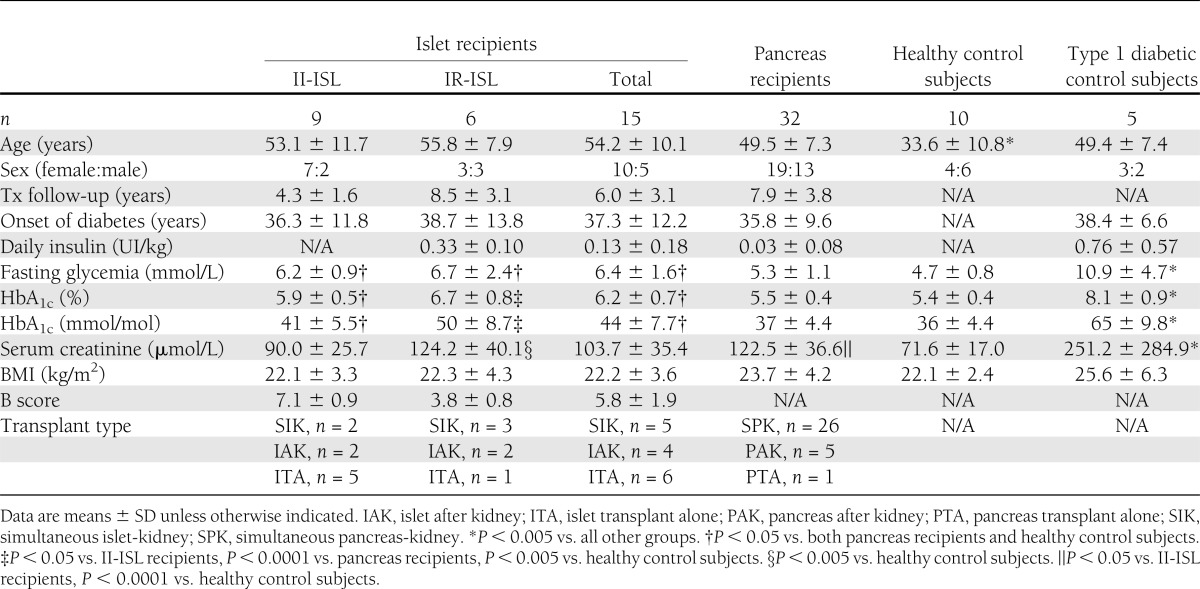
Study population characteristics are shown in Table 1. Fifteen islet transplant recipients were enrolled in the islet group, which was further subdivided into an insulin-independent group (II-ISL) and an insulin-requiring group (IR-ISL). Thirty-two pancreas transplant recipients were recruited for the pancreas group. Ten healthy nondiabetic volunteers were recruited to serve as control subjects. Finally, five patients with type 1 diabetes awaiting pancreas or islet transplant, all of whom demonstrated no C-peptide production at the time of study inclusion, served as negative control subjects. Written informed consent was obtained from all patients and control subjects prior to their participation in the study.
Table 1.
Demographics and population characteristics
Sample collection
All samples were obtained from patients during either their annual posttransplantation follow-up or one of their regularly scheduled outpatient consultations. Blood samples were collected in anonymously labeled EDTA tubes. All samples were immediately centrifuged for serum separation. The serum volumes obtained were divided into three aliquots and stored at −80°C. The serum was labeled using the same anonymous codes assigned to the tubes at the time of the test. The serum remained stored at −80°C until the assays were performed.
