Abstract
OBJECTIVE
The prognostic importance of carotid-femoral pulse wave velocity (PWV), the gold standard measure of aortic stiffness, has been scarcely investigated in type 2 diabetes and never after full adjustment for potential confounders. The aim was to evaluate the prognostic impact of carotid-femoral PWV for cardiovascular morbidity and all-cause mortality in a cohort of 565 high-risk type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
Clinical, laboratory, ambulatory blood pressure (BP) monitoring, and carotid-femoral PWV data were obtained at baseline. The primary end points were a composite of fatal and nonfatal cardiovascular events and all-cause mortality. Multiple Cox survival analysis was used to assess the associations between carotid-femoral PWV, as a continuous variable and categorized at 10 m/s, and the end points.
RESULTS
After a median follow-up of 5.75 years, 88 total cardiovascular events and 72 all-cause deaths occurred. After adjustments for potential cardiovascular risk factors, including micro- and macrovascular complications, ambulatory BP, and metabolic control, carotid-femoral PWV was predictive of the composite end point but not of all-cause mortality both as a continuous variable (hazard ratio 1.13 [95% CI 1.03–1.23], P = 0.009 for increments of 1 m/s) and as categorized at 10 m/s (1.92 [1.16–3.18], P = 0.012). On sensitivity analysis, carotid-femoral PWV was a better predictor of cardiovascular events in younger patients (<65 years), in those with microvascular complications, and in those with poorer glycemic control (HbA1c ≥7.5% [58.5 mmol/mol]).
CONCLUSIONS
Carotid-femoral PWV provides cardiovascular risk prediction independent of standard risk factors, glycemic control, and ambulatory BPs and improves cardiovascular risk stratification in high-risk type 2 diabetes.
In the past decade, knowledge of the importance of arterial stiffness in the pathogenesis of cardiovascular diseases grew (1,2). Arterial stiffness depends on the structural and geometric properties of the arterial wall and on the distending pressure, and aging and blood pressure (BP) are its main determinants (1,2). The measurement of carotid-femoral pulse wave velocity (PWV) is considered the gold standard evaluation of central aortic stiffness (1). Furthermore, aortic stiffness has been demonstrated to predict cardiovascular morbidity and mortality above and beyond other traditional cardiovascular risk factors in patients with end-stage renal disease (3) and hypertension (4), elderly individuals (5), and general population-based samples (6,7). This prognostic importance has also been recently confirmed in a meta-analysis (8).
Type 2 diabetic patients have increased arterial stiffness (9–11) and are at particular risk for augmented cardiovascular morbidity and mortality. This high cardiovascular risk is not completely explained by clustering of traditional risk factors, and increased arterial stiffness may be one pathophysiological mechanism that links diabetes to increased cardiovascular morbidity and mortality (12). Nevertheless, only one previous study investigated the prognostic impact of increased aortic stiffness for cardiovascular outcomes in type 2 diabetes (13), but because of a smaller sample size (397 diabetic individuals), the study could not completely adjust for traditional cardiovascular risk factors, chronic diabetes complications, or metabolic control parameters. Therefore, we aimed to investigate in a prospective follow-up cohort of high-risk type 2 diabetic patients the prognostic impact of increased aortic stiffness for cardiovascular morbidity and mortality and for all-cause mortality. In particular, we evaluated whether aortic stiffness was able to add prognostic information beyond traditional cardiovascular risk markers and whether there were interactions between aortic stiffness and other important covariates, such as age, sex, presence of diabetes complications, and glycemic control.
RESEARCH DESIGN AND METHODS
Patients and baseline procedures
This report includes the first 565 patients from the Rio de Janeiro Type 2 Diabetes Cohort Study enrolled between August 2004 and December 2008 in the type 2 diabetes outpatient clinic of our tertiary care university hospital and followed up until June 2012. All participants gave written informed consent, and the local ethics committee approved the study protocol. The enrollment criteria, baseline protocol, and diagnostic definitions have been detailed previously (14–17). In brief, inclusion criteria were all adult type 2 diabetic individuals up to 80 years old with either any microvascular or macrovascular complication or at least two other modifiable cardiovascular risk factors. Exclusion criteria were morbid obesity (BMI ≥40 kg/m2), advanced renal failure (serum creatinine >180 μmol/L or estimated glomerular filtration rate <30 mL/min/1.73 m2), or the presence of any serious concomitant disease limiting life expectancy. Specifically for this analysis, patients with aortoiliac occlusive disease were excluded because of the condition’s effect on PWV measurement (15). None of the patients had a left ventricular ejection fraction <40%. All were submitted to a standard protocol that included a complete clinical examination, laboratory evaluation, 24-h ambulatory BP monitoring (ABPM), and carotid-femoral PWV measurement. Diagnostic criteria for chronic diabetic complications were detailed previously (14–17). Coronary heart disease was diagnosed by clinical criteria, electrocardiographic criteria, or positive ischemic stress tests. Cerebrovascular disease was diagnosed by history and physical examination and peripheral arterial disease by an ankle-brachial index <0.9. Diabetic retinopathy was evaluated by an ophthalmologist. The diagnosis of nephropathy needed at least two albuminuria measures of ≥30 mg in 24 h or proteinuria measures of ≥0.5 g in 24 h or a confirmed reduction of glomerular filtration rate (<60 mL/min/1.73 m2 or serum creatinine >130 μmol/L). Peripheral neuropathy was ascertained by clinical examination (knee and ankle reflex activities and feet sensation determined by Semmes-Weinstein monofilament and vibration examination with a 128-Hz tuning fork).
