Abstract
OBJECTIVE
To establish the prevalence of disturbed eating behavior (DEB) and insulin omission among adolescents with type 1 diabetes using intensive insulin treatment in a nationwide population-based study.
RESEARCH DESIGN AND METHODS
The Diabetes Eating Problem Survey–Revised (DEPS-R) is a diabetes-specific screening tool for DEB. Clinical data and HbA1c were obtained from the Norwegian Childhood Diabetes Registry.
RESULTS
A total of 770 children and adolescents 11–19 years of age with type 1 diabetes completed the DEPS-R. A total of 27.7% of the females and 8.6% of the males scored above the DEPS-R cutoff. Participants scoring above the cutoff had significantly higher HbA1c (9.2% [77 mmol/mol]; SD, 1.6) than participants scoring below the cutoff (8.4% [68 mmol/mol]; SD, 1.3; P < 0.001). The prevalence of DEB increased significantly with age and weight, from 7.2% in the underweight group to 32.7% in the obese group, and from 8.1% in the youngest age-group (11–13 years) to 38.1% in the oldest age-group (17–19 years). A total of 31.6% of the participants reported insulin restriction and 6.9% reported insulin omission after overeating. Patients reporting insulin restriction had significantly higher HbA1c (9.0% [75 mmol/mol]; SD, 1.7) than nonrestrictors (8.3% [67 mmol/mol]; SD, 1.2; P < 0.001).
CONCLUSIONS
One-fourth of girls with type 1 diabetes scored above the cutoff for DEB and one-third reported skipping their insulin dose entirely at least occasionally after overeating. Both DEB and insulin restriction were associated with poorer metabolic control, which may increase the risk of serious late diabetes complications.
Type 1 diabetes appears to be a risk factor for the development of disturbed eating behavior (DEB) (1,2). Estimates of the prevalence of DEB among individuals with type 1 diabetes range from 10 to 49% (3,4), depending on methodological issues such as the definition and measurement of DEB. Some studies only report the prevalence of full-threshold diagnoses of anorexia nervosa, bulimia nervosa, and eating disorders not otherwise specified, whereas others also include subclinical eating disorders (1). The term “DEB” is used here to refer to both clinical and subclinical eating pathology.
Although different terminology complicates the interpretation of prevalence rates across studies, the findings are sufficiently robust to indicate that there is a higher prevalence of DEB in type 1 diabetes compared with healthy controls. A meta-analysis reported a three-fold increase of bulimia nervosa, a two-fold increase of eating disorders not otherwise specified, and a two-fold increase of subclinical eating disorders in patients with type 1 diabetes compared with controls (2). No elevated rates of anorexia nervosa were found. DEBs have been found to be associated with higher weight and older adolescence (5,6). Although most studies focus on females, some research suggests that males with type 1 diabetes also may have an increased risk of development of DEB (7). However, the bulk of research investigating the prevalence of DEB in type 1 diabetes uses generic measures of DEB or diabetes-adapted generic measures with an additional question about insulin misuse. Generic measures of DEB may not be appropriate in a type 1 diabetes population because of their assessment of behaviors that are a natural part of diabetes care (for example, restriction of carbohydrate intake and eating when not hungry). This may lead to inflated prevalence estimates; therefore, diabetes-specific measures are warranted (8).
When DEB and type 1 diabetes co-occur, rates of morbidity and mortality are dramatically increased. A Danish study of comorbid type 1 diabetes and anorexia nervosa showed that the crude mortality rate at 10-year follow-up was 2.5% for type 1 diabetes and 6.5% for anorexia nervosa, but the rate increased to 34.8% when occurring together (the standardized mortality rates were 4.06, 8.86, and 14.5, respectively) (9). The presence of DEB in general also can severely impair metabolic control and advance the onset of long-term diabetes complications (4). Insulin reduction or omission is an efficient weight loss strategy uniquely available to patients with type 1 diabetes and has been reported in up to 37% of patients (10–12). Insulin restriction is associated with poorer metabolic control, and previous research has found that self-reported insulin restriction at baseline leads to a three-fold increased risk of mortality at 11-year follow-up (10).