Arginine stimulation test
An arginine stimulation test was performed on all subjects to determine basal and stimulated pancreatic hormone levels. Blood was drawn at −10, 0, 2, 3, 4, 5, 7, and 10 min from intravenous injection of a 5 g arginine bolus. All subjects were required to fast overnight. Patients who were back on insulin were required to discontinue slow-acting insulin at least 24 h prior to the test and rapid-acting insulin at least 6 h before the test. Serum levels of insulin, C-peptide, total proinsulin, and intact proinsulin (IPI) were then measured using commercially available ELISAs as described below. Acute insulin response (AIR) was calculated as the mean of the three highest values between 2 and 5 min minus the mean of the basal values at −10 and 0 min. Acute responses for C-peptide were calculated similarly. The area under the curve (AUC) between 0 and 10 min postinjection for insulin and C-peptide was calculated by the trapezoidal rule with the mean of the baseline values subtracted.
Proinsulin fragments, including split-32,33 proinsulin, des-31,32 proinsulin, split-65,66 proinsulin, and des-64,65 proinsulin, were obtained by subtracting IPI from total proinsulin. Ratios of insulin to total proinsulin and insulin [insulin/(total proinsulin+insulin)] as well as proinsulin fragments to total proinsulin (proinsulin fragments/total proinsulin) were calculated to quantify the rate of proinsulin processing. Baseline fasting state ratios were calculated for levels observed 10 min before arginine injection. Ratios representing peak stimulation were calculated as the mean of ratios for the three highest time points between 2 and 5 min after arginine stimulation. Insulin/(total proinsulin+insulin) ratio represents the quantity of fully processed insulin with respect to all circulating forms of the hormone. Proinsulin fragments/total proinsulin represents the percentage of proinsulin that is partially processed.
Assays
Commercially available ELISAs were used to measure hormone levels for insulin, C-peptide, total proinsulin, and IPI. The following kits were used: insulin (Mercodia, Uppsala, Sweden), C-peptide (Mercodia), total proinsulin (Millipore, Zug, Switzerland), and IPI (Millipore).
All fasting serum levels were assayed in duplicate. No dilution of serum samples was performed. Concentrations were determined using a standard curve with high- and low-level control subjects.
Cross-reactivities of the assays were provided by the manufacturer and are as follows: The insulin assay cross-reacts 98% with des-64,65 proinsulin, <0.01% with C-peptide and proinsulin, and <0.05% with des-31,32 proinsulin and split-32,33 proinsulin; the C-peptide ELISA cross-reacts <0.0006% with insulin, <1.8% with proinsulin, 10% with split-65,66 proinsulin, 74% with des-64,65 proinsulin, 3% with des-31,32 proinsulin, and 2% with split-32,33 proinsulin; the total proinsulin ELISA cross-reacts 100% with intact and des-31,32 proinsulin, and 81% with des-64,65 proinsulin; the IPI ELISA cross-reacts 36% with des-64,54 proinsulin, and it shows no cross-reactivity with des-31,32 proinsulin.
The lower limits of detection of the assays were provided by the manufacturer and are as follows: the insulin assay has a detection limit of 6 pmol/L, the C-peptide ELISA has a limit of 15 pmol/L, the total proinsulin assay has a limit of 0.5 pmol/L, and the IPI assay has a lower limit of 0.1 pmol/L.
Statistical analysis
All statistical analyses were performed using Prism 6 software (Graphpad Software, La Jolla, CA). For all analyses, values obtained from healthy subjects were used as control subjects. For data that were normally distributed, Student t test was used to compare two groups, and one-way ANOVA was used to compare between more than two groups. For data that had non-Gaussian distributions, we used the Mann-Whitney test to compare between two groups and the Kruskal-Wallis test to compare between more than two groups. Significance was considered for values of P < 0.05.
RESULTS
Demographic characteristics
The study population characteristics are shown in Table 1. Subjects in the healthy control group were slightly younger than the rest of the study population (P < 0.01). Islet recipients exhibited higher fasting glycemia and HbA1c levels than other groups (P < 0.05). Nonetheless, HbA1c levels remained in the normal range for the II-ISL group. Creatinine levels were elevated in the pancreas and IR-ISL groups with respect to the II-ISL and healthy control groups (P < 0.005 vs. control, P < 0.05 vs. II-ISL).