Clinic BP was measured three times with a digital oscillometric BP monitor (HEM-907XL; Omron Healthcare, Kyoto, Japan) with a suitable-size cuff on two occasions 2 weeks apart at study entry. The first measure of each visit was discarded, and the mean of the last two BP readings of each visit was used. Arterial hypertension was diagnosed if the mean systolic BP (SBP) was ≥130 mmHg or the mean diastolic BP (DBP) was ≥80 mmHg or if antihypertensive drugs were prescribed. ABPM was recorded in the following month with use of Mobil-O-Graph version 12 equipment. Parameters evaluated were 24-h SBP, DBP, pulse pressure (PP), and circadian BP variability pattern (normal dipping defined as ≥10% nocturnal BP fall in relation to daytime levels). Laboratory evaluation included fasting glycemia, glycated hemoglobin, serum creatinine, and lipids. Albuminuria and proteinuria were evaluated in two nonconsecutive sterile 24-h urine collections.
Aortic stiffness measurement
Immediately after the 24-h ABPM recording, a single trained independent observer unaware of patient data measured PWV along the descending thoracoabdominal aorta (central arterial stiffness) by the foot-to-foot velocity method with Complior equipment (Artech-Medical, Val De Marne, France), as previously validated (18). Briefly, waveforms were obtained transcutaneously over the right-side common carotid and femoral arteries simultaneously during a minimum period of 10–15 s. The time delay (t) in seconds was measured between the feet of the two waveforms, and the distance (D) in meters covered by the waves was measured directly between femoral and carotid recording sites. PWV was calculated as D / t. Three consecutive readings were obtained, and PWV was considered to be the mean between them. We used the recommended scaling factor of 0.8 to convert PWV obtained through direct distances to real PWV (19). The cutoff value for considering increased aortic PWV was 10 m/s (19).
Follow-up and end points
The patients were followed up regularly at least three to four times a year until June 2012. The observation period for each patient was the number of months from the date of the first clinical examination to the date of the last clinical visit in 2012 or the date of the first end point. Except for those who died, no patient was lost to follow-up. The primary end points comprised a composite of all fatal or nonfatal cardiovascular events and all-cause mortality. Cardiovascular events were as follows: fatal or nonfatal acute myocardial infarction (AMI), sudden cardiac death, new-onset heart failure, death from progressive heart failure, any myocardial revascularization procedure (surgical or not), fatal or nonfatal stroke, any aortic or lower-limb revascularization procedure (surgical or not), any amputation above the ankle, and death from aortic or peripheral arterial disease. End points were ascertained from medical records, death certificates, and interviews with attending physicians and patient families through a standard questionnaire reviewed by two independent observers.
Statistical analysis