Few population-based studies have specifically investigated the prevalence of and relationship between DEBs and insulin restriction. The generalizability of existing research remains limited by relatively small samples and a lack of males. Further, many studies have relied on generic measures of DEBs, which may not be appropriate for use in individuals with type 1 diabetes. The Diabetes Eating Problem Survey–Revised (DEPS-R) is a newly developed and diabetes-specific screening tool for DEBs. A recent study demonstrated satisfactory psychometric properties of the Norwegian version of the DEPS-R among children and adolescents with type 1 diabetes 11–19 years of age (13). The DEPS-R has not yet been used to assess prevalence of DEBs in children and adolescents with type 1 diabetes.This study aimed to assess young patients with type 1 diabetes to assess the prevalence of DEBs and frequency of insulin omission or restriction, to compare the prevalence of DEB between males and females across different categories of weight and age, and to compare the clinical features of participants with and without DEBs and participants who restrict and do not restrict insulin.
RESEARCH DESIGN AND METHODS
Design
This is a cross-sectional epidemiological survey of the nationwide, population-based Norwegian Childhood Diabetes Registry (NCDR). The NCDR includes all children with newly diagnosed diabetes since 1989. All pediatric departments in Norway perform and report the results of annual standardized examinations to NCDR. The completeness of ascertainment in the NCDR is high at 92% (14).
Participants
The participants were recruited from the NCDR between 1 April 2010 and 31 March 2011. A total of 1,816 individuals with type 1 diabetes 11–19 years of age in the NCDR were invited to participate in the study. The final sample consisted of 770 (42.4% response rate) children and adolescents with type 1 diabetes 11–19 years of age. There were 380 (49.4%) males and 390 (50.6%) females. Participants were similar to nonparticipating patients in the NCDR, but a few differences were found. Participants were somewhat younger than nonparticipants (14.6 vs. 15.1 years; P < 0.001), had slightly lower HbA1c (8.5 vs. 8.7%; P < 0.01), and had a somewhat shorter duration of type 1 diabetes (5.3 vs. 6.1 years; P < 0.001). However, the effect sizes were small (−0.2, −0.1, and −0.2, respectively). Participants and nonparticipants did not differ regarding BMI or age at onset of type 1 diabetes.
Procedure
The regional ethics committee approved the study. Written informed consent was obtained from all participants and their parents if the participant was younger than 16 years of age. Questionnaires were distributed to the participants at their regularly scheduled appointments at their local outpatient diabetes clinic.
Measures
The Diabetes Eating Problem Survey (DEPS) (15) was the first measure designed to screen for DEB specific to type 1 diabetes and also includes insulin omission. The original DEPS consisted of 28 items but recently has been revised (DEPS-R). The DEPS-R is a brief 16-item version of the DEPS and has demonstrated good psychometric properties (16). Responses are scored on 6-point Likert items scale and higher scores indicate greater pathology. Items tap concepts such as drive for thinness, such as “I would rather be thin than have good control of my diabetes,” and eating pathology, such as “I skip meals and/or snacks” or “I make myself vomit.” A recommended cutoff score of ≥20 has been empirically established as a threshold indicating the need for further clinical assessment of eating pathology (16). This cutoff was determined by examining a general population of youth with type 1 diabetes without a known diagnosis of an eating disorder and external validity was confirmed against reports by medical providers of insulin restriction. In our study, DEB was operationally defined as a DEPS-R score above the cutoff of ≥20. Further, we operationally defined insulin restriction and insulin omission according to the following two DEPS-R items: “When I overeat, I do not take enough insulin to cover the food” and “After I overeat, I skip my next insulin dose.”
The Eating Attitudes Test (EAT) (17) is a generic screening measure of eating pathology used internationally to detect pathologic eating attitudes and behaviors. A 12-item Norwegian version of EAT (EAT-12) has been developed (18) and has demonstrated adequate psychometric properties (19). Answers are ranged on a 4-point scale and higher scores indicate greater pathology. It was used here to validate the cutoff score on the DEPS-R.
Clinical data were obtained from NCDR. HbA1c was determined for all participants by high-performance liquid chromatography (Tosoh G7; Tosoh Europe N.V.). All samples were analyzed in the same central laboratory and standardized according to the Diabetes Control and Complications Trial standards. The reference range was 4.0–6.0%; the analytical coefficient of variation was <1%. BMI was calculated based on weight and height (kg/m2) and standardized to a z-score according to age and sex using the Centers for Disease Control and Prevention Growth Charts 2000 because the participants were primarily younger than 18 years of age (zBMI) (20). Weight was categorized into the following four groups according to the World Health Organization classification scheme (21): underweight (BMI <18.5); normal weight (BMI ≥18.5–24.9); overweight (BMI ≥25–29.9); and obese (BMI ≥30). The BMI for all participants were adjusted for age and sex to generate these groups. Height and weight were assessed by a medical provider as part of a routine control at the local diabetes outpatient clinics. Pubertal state was presented as Tanner stages 1–5 and was defined as follows: prepubertal = Tanner 1; pubertal = Tanner 2–4; and postpubertal = Tanner 5.