Insulin and C-peptide levels
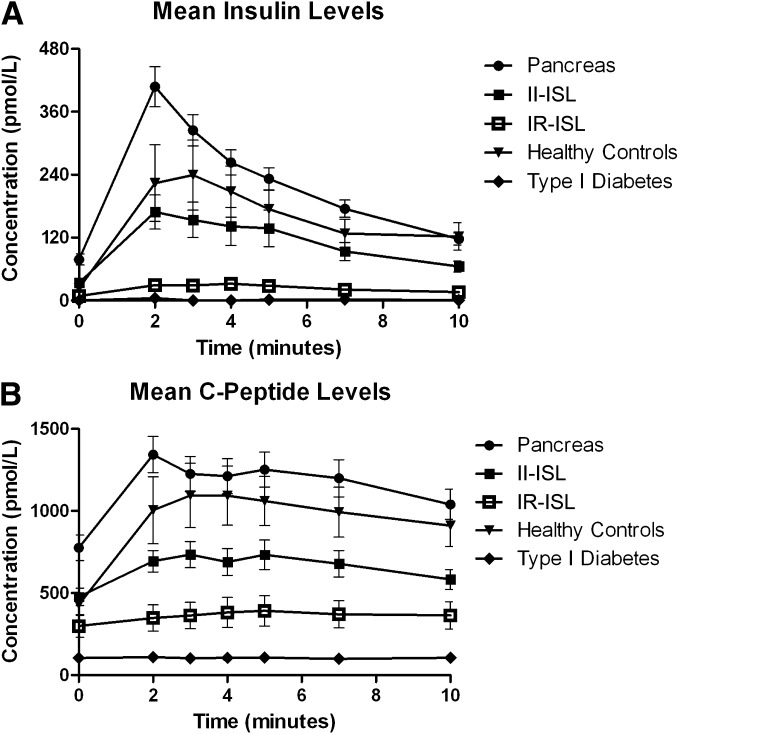
Figure 1A and B shows the insulin and C-peptide response curves for all five groups. The insulin and C-peptide response curves maintain the same overall shape in all groups, with the exception of the diabetic control subjects who demonstrate no response to arginine stimulation. Insulin levels were higher in pancreas recipients but lower in islet recipients compared with healthy control subjects. Insulin levels in the pancreas group were significantly higher than those of the healthy control group. AIRs for healthy control subjects, IR-ISL, II-ISL, and pancreas groups were 217 ± 163, 23 ± 13, 125 ± 90, and 266 ± 129 pmol/L, respectively. The C-peptide acute responses in the same groups were 717 ± 362, 91 ± 53, 278 ± 124, and 550 ± 244 pmol/L, respectively (Supplementary Table 1). Mean acute C-peptide responses were similar in the healthy control group and pancreas group but significantly lower in the islet groups. The IR-ISL transplantees showed a significantly decreased acute insulin and C-peptide response with respect to all other groups. There was no significant difference in AIR between the II-ISL and control groups. As expected, the diabetic control group showed negligible insulin and C-peptide levels.
Figure 1.
A: Mean insulin levels during arginine stimulation of healthy control group, IR-ISL group, II-ISL group, pancreas group, and diabetic control group. B: Mean C-peptide levels during arginine stimulation of healthy control group, IR-ISL group, II-ISL group, pancreas group, and diabetic control group. Whiskers = SEM.
AUC values demonstrated similar distributions to acute responses in the various study groups (Supplementary Table 1). AUC values for insulin response in healthy control subjects, IR-ISL, II-ISL, and pancreas groups were 1,332 ± 959, 152 ± 91, 774 ± 528, and 1,540 ± 707 pmol ⋅ min/L, respectively. These values were higher in pancreas recipients and lower in islet recipients compared with healthy control subjects. AUC values for C-peptide response in healthy control subjects, IR-ISL, II-ISL, and pancreas groups were 4,955 ± 3,158, 630 ± 428, 1,843 ± 875, and 3,859 ± 2,061 pmol ⋅ min/L, respectively. The AUC of C-peptide for the IR-ISL group was significantly lower than in all other groups.