Continuous variables are described as mean and SD or median and interquartile range (IQR). Survival analyses were performed by Kaplan-Meier estimation of event-free survival curves and compared by log-rank tests (with patients divided according to carotid-femoral PWV >10 m/s) and multivariate Cox proportional hazards regression. For patients with multiple events, analysis was restricted to the first event under study. Results are presented as hazard ratios (HRs) with their 95% CIs. Carotid-femoral PWV was analyzed both as a continuous variable (for 1 m/s and 1-SD increments) and as divided at 10 m/s. First, carotid-femoral PWV was adjusted for age and sex and then fully adjusted for all potential risk factors: age, sex, BMI, diabetes duration, smoking status, physical inactivity, number of antihypertensive drugs in use, presence of macro- and microvascular complications at baseline, 24-h SBP, glycated hemoglobin, HDL and LDL cholesterol, and statin and aspirin use. A separate analysis was performed by entering 24-h PP instead of 24-h SBP into the same multivariate-adjusted model. Additionally, other analyses were carried out separately for cardiac and cerebrovascular-peripheral events and for cardiovascular and noncardiovascular mortality. We compared the predictive performance of Cox models with and without aortic PWV by calculating the Akaike information criterion (AIC), which carries a penalty for the number of variables used in the model and, therefore, can be compared directly across models with differing numbers of variables (20). A lower AIC value indicates a better prediction. For assessing the improvement of discrimination performance after the addition of aortic PWV to the models, we used the C statistic (analogous to the area under the receiver operating characteristic curve applied to time-to-event analysis), compared by the method proposed by DeLong et al. (21), and the integrated discrimination improvement (IDI) index (22). The IDI is equivalent to the difference in discrimination slopes between models with and without the new variable, and its calculation is based on continuous differences in predicted risk in new and old models in individual cases and controls. Thus, the IDI is free from the dependence on empirical risk categories that is inherent to reclassification tables and can be used as an objective indicator of reclassification improvement (7,22). In sensitivity and interaction analyses, interaction terms were tested between aortic PWV and age (<65 or ≥65 years), sex, presence of macro- and microvascular complications, and glycemic control (HbA1c <7.5% or ≥7.5% [58.5 mmol/mol]), and stratified survival analyses were performed for the composite end point. Additionally, patients with events during the first year of follow-up were excluded to examine for possible reverse causality between aortic stiffness and outcomes. A two-tailed P < 0.05 was considered significant. Statistical analyses were performed with SPSS version 19.0 (IBM Corporation, Chicago, IL) and R version 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics and follow-up end points
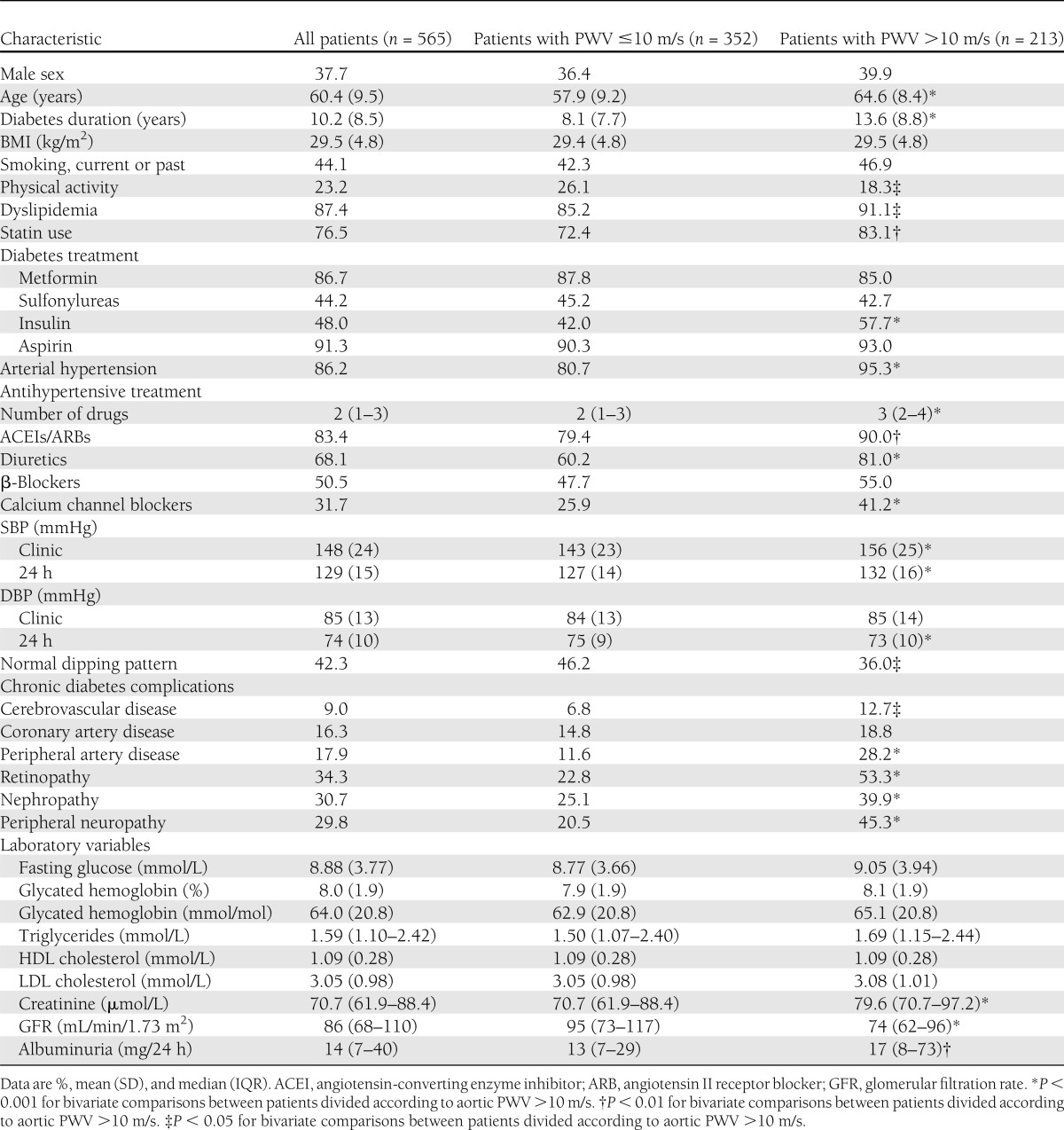
Mean carotid-femoral PWV was 9.6 (SD 2.1) m/s (median 9.3 [IQR 8.1–10.8] m/s). A total of 213 (37.7%) patients had increased (>10 m/s) aortic stiffness. Table 1 shows the characteristics of all patients enrolled and divided according to aortic PWV >10 m/s. Patients with high central PWV were older, had a higher prevalence of arterial hypertension and dyslipidemia, had longer diabetes duration, used insulin more frequently, and were less physically active than those with aortic PWV ≤10 m/s. Despite being under a more intense antihypertensive treatment, they had higher clinic and ambulatory BPs and had the nondipping pattern more frequently than patients with lower aortic PWV. They also had a higher prevalence of all chronic degenerative diabetes complications, except for coronary artery disease, than patients with lower aortic stiffness.