Statistical analyses
Prevalence of DEBs (≥20 on the DEPS-R) and frequency of insulin restriction are presented as percentages. Correlations were performed to explore relationships between prevalence of DEB and the following: HbA1c; EAT-12 score; zBMI; age; pubertal stage; duration of type 1 diabetes; onset of type 1 diabetes; number of ketoacidosis episodes; and number of consultations with the diabetes team at the local outpatient clinic. To correct for multiple comparisons, the α level was set to <0.01 for all analyses. Group differences were investigated using t tests. Pearson χ2 tests were used for categorical variables. Effect sizes were calculated by means of Cohen d. Following the guidelines by Cohen (22), effect sizes >0.2 were interpreted as small, effect sizes >0.5 were interpreted as medium, and effect sizes >0.8 were interpreted as large. Statistical analyses were conducted using SPSS version 18 (SPSS IBM).
RESULTS
Participant characteristics
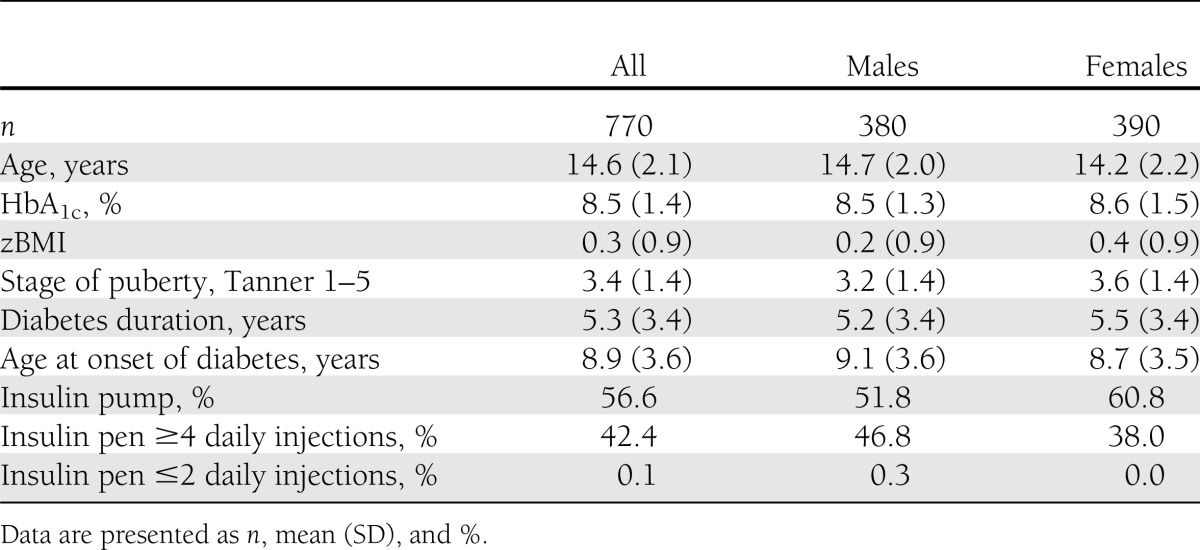
Table 1 illustrates sample characteristics. Mean age of the 770 (390 females) participants was 14.6 years (SD, 2.1) and age at onset of type 1 diabetes was 8.9 years (SD, 3.6). Mean type 1 diabetes duration was 5.3 years (SD, 3.4), mean zBMI was 0.3 (SD, 0.9), mean HbA1c was 8.5% (69 mmol/mol; SD, 1.4), and mean pubertal stage was 3.4 (SD, 1.4); 98% used intensified insulin treatment, defined as four or more insulin injections per day or using an insulin pump. A total of 56% used insulin pumps.
Table 1.
Participant characteristics
Prevalence of DEB
The proportion of participants scoring above the predetermined threshold of DEB is presented in Table 2. A total of 27.7% of the females and 8.6% of the males scored above cutoff on the DEPS-R (P < 0.001). Significant differences emerged across age and weight categories, and notable sex-specific trends were observed.