Proinsulinemia
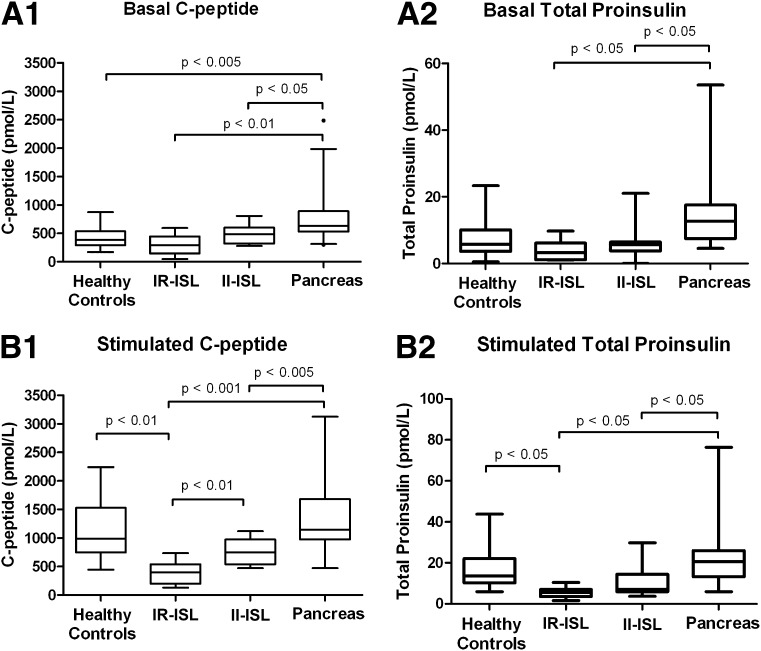
Mean values for C-peptide and total proinsulin before and after arginine stimulation are shown in Fig. 2. These figures show that total proinsulin levels are reduced in the IR-ISL group with respect to all other groups and follow a distribution similar to that of C-peptide.
Figure 2.
A: Box plots of basal C-peptide and total proinsulin levels in healthy control group, IR-ISL group, II-ISR group, and pancreas group before arginine injection (basal values). B: Box plots of stimulated C-peptide and total proinsulin levels in healthy control group, IR-ISL group, II-ISL group, and pancreas group during peak stimulation after arginine injection (stimulated values). Data are expressed as median (solid line), interquartile range (box), and range (whiskers).
Basal proinsulin processing
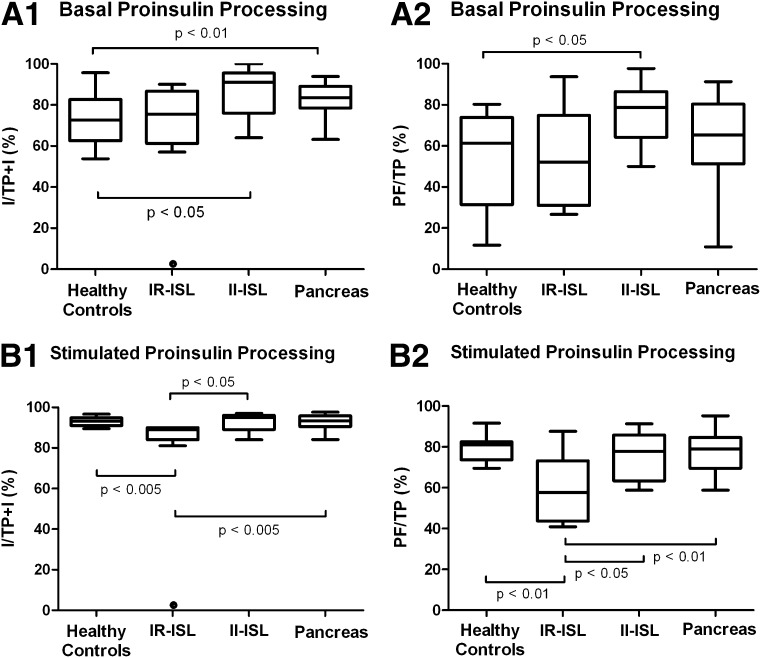
Basal proinsulin-processing ratios are shown in Fig. 3A. The healthy control group demonstrated lower basal proinsulin-processing rates than the pancreas (P < 0.01) and II-ISL (P < 0.05) groups [74 ± 14% vs. 83 ± 7% vs. 86 ± 12%, respectively, for insulin/(total proinsulin+insulin) and 54 ± 25% vs. 67 ± 18% vs. 76 ± 16%, respectively, for proinsulin fragments/total proinsulin]. The IR-ISL group displayed a basal insulin/(total proinsulin+insulin) rate of 75 ± 14% and a basal proinsulin fragments/total proinsulin rate of 54 ± 25%.
Figure 3.
A: Box plots of the proinsulin-processing ratios [(insulin/total proinsulin+insulin) and (proinsulin fragments/total proinsulin)] in healthy control group, IR-ISL group, II-ISL group, and pancreas group before arginine injection (basal values). B: Box plots of the proinsulin-processing ratios [(insulin/total proinsulin+insulin) and (proinsulin fragments/total proinsulin)] in healthy control group, IR-ISL group, II-ISL group, and pancreas group during peak stimulation after arginine injection (stimulated values). Data are expressed as median (solid line), interquartile range (box), and range (whiskers). I/TP+I, insulin/total proinsulin+insulin; PF/TP, proinsulin fragments/total proinsulin.