Table 1.
Characteristics of all type 2 diabetic patients divided according to carotid-femoral PWV categorized at 10 m/s
After a median follow-up of 5.75 years (range 4–94 months), which corresponds to 3,134 patient-years of follow-up, 88 (15.6%) patients presented with a first fatal or nonfatal cardiovascular event (12 nonfatal strokes, 8 nonfatal AMIs, 14 myocardial revascularizations, 4 new-onset heart failures, 12 nonfatal peripheral artery events [amputation or revascularization], and 38 cardiovascular deaths [7 strokes, 14 AMIs, 4 heart failures, 11 sudden deaths, and 2 deaths from peripheral artery disease]). The crude incidence rate of total cardiovascular events was 2.77 per 100 patient-years of follow-up. There were 72 all-cause deaths (12.7%, incidence rate 2.26 per 100 patient-years) and 38 deaths from cardiovascular disease, 15 from cancer, 12 from infectious diseases, and 7 from other causes. Patients with increased aortic stiffness had a higher incidence of cardiovascular events (4.75 vs. 1.82 per 100 patient-years, P < 0.001) and of all-cause deaths (3.67 vs. 1.57 per 100 patient-years, P < 0.001) than patients with lower aortic PWV.
Survival analyses
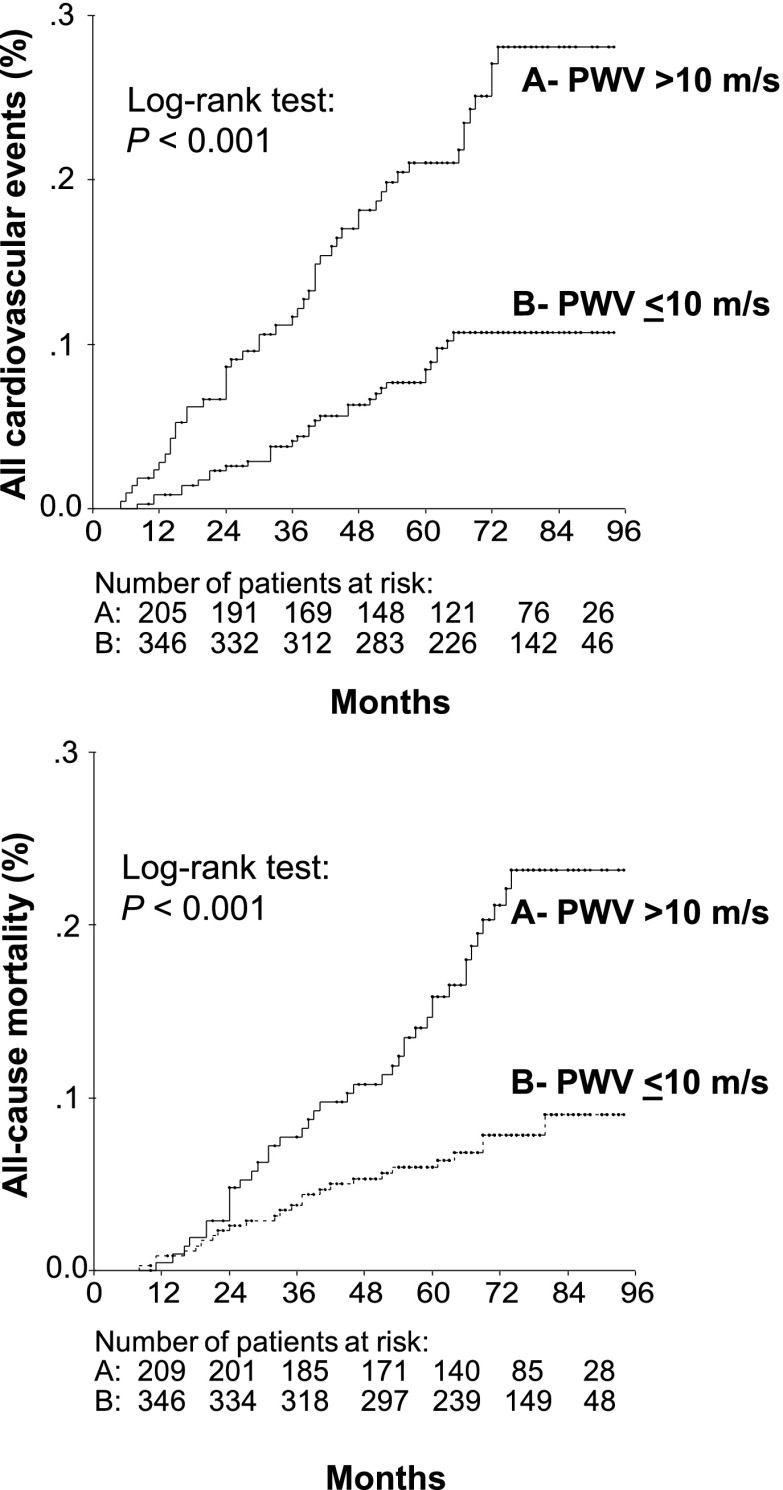
Table 2 shows the results of Cox proportional hazards regression for carotid-femoral PWV analyzed as a continuous variable and categorized at 10 m/s as a predictor of the two primary end points. Aortic PWV was a predictor of total cardiovascular events but not of all-cause deaths after full statistical adjustments, with a 13% excess risk for each 1 m/s increase in aortic PWV (corresponding to a 32% excess risk