Table 2.
Prevalence rates of DEB in type 1 diabetes based on a predetermined cutoff score of ≥20 on the DEPS-R, categorized by age and weight
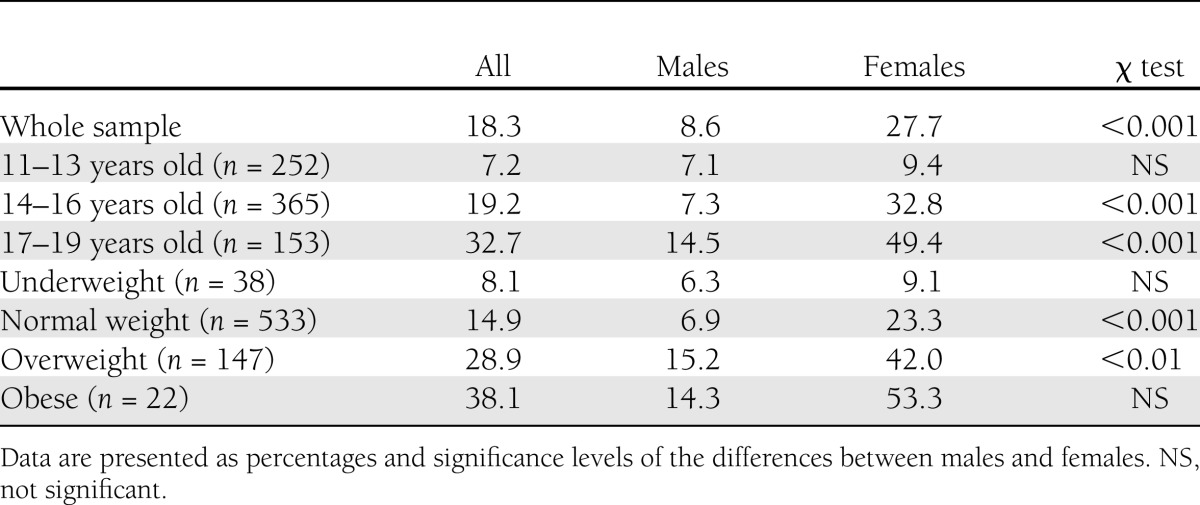
For the youngest (11–13 years) and underweight (BMI <18.5) categories, the proportion of DEB was <10% for both sexes (not significant). Among females, the prevalence of DEB increased dramatically with age to ∼33% among 14 to 16 year olds and to nearly 50% among 17 to 19 year olds. Among males, the rate remained low at 7% for 14 to 16 year olds and doubled to ∼15% for 17 to 19 year olds.
A similar sex-specific pattern was detected across weight categories. Among females, the prevalence of DEB increased steadily and significantly from 9% among the underweight category to 23% for normal weight, 42% for overweight, and 53% for the obese categories, respectively. Among males, ∼6–7% of both the underweight and normal weight groups reported DEB, with rates increasing to ∼15% for both the overweight and obese groups.