Stimulated proinsulin processing
Stimulated proinsulin-processing ratios are shown in Fig. 3B. Interestingly, healthy control subjects, pancreas recipients, and II-ISL recipients all attain similarly elevated processing ratios after stimulation (93 ± 2% vs. 93 ± 3% vs. 93 ± 5%, respectively, for insulin/(total proinsulin+insulin) and 80 ± 7% vs. 77 ± 9% vs. 76 ± 12%, respectively, for proinsulin fragments/total proinsulin), while IR-ISL transplant recipients’ ratios do not demonstrate a comparable increase. Although IR-ISL proinsulin-processing ratios do increase slightly after stimulation to 87 ± 4% and 58 ± 18% for insulin/(total proinsulin + insulin) and proinsulin fragments/total proinsulin, respectively, these values remain significantly lower than all other groups (P < 0.01 vs. control and pancreas groups, P < 0.05 vs. II-ISL).
CONCLUSIONS
This study explores for the first time proinsulin processing in islet transplant recipients, not only in comparison with healthy control subjects, but also with respect to pancreas transplant recipients. This study also explores the impact of endocrine cell stimulation on proinsulin processing. We have shown, first, that the exposure to arginine stimulation is in fact similar in II-ISL transplant recipients and healthy control subjects. As expected, recipients of pancreas transplants with systemic venous drainage demonstrate higher insulin response to arginine than healthy control subjects. Second, there is a noticeable difference in the rate of basal proinsulin processing comparing II-ISL and IR-ISL transplant recipients. II-ISL patients have proinsulin-processing ratios similar to those of pancreas transplant recipients, which are significantly higher than those of healthy control and IR-ISL patients. Third, after stimulation, II-ISL, pancreas, and healthy control subjects all increase their proinsulin-processing ratios to a similar level. In contrast, IR-ISL patients are unable to achieve the same increase in proinsulin-processing rate.