for a 1-SD increment) and a nearly twofold higher risk for those with increased (>10 m/s) aortic stiffness. Adjusting to ambulatory PP instead of SBP did not change the results. Furthermore, as assessed by AIC, the addition of aortic PWV to all survival models improved their predictive performance, except in the fully adjusted model for all-cause mortality. Assessed as the C statistic, on the other hand, only the sex- and age-adjusted models for total cardiovascular events showed improved discrimination after the addition of aortic PWV (Table 2). However, when assessed by the IDI, the addition of aortic PWV to sex- and age-adjusted models resulted in a discrimination improvement of 2.3% (95% CI 0.3–5.7%, P < 0.001) and to fully adjusted models, 2.2% (0.1–5.3%, P = 0.03) for total cardiovascular events prediction. For all-cause mortality, the addition of aortic PWV marginally improved integrated discrimination by 1.0% (0–4.0%, P = 0.05) in sex- and age-adjusted models but did not improve discrimination in fully adjusted models (0.1% [−0.3 to 2.6%], P = 0.48). Kaplan-Meier survival curve analysis confirmed the worse prognosis associated with increased aortic stiffness (Fig. 1). Together, these findings indicate that the addition of aortic PWV to standard cardiovascular risk factors actually improved model prediction and discrimination, except for all-cause mortality in a previously fully adjusted model.
Table 2.
Results of multivariate Cox survival analyses for associations between aortic PWV analyzed as a continuous variable or categorized at >10 m/s and end points
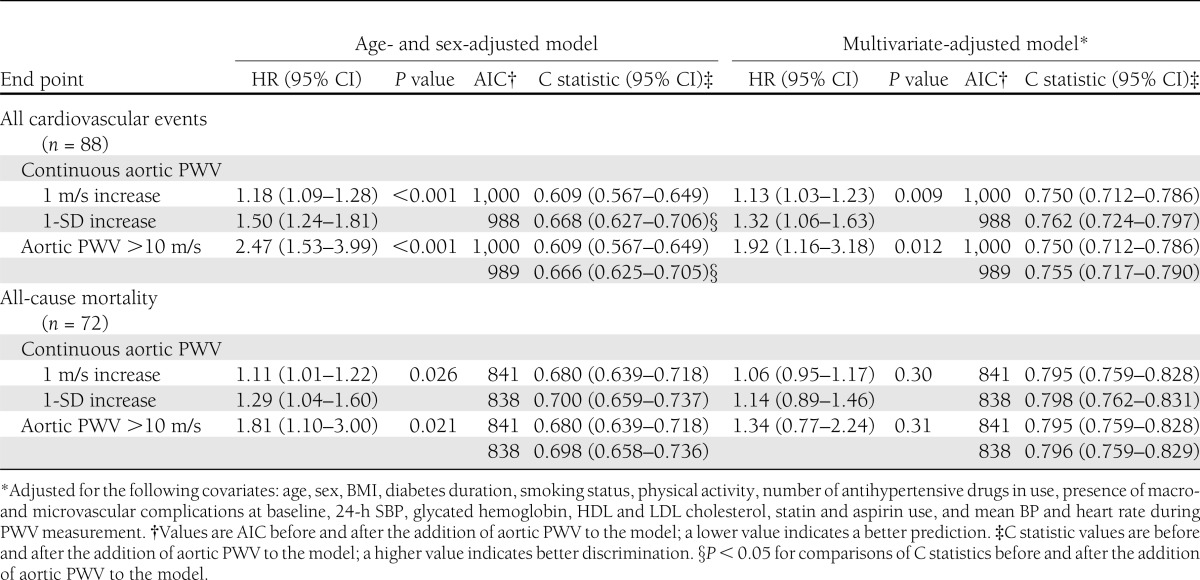
Figure 1.