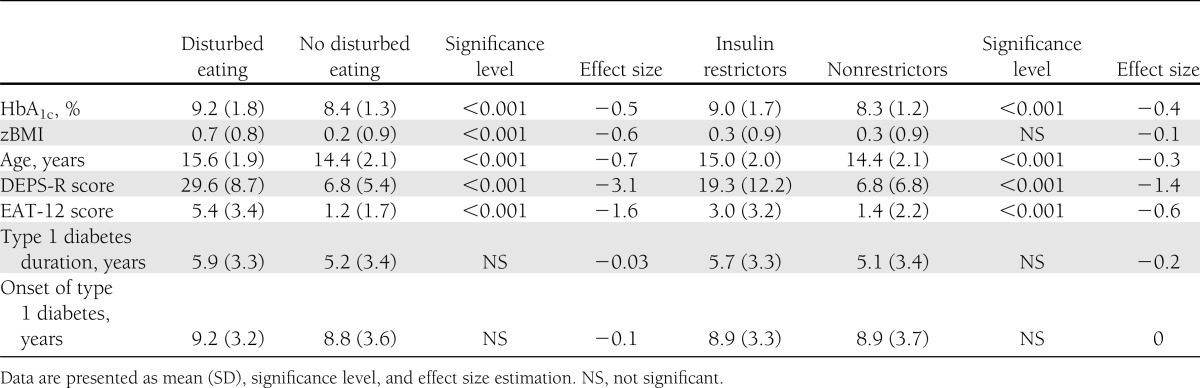
Table 3 presents the comparisons of clinical features between participants scoring below and above the cutoff on DEPS-R. For the total sample, participants scoring above cutoff on the DEPS-R also had higher scores on the EAT-12 (5.4 [SD, 3.4] vs. 1.2 [SD, 1.7]; P < 0.001), higher HbA1c (9.2 [SD, 1.8] vs. 8.4 [SD, 1.3]; P < 0.001), higher zBMI (0.7 [SD, 0.8] vs. 0.2 [SD, 0.9]; P < 0.001), older age (15.6 [SD, 1.9] vs. 14.4 [SD, 2.1]; P < 0.001), higher pubertal stage (4.1 [SD, 1.1] vs. 3.2 [SD, 1.4]; P < 0.001), and a higher number of consultations with the diabetes team (4.5 [SD, 2.3] vs. 3.8 [SD, 1.5]; P < 0.01). The effect sizes for HbA1c, zBMI, and age were considered medium (−0.5, −0.6, and −0.7, respectively) and the effect size for the number of consultations with a diabetes team was considered small (−0.3), whereas the effect sizes for EAT-12 and pubertal stage were considered high (−1.6 and −0.8). The groups were not significantly different with respect to duration of type 1 diabetes, age at onset of type 1 diabetes, or episodes of diabetes ketoacidosis. When separated by sex, females scoring above the cutoff on the DEPS-R had significantly higher HbA1c (9.2% [SD, 1.9]) than females scoring below the cutoff (8.4% [SD, 1.3]; P < 0.001). The same trend was observed among males (9.2% [SD, 1.6] vs. 8.4% [SD, 1.3]; P < 0.01).
Table 3.
Comparisons between participants with and without DEB and between insulin restrictors and nonrestrictors
Frequency of insulin restriction and omission
A total of 31.6% of the participants reported using less insulin and 6.9% reported skipping their insulin dose entirely at least occasionally after overeating. When assessing the sexes separately, we found that 36.8% of females reported restricting and 26.2% reported skipping insulin because of overeating. The rates for males were 9.4 and 4.5%, respectively. As described in Table 3, insulin restrictors were significantly older (15.0 [SD, 2.0] vs. 14.4 [SD, 2.1]; P < 0.001), had significantly higher HbA1c (9.0 [SD, 1.7] vs. 8.3 [SD, 1.2]; P < 0.001), and had significantly higher scores on both the DEPS-R (19.3 [SD, 12.2] vs. 6.8 [SD, 6.8]; P < 0.001) as well as the EAT-12 (3.0 [SD, 3.2] vs. 1.4 [SD, 2.2]; P < 0.001) than nonrestrictors. The effect sizes were considered small for age (−0.29) and HbA1c (−0.42), medium for the EAT-12 (−0.56), and large for the DEPS-R (−0.97). When separated by sex, the females who restrict insulin had significantly higher HbA1c (9.1% [SD, 1.7]) than females not restricting insulin (8.4% [SD, 1.3]; P < 0.001). The same trend was observed among males (8.8% [SD, 1.6] vs. 8.3% [SD, 1.2]; P < 0.01).
A total of 41.1% of the insulin restrictors also scored above the cutoff on the DEPS-R. However, this should be interpreted cautiously because insulin restriction is likely to contribute to a higher score on the DEPS-R.
CONCLUSIONS
This study found that 27.7% of female and 9% of male children and adolescents with type 1 diabetes receiving intensified insulin treatment scored above the predetermined cutoff on the DEPS-R, suggesting a level of disturbed eating that warrants further attention by treatment providers. The prevalence of DEB increased considerably with increasing age and weight, especially for females. Also, 31.6% of the total participants admitted to restricting insulin at least on an occasional basis after overeating. Among females, rates of insulin restriction and insulin omission were 37 and 26%. Importantly, patients scoring above the DEPS-R cutoff and patients who engaged in insulin restriction had higher HbA1c, indicating poorer metabolic control.