These data suggest that fully functional islet grafts are able to attain optimal proinsulin processing when stimulated but are required to sustain high levels of processing even during a metabolic fasting state in order to maintain glycemic control. Islet grafts requiring supplementation with insulin therapy, on the other hand, demonstrate low basal proinsulin-processing rates, which are unable to reach optimal levels even when stimulated. Interestingly, total proinsulin levels in this group (IR-ISL) are low compared with other groups both before and after stimulation. However, the ratio of proinsulin to insulin in the IR-ISL group after stimulation is disproportionately high. This suggests that partially functional islet grafts have a defect in proinsulin processing, which manifests itself as relative and not absolute hyperproinsulinemia.
It should be noted that the calculation of proinsulin fragments may be slightly biased owing to ELISA cross-reactivities. In theory, when we calculate proinsulin fragments as proinsulin fragments = total proinsulin − IPI, we are assuming that all of the des-64,65 proinsulin has been measured by the total proinsulin assay and that none of it has been measured by the intact assay. In reality, we measure only 81% of des-64,65 with the total proinsulin assay and 36% with the IPI assay. So, theoretically we are underestimating the level of des-64,65 proinsulin by ~50%. However, since the PC2 pathway responsible for the formation of des-64,65 proinsulin is not the primary pathway for the production of insulin, unmeasured levels of des-64,65 proinsulin are relatively insignificant with respect to des-31,32 proinsulin levels.
It should also be noted that clearance of proinsulin and proinsulin fragments could be altered by diminished renal function. As seen in Table 1, our IR-ISL group demonstrated creatinine levels that were elevated compared with healthy control subjects. However, this difference was not significant comparing the IR-ISL group to II-ISL and pancreas groups. In contrast, the reduced processing ratios observed in the IR-ISL group are significantly lower than all other groups. This suggests that diminished renal function alone cannot explain the observed relative hyperproinsulinemia.
Both absolute and relative hyperproinsulinemia have already been observed in settings of impaired glucose metabolism (12–14). Two theories have been proposed to explain this. First, it has been suggested that dysfunctional β-cells present a fundamental defect in the pathway responsible for processing of proinsulin to insulin (18). It is possible in the case of islet transplantation that manipulation of islets, introduction of β-cells into a foreign microenvironment, or immunosuppressive drugs may render these cells inherently dysfunctional. A second theory suggests that hyperproinsulinemia occurs because of increased demand on β-cells, which leads to insufficient time to complete proinsulin processing intracellularly before granule secretion occurs (15,19,20). It seems reasonable to suppose that a combination of insulin resistance and decreased β-cell mass could lead to unattainable insulin needs and eventual β-cell deterioration via exhaustion. Our data show that islet grafts initially present increased basal proinsulin processing with respect to healthy control subjects and that this processing deteriorates as graft function declines, a phenomenon that more readily supports the latter theory. We cannot, however, exclude the effect of prolonged glucotoxicity on β-cell function as an additional possible explanation for the observed processing defects. Hyperglycemia alone can be sufficient to provoke secretion of immature proinsulin (21). Although our two islet groups had similar fasting glucose levels, HbA1c was slightly increased in the IR-ISL group, indicating that chronic glucotoxicity may have played a role in the functional deterioration of β-cells within this subgroup. Ultimately, a longitudinal study on the same or similar populations might help to verify this hypothesis.
Two studies have previously investigated proinsulin processing in islet transplant recipients (16,22). However, neither study used arginine stimulation to explore hormonal processing when under simulated metabolic stress and neither study compared islet and pancreas transplant recipients. It is interesting to note that these studies produced conflicting data and presented contrasting conclusions.