Kaplan-Meier curves of all incident cardiovascular events and of all-cause mortality in type 2 diabetic patients grouped according to aortic PWV categorized at 10 m/s.
When analyzed separately for cardiovascular (38 events) and noncardiovascular mortality (34 events), continuous aortic PWV was a predictor of only cardiovascular mortality, with HRs greater than those for all-cause mortality (1.16 [95% CI 1.02–1.32], P = 0.024 after adjustment for age and sex, and 1.15 [0.99–1.34], P = 0.072 after full statistical adjustments), whereas no association was found between aortic PWV and noncardiovascular mortality (1.07 [0.94–1.23], P = 0.26, and 0.95 [0.82–1.11], P = 0.54, after age and sex adjustment and complete statistical adjustment, respectively). Additionally, when analyzed separately, an increased aortic PWV equally predicted cardiac (1.17 [1.06–1.30], P = 0.002) and cerebrovascular-peripheral (1.14 [1.02–1.27], P = 0.022) events for increments of 1 m/s after age and sex adjustments.
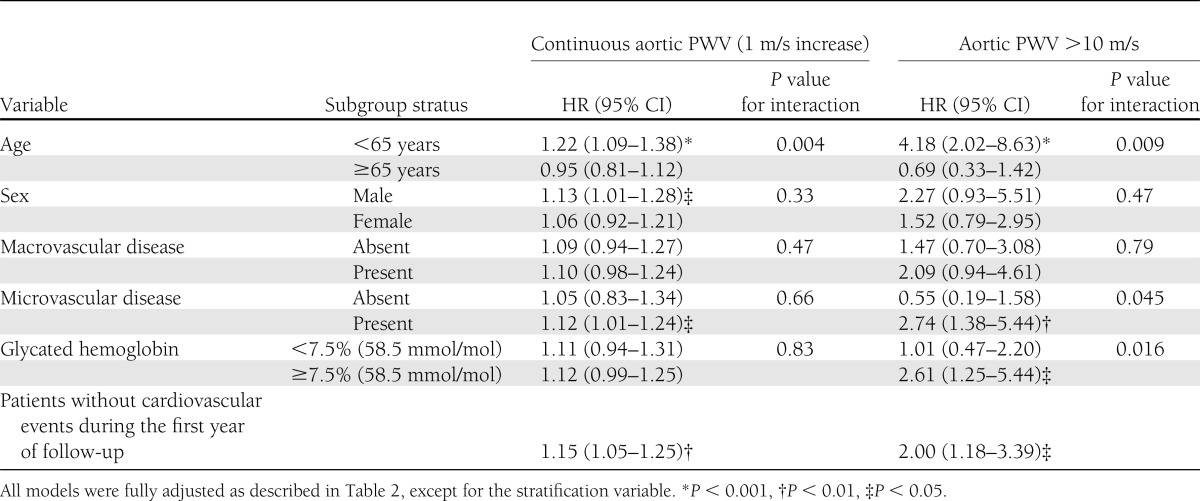
Table 3 shows the results of sensitivity and interaction analyses. Age significantly influenced the prognostic value of aortic PWV in that younger patients (<65 years) with increased aortic stiffness had the highest cardiovascular risk (fourfold), whereas in elderly patients, aortic PWV was not a predictor of cardiovascular events. The significant interaction with age persisted when end points were evaluated separately for cardiac and cerebrovascular-peripheral events. When analyzed as a dichotomous variable, patients with microvascular complications and poorer glycemic control (HbA1c ≥7.5% [58.5 mmol/mol]) also had greater cardiovascular risks associated with increased aortic stiffness than their counterparts without microvascular complications and with better metabolic control. Excluding patients with cardiovascular events during the first year of follow-up did not change the prognostic impact of carotid-femoral PWV, suggesting no reverse causality between aortic stiffness and cardiovascular events.
Table 3.
Sensitivity and interaction analyses for total cardiovascular events end point
CONCLUSIONS
This prospective cohort study shows that increased aortic stiffness, measured by carotid-femoral PWV, is a predictor of future fatal or nonfatal cardiovascular events in high-risk type 2 diabetic patients over and beyond traditional cardiovascular risk factors, presence of micro- or macrovascular diabetes complications, metabolic control parameters, and ambulatory BP levels. Furthermore, it shows that the addition of arterial stiffness improved cardiovascular risk stratification in relation to a prediction model with standard risk markers and that its predictive power is higher in younger individuals and patients with microvascular complications and poor glycemic control. Overall, this study suggests that aortic stiffness measurement should be incorporated into cardiovascular risk assessment of high-risk type 2 diabetic patients.