The finding that DEBs are common in young patients with type 1 diabetes is in line with previous literature (2). However, because of different assessment methods and different definitions of DEB, direct comparison with other studies is complicated, especially because this is the first study to have used the DEPS-R in a prevalence study. However, two studies using the original DEPS have reported similar results, with 37.9% (23) and 53.8% (24) of the participants reporting engaging in unhealthy weight control practices. In our study, females scored significantly higher than males, which is not surprising given previous studies demonstrating an increased risk of development of DEB in nondiabetic females compared with males. In addition, the prevalence rates increased considerably by increasing age and weight. A relationship between eating pathology and older age and higher BMI also has been demonstrated in previous research conducted in both diabetic and nondiabetic adolescent populations. For example, Olmsted et al. (6) conducted a longitudinal study and found that higher BMI predicted the onset of DEB among patients with type 1 diabetes. Further, Colton et al. (5) investigated a younger sample of 101 girls with type 1 diabetes aged 9–14 years and reported that 8% of the girls had eating disorders not otherwise specified or subthreshold eating problems. No full-threshold cases of anorexia nervosa or bulimia nervosa were identified. The prevalence rates among preteen and early-teen girls with type 1 diabetes appear to be lower than the prevalence rates reported in studies of older samples (4,12).
The level of stringency applied when determining the cutoff value influences the prevalence rates. It has been suggested that the cutoff for identifying girls with type 1 diabetes at risk for development of DEB should be set low because early intervention is crucial to prevent the development of eating disorders (6). In line with this advice, the cutoff on the DEPS-R is not diagnostic but rather a threshold to identify at-risk individuals in need of further evaluation. This measurement issue likely contributes to the relatively high prevalence rates reported in this study. In light of the finding that 50% of the females 17–19 years of age scored above threshold for identifying at-risk individuals, it could be argued that the DEPS-R cutoff was too low. However, the finding that individuals scoring above the cutoff also demonstrated higher scores on the EAT-12, and higher zBMI provides some evidence of concurrent validity and suggests the presence of eating, weight, or shape concerns that may adversely affect the prognosis of type 1 diabetes. Future studies with clinical eating disorders samples are necessary for additional validation of the DEPS-R cutoff and to provide a broader context for interpreting our results.
In addition to methodological issues involved, the operational definition of “at-risk” prevalence rates is influenced by the choice of measurement. A range of different assessment tools has been used to assess DEB in type 1 diabetes, including generic measures (25), diabetes-adapted measures (4), self-report measures (26), and diagnostic interviews (6). Whether a generic or diabetes-adapted assessment tool has been implemented may affect the observed prevalence rates. Young et al. (8) demonstrated in their recent meta-analysis that the administration of generic measures to patients with type 1 diabetes resulted in inflated prevalence estimates because these instruments assess the extent to which patients worry about their diets, reduce the intake of certain food groups, or eat when they are not hungry. Individuals with type 1 diabetes without DEB may score high on such items because of their daily dietary diabetes management, thereby leading to inaccurate and inflated prevalence rates.
Consistent with existent literature (10–12,27), we found a high frequency of insulin restriction. For example, Bryden et al. (11) assessed 113 males and females (aged 17–25 years) with type 1 diabetes and found that a total of 37% of the females (no males) reported a history of insulin omission or reduction for weight control purposes. Peveler et al. (12) investigated 87 females with type 1 diabetes aged 11–25 years, and 36% reported intentionally reducing or omitting their insulin doses to control their weight. Finally, Goebel-Fabbri et al. (10) examined 234 females 13–60 years of age and found that 30% reported insulin restriction. Similarly, 36.8% of the participants in our study reported reducing their insulin doses occasionally or more often after overeating. However, it is important to note that it was not specified in the two DEPS-R questions that tapped insulin restriction and omission that such behavior was for the purpose of weight loss. There are other possible reasons for why individuals reduce or omit insulin, for example, fear of hypoglycemia, interference with activities of daily living, injection pain, injection embarrassment, or negative affect toward injections (28). Nevertheless, the clinical effects of insulin restriction are detrimental on the total physiology and are important to the pathogenesis of short-term and long-term consequences. Further, the term “overeating” is complex and may be interpreted differently by participants. In the absence of a structured interview, it is unknown how participants have defined this term. This issue may have resulted in a biased estimate, and possibly an overestimation, of insulin restriction.