Our study tends to agree with those of McDonald et al. (16) and explains why Klimek et al. (22) found seemingly “contrasting” results. We found that functional islet grafts display increased processing and relatively lower proinsulinemia at rest, with an inability to increase their processing rates further when stimulated. These grafts are essentially behaving the same during the metabolic fasting state as fully stimulated healthy control subjects. As islet grafts lose function, however, processing—even during the fasting state—becomes less effective and proinsulinemia increases. These findings directly correlate with the findings of McDonald et al. and are supported by the data of Klimek at al., which include mostly IR-ISL transplant recipients. Two other groups, Fiorina et al. (17) and Rickels et al. (21), found similar results. It would seem that there is a threshold level of demand beyond which β-cells can no longer process proinsulin effectively. As stated earlier, we presume this to be due to an increased demand on decreased β-cell mass. Further investigation is warranted to explain the exact mechanism responsible for the increased basal proinsulin processing seen in II-ISL grafts. Again, a longitudinal study of these patients might help shed more light on the issue.
No study previously analyzed the stimulated hormonal response of pancreas grafts with respect to islet grafts. Our data show that pancreas grafts behave quite similarly to healthy control subjects but with elevated overall hormone levels. This is undoubtedly a reflection of the systemic venous pancreatic drainage of these patients, in whom there is no hepatic first-pass metabolism of pancreatic hormonal secretions (23). Pancreas grafts tend to show similar patterns of proinsulin processing to fully functional islet grafts. Whether this is peculiar to systemically drained pancreata cannot be elucidated from this data.
This study used arginine stimulation rather than oral or intravenous glucose tolerance tests to test secretory reserve in our patient populations. Arginine stimulation provides the advantage of not inducing hyperglycemia, thus reducing the confounding influence of glucotoxicity to a minimum. Other investigators have found the intravenous glucose tolerance tests to be more robust and a better indicator of β-cell status (24), but this method does not account for host insulin resistance.
In summary, II-ISL transplant recipients can maintain basal metabolic parameters similar to healthy control subjects at the cost of a higher rate of basal proinsulin processing. IR-ISL transplant recipients demonstrate both lower insulin response and lower basal rates of proinsulin processing, which remain suboptimal even after arginine stimulation (i.e., loss of graft function is associated with less effective processing and relative hyperproinsulinemia). Finally, the higher AIRs of pancreas transplant recipients are a reflection of systemic venous drainage of endocrine secretions.
Supplementary Material
Acknowledgments
This research was partially supported by a grant from the Insuleman Foundation. C.T. was supported by the Swiss National Science Foundation. This research was also supported by an unrestricted educational grant from Astellas. No other potential conflicts of interest relevant to this article were reported.
N.M.E. researched data, contributed to discussion, and wrote the manuscript. S.B. contributed to discussion and reviewed and edited the manuscript. P.M. reviewed and edited the manuscript. S.D.-M. researched data. L.G. researched data and reviewed and edited the manuscript. C.T. contributed to discussion and reviewed and edited the manuscript. D.B. reviewed and edited the manuscript. T.B. designed study, contributed to discussion, and wrote the manuscript. T.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2710/-/DC1.