Previous studies established the prognostic value of aortic stiffness in patients with end-stage renal disease (3,23), arterial hypertension (4,24), and chest pain (25) and in population-based samples of elderly (5,26) and middle-aged individuals (6,7). Nevertheless, only one study (13) evaluated the prognostic impact of aortic stiffness in 397 type 2 diabetic patients and 174 nondiabetic individuals (55 with glucose intolerance) followed for a mean of 10 years. The study showed that central aortic stiffness measured by flow Doppler PWV was a predictor of cardiovascular mortality (HR 1.08 [95% CI 1.03–1.14] for each 1 m/s increment) together with age and sex in the diabetes subgroup. In the analysis with all individuals combined, aortic stiffness remained an independent predictor of mortality together with age, sex, glucose tolerance status, smoking, and ethnicity. However, this pioneering study did not adjust for important covariates, such as serum lipids, presence of diabetes complications, and metabolic control parameters. Hence, the present study is the first in our knowledge to perform a comprehensive statistical adjustment for potential confounders of the association between arterial stiffness and occurrence of cardiovascular events. In this sense, we also adjusted for ambulatory BPs, which have been consistently demonstrated to be better risk markers than clinical BPs (27,28). Only one previous study (6) adjusted survival analysis for ambulatory BPs. Therefore, the present study confirmed and advanced previous results (13) by showing that increased aortic stiffness added prognostic information over standard risk factors and may be considered a valuable biomarker of cardiovascular disease risk in type 2 diabetic individuals.
The finding that the predictive performance of aortic stiffness seemed to be stronger in younger patients than in older ones had been previously described in patients with end-stage renal failure (8) and could possibly reflect selective survival bias in which very high-risk individuals with increased aortic stiffness died earlier and were underrepresented in cohort studies or elderly survivors were less vulnerable to the adverse effects of increased arterial stiffening. Otherwise, the differential prognostic influence of aortic stiffness in patients with microvascular complications and poor glycemic control, observed exclusively in the analyses with dichotomized aortic PWV, is a new finding that needs confirmation from other large cohorts of diabetic patients. However, we had previously demonstrated in cross-sectional analysis (15) that aortic stiffness was independently associated with diabetic microvascular disease, particularly with retinopathy, raising a hypothesis of cross talk between macro- and microcirculatory disease in diabetes (12).
The physiopathological mechanisms linking increased aortic stiffness to cardiovascular events occurrence are still largely unclear. The widely accepted concept that a high aortic PWV leads to an early backward pulse wave return and higher systolic and lower diastolic central pressures, causing a rise in left ventricular workload and subsequent hypertrophy and a decrease in coronary perfusion with consequent myocardial oxygen demand/perfusion imbalance, has been questioned in a study from the Framingham cohort (7). The study showed that aortic PWV, but not measures of central aortic pressures or of backward pulse wave reflex (augmentation index), was a strong cardiovascular risk predictor. Otherwise, aortic PWV may be an integrate indicator not only of the effects of aging and genetic background, but also of the cumulative damage of cardiovascular risk factors on the arterial wall over time (8). Particularly in diabetes, aortic stiffness may be accelerated by long-term hyperglycemia and formation of advanced glycation end products on the arterial wall, with loss of elastin and increased collagen cross-links (29). Nevertheless, whether arterial stiffening can be reduced by treatment and, most important, whether this improvement will be translated into a better prognosis still remains to be demonstrated. Some studies suggested that aortic stiffness can be improved independent of BP changes by interventions such as salt restriction and use of renin-angiotensin system blockers (30,31) and in diabetes, by breakers of advanced glycation end product cross-links (32). Moreover, one study reported that aortic stiffness attenuation may be associated with improved survival in patients with end-stage renal failure (33). If these results were more widely confirmed, then decreasing aortic stiffness may be a potential treatment target, at least in high-risk patients.
We acknowledge some limitations of the study. An observational cohort study cannot definitely demonstrate that there is a causal link underlying the association between aortic stiffness and cardiovascular events. We also cannot rule out residual confounding by duration or severity of associated risk factors. In addition, the separate analyses of cardiac and cerebrovascular-peripheral events and of cardiovascular and noncardiovascular mortality should be met with caution because of the small number of events and consequent overfitting. We evaluated a middle-aged to elderly high-risk type 2 diabetes cohort; therefore, the results may not be generalizable to younger or lower-risk type 2 diabetic individuals. Otherwise, the strengths of the study are that it was the first to use the recently recommended 80% correction factor for the direct carotid-femoral distance measurement (19), which seems to be the most accurate body surface estimate of the true distance, although it did not influence the associations between aortic stiffness and cardiovascular risk. More important, we obtained a complete and standardized evaluation of risk factors, ambulatory BPs, metabolic control parameters, and presence of diabetes complications of the whole studied group that could potentially influence the prognostic impact of aortic stiffness.