References
- 1.Vardanyan M, Parkin E, Gruessner C, Rodriguez Rilo HL. Pancreas vs. islet transplantation: a call on the future. Curr Opin Organ Transplant 2010;15:124–130 [DOI] [PubMed] [Google Scholar]
- 2.Rickels MR. Recovery of endocrine function after islet and pancreas transplantation. Curr Diab Rep 2012;12:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borot S, Niclauss N, Wojtusciszyn A, et al. GRAGIL Network Impact of the number of infusions on 2-year results of islet-after-kidney transplantation in the GRAGIL network. Transplantation 2011;92:1031–1038 [DOI] [PubMed] [Google Scholar]
- 5.Berney T, Ferrari-Lacraz S, Bühler L, et al. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am J Transplant 2009;9:419–423 [DOI] [PubMed] [Google Scholar]
- 6.Lacotte S, Berney T, Shapiro AJ, Toso C. Immune monitoring of pancreatic islet graft: towards a better understanding, detection and treatment of harmful events. Expert Opin Biol Ther 2011;11:55–66 [DOI] [PubMed] [Google Scholar]
- 7.Harlan DM, Kenyon NS, Korsgren O, Roep BO, Immunology of Diabetes Society Current advances and travails in islet transplantation. Diabetes 2009;58:2175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 9.Henriksnäs J, Lau J, Zang G, Berggren PO, Köhler M, Carlsson PO. Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: disruption of islet integrity necessary for islet revascularization. Diabetes 2012;61:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berney T, Buhler LH, Majno P, Mentha G, Morel P. Immunosuppression for pancreatic islet transplantation. Transplant Proc 2004;36(Suppl.):362S–366S [DOI] [PubMed] [Google Scholar]
- 11.Uchizono Y, Alarcón C, Wicksteed BL, Marsh BJ, Rhodes CJ. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead? Diabetes Obes Metab 2007;9(Suppl. 2):56–66 [DOI] [PubMed] [Google Scholar]
- 12.Røder ME, Porte D, Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:604–608 [DOI] [PubMed] [Google Scholar]
- 13.Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes 1997;46:1725–1732 [DOI] [PubMed] [Google Scholar]
- 14.Grill V, Dinesen B, Carlsson S, Efendic S, Pedersen O, Ostenson CG. Hyperproinsulinemia and proinsulin-to-insulin ratios in Swedish middle-aged men: association with glycemia and insulin resistance but not with family history of diabetes. Am J Epidemiol 2002;155:834–841 [DOI] [PubMed] [Google Scholar]
- 15.Davalli AM, Perego L, Bertuzzi F, et al. Disproportionate hyperproinsulinemia, beta-cell restricted prohormone convertase 2 deficiency, and cell cycle inhibitors expression by human islets transplanted into athymic nude mice: insights into nonimmune-mediated mechanisms of delayed islet graft failure. Cell Transplant 2008;17:1323–1336 [DOI] [PubMed] [Google Scholar]
- 16.McDonald CG, Ryan EA, Paty BW, et al. Cross-sectional and prospective association between proinsulin secretion and graft function after clinical islet transplantation. Transplantation 2004;78:934–937 [DOI] [PubMed] [Google Scholar]
- 17.Fiorina P, Vergani A, Petrelli A, et al. Metabolic and immunological features of the failing islet-transplanted patient. Diabetes Care 2008;31:436–438 [DOI] [PubMed] [Google Scholar]
- 18.Porte D, Jr, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes 2001;50(Suppl. 1):S160–S163 [DOI] [PubMed] [Google Scholar]
- 19.Leahy JL, Halban PA, Weir GC. Relative hypersecretion of proinsulin in rat model of NIDDM. Diabetes 1991;40:985–989 [DOI] [PubMed] [Google Scholar]
- 20.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. beta-Cell function following human islet transplantation for type 1 diabetes. Diabetes 2005;54:100–106 [DOI] [PubMed] [Google Scholar]
- 21.Rickels MR, Naji A. Proinsulin processing and transplanted islets. Am J Transplant 2010;10:1495. [DOI] [PubMed] [Google Scholar]
- 22.Klimek AM, Soukhatcheva G, Thompson DM, et al. Impaired proinsulin processing is a characteristic of transplanted islets. Am J Transplant 2009;9:2119–2125 [DOI] [PubMed] [Google Scholar]
- 23.Frystyk J, Ritzel RA, Maubach J, et al. Comparison of pancreas-transplanted type 1 diabetic patients with portal-venous versus systemic-venous graft drainage: impact on glucose regulatory hormones and the growth hormone/insulin-like growth factor-I axis. J Clin Endocrinol Metab 2008;93:1758–1766 [DOI] [PubMed] [Google Scholar]
- 24.Hirsch D, Odorico J, Danobeitia JS, et al. Early metabolic markers that anticipate loss of insulin independence in type 1 diabetic islet allograft recipients. Am J Transplant 2012;12:1275–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.