In conclusion, we demonstrate that carotid-femoral PWV, a noninvasive, safe, and readily implemented method of assessing aortic stiffness in an office setting with relatively inexpensive equipment and modest training, provided prognostic information beyond traditional and less traditional risk factors in a high-risk type 2 diabetes population. Whether measures to decrease aortic stiffness will translate into reduction of cardiovascular risk demands future studies.
Acknowledgments
This study was partially supported by research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico and the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro.
No potential conflicts of interest relevant to this article were reported.
C.R.L.C. contributed to the interpretation of the results and drafted the manuscript. C.R.L.C., N.C.L., and G.F.S. contributed to the study concept and design, patient follow-up, data collection and analysis, interpretation of the results, and review of the manuscript. M.T.F. performed all PWV measurements. C.R.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 23rd European Meeting on Hypertension and Cardiovascular Protection, Milan, Italy, 14–17 June 2013.
The authors thank Emilia M. Nascimento, PhD, and Basilio B. Pereira, PhD, Federal University of Rio de Janeiro, for statistical support during manuscript revision.
References
- 1.Laurent S, Cockcroft J, Van Bortel L, et al. European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007;49:1202–1206 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999;99:2434–2439 [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001;37:1236–1241 [DOI] [PubMed] [Google Scholar]
- 5.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113:657–663 [DOI] [PubMed] [Google Scholar]
- 6.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113:664–670 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–1327 [DOI] [PubMed] [Google Scholar]
- 9.Taniwaki H, Kawagishi T, Emoto M, et al. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Vessel wall properties in type 2 diabetes. Diabetes Care 1999;22:1851–1857 [DOI] [PubMed] [Google Scholar]
- 10.Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003;52:448–452 [DOI] [PubMed] [Google Scholar]
- 11.De Angelis L, Millasseau SC, Smith A, et al. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension 2004;44:67–71 [DOI] [PubMed] [Google Scholar]
- 12.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 2008;51:527–539 [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–2090 [DOI] [PubMed] [Google Scholar]
- 14.Cardoso CR, Leite NC, Freitas L, Dias SB, Muxfeld ES, Salles GF. Pattern of 24-hour ambulatory blood pressure monitoring in type 2 diabetic patients with cardiovascular dysautonomy. Hypertens Res 2008;31:865–872 [DOI] [PubMed] [Google Scholar]
- 15.Cardoso CR, Ferreira MT, Leite NC, Barros PN, Conte PH, Salles GF. Microvascular degenerative complications are associated with increased aortic stiffness in type 2 diabetic patients. Atherosclerosis 2009;205:472–476 [DOI] [PubMed] [Google Scholar]
- 16.Salles GF, Teixeira GB, Leite NC, Muxfeldt ES, Cardoso CR. Uncontrolled isolated office hypertension is associated with subclinical markers of cardiovascular disease in hypertensive type 2 diabetic patients. Hypertens Res 2010;33:819–824 [DOI] [PubMed] [Google Scholar]
- 17.Cardoso CR, Leite NC, Muxfeldt ES, Salles GF. Thresholds of ambulatory blood pressure associated with chronic complications in type 2 diabetes. Am J Hypertens 2012;25:82–88 [DOI] [PubMed] [Google Scholar]
- 18.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 1995;26:485–490 [DOI] [PubMed] [Google Scholar]
- 19.Van Bortel LM, Laurent S, Boutouyrie P, et al. Artery Society. European Society of Hypertension Working Group on Vascular Structure and Function. European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–448 [DOI] [PubMed] [Google Scholar]
- 20.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–935 [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 23.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension 2005;45:592–596 [DOI] [PubMed] [Google Scholar]
- 24.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002;39:10–15 [DOI] [PubMed] [Google Scholar]
- 25.Choi CU, Park EB, Suh SY, et al. Impact of aortic stiffness on cardiovascular disease in patients with chest pain: assessment with direct intra-arterial measurement. Am J Hypertens 2007;20:1163–1169 [DOI] [PubMed] [Google Scholar]
- 26.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Health ABC Study Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–3390 [DOI] [PubMed] [Google Scholar]
- 27.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005;46:156–161 [DOI] [PubMed] [Google Scholar]
- 28.Boggia J, Li Y, Thijs L, et al. International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007;370:1219–1229 [DOI] [PubMed] [Google Scholar]
- 29.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003;21:3–12 [DOI] [PubMed] [Google Scholar]
- 30.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004;44:35–41 [DOI] [PubMed] [Google Scholar]
- 31.Karalliedde J, Smith A, DeAngelis L, et al. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension 2008;51:1617–1623 [DOI] [PubMed] [Google Scholar]
- 32.Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2001;104:1464–1470 [DOI] [PubMed] [Google Scholar]
- 33.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001;103:987–992 [DOI] [PubMed] [Google Scholar]