Despite these issues, poorer metabolic control was found among patients who restrict insulin compared with nonrestrictors. This represents an important finding in the current study with direct clinical implications. When patients engage in insulin restriction, blood glucose control is not yet optimal despite receiving intensive insulin treatment, free of charge, with the newest insulin analogs or insulin pumps. In fact, only one-third of the patients in the NCDR manage the international target of HbA1c values <7.5% (29). Our study indicates that DEB and insulin restriction contribute to the failure to achieve optimal metabolic control.
Similar to previous reports, this study has identified relatively high rates of both DEB and insulin restriction among children and adolescents with type 1 diabetes. The clinical implications are serious and place the individual at risk for the development of a variety of health problems. Continuous high blood glucose levels can cause short-term effects such as dehydration, fatigue, and reduction of muscle tissue. Over longer periods of time, individuals are at risk for development of serious late complications such as blindness, kidney failure, and cardiovascular disease. It is important to raise awareness among health personnel to improve the ability to recognize symptoms of this risky behavior. Our findings that DEB was associated with female sex, older adolescence, and overweight status should be considered by health providers when working with this population.
DEBs have shown to be persistent among individuals with type 1 diabetes, and routine screening and subsequent early intervention are important to improve the poor prognosis associated with this type of comorbidity. Treatment of this patient group is complicated, and there is a scarcity of controlled treatment studies and no consensus on how to combine treatments for both type 1 diabetes and eating disorders. However, it is likely that increased and direct focus on psychosocial issues among young people and their families may be beneficial. Multidisciplinary treatment teams are recommended to best-meet the complexity of this comorbidity (30). Randomized controlled studies are needed to identify efficient treatment approaches for patients with concurrent type 1 diabetes and DEB. Another consideration for future research is the association between DEB and psychosocial factors such as parent involvement in their children’s daily diabetes management, aspects of autonomy for young patients with type 1 diabetes, and comorbidities such as anxiety and depression. The large population-based sample recruited from 22 of 27 of pediatric departments nationwide is considered a strength of this study. In addition, we included both males and females and used both standardized clinical data and a diabetes-specific assessment tool. A total of 98% used intensified insulin treatment. A major limitation is the relatively low response rate (42%). It should be noted that our calculation of the response rate is conservative because it is based on the total eligible population. A less conservative approach would have substantially increased the rate. Our participants were compared with nonparticipants and, from a clinical point of view, are representative of children and adolescents with type 1 diabetes in Norway. Finally, the clinics reported that >200 patients nationwide were never approached because of work overload. This occurred randomly, however, and therefore is not believed to constitute a selection bias. Other limitations include the uncertainty of the cutoff score of the DEPS-R and the potential various reasons for restricting insulin besides losing weight, in addition to the term “overeating,” which can leave room for interpretation.
In conclusion, our study found that ∼28% of female children and adolescents with type 1 diabetes scored above the cutoff on the DEPS-R, indicating some level of DEB. In addition, almost 38% of the females reported restriction of insulin. DEB and insulin restriction were associated with higher HbA1c, indicating poorer metabolic control. Poor metabolic control may place individuals at risk for increased morbidity and mortality. Routine and annual screenings of disturbed eating are recommended in young patients with type 1 diabetes, especially among females, older adolescents, and individuals with higher BMI, to secure early intervention and hopefully to improve the poor prognosis associated with comorbid type 1 diabetes and disturbed eating.
Acknowledgments
The Research Council of Norway funded this work. The Norwegian Childhood Diabetes Registry is fully funded by the Health Region South-East.
No potential conflicts of interest relevant to this article were reported.
L.W. contributed to the planning of the study, analyzed the data, and wrote the manuscript. D.H.F. planned the study, collected data for DEB, and contributed to the data analyses and the writing of the manuscript. T.S. is the leader of the NCDR, contributed to the planning of the study, collected clinical data from the NCDR, and contributed to the manuscript. K.D.-J. was one of the initiators of the NCDR, had the idea for this study, supervised D.H.F., contributed to the planning of the study, and contributed to the manuscript. Ø.R. supervised L.W. and contributed to the data analyses and the writing of the manuscript. L.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented orally at the 10th London International Eating Disorders Conference, London, United Kingdom, 29–31 March 2011.
The authors thank all members of the Norwegian Childhood Diabetes Study Group who provided data to the NCDR, and the staff and patients from the outpatient clinics in Norway for participating in the study. The authors also thank the Norwegian Childhood Diabetes Registry and Regional Eating Disorders Service, Oslo University Hospital, for contributing to the study. Finally, the authors thank Deborah Lynn Reas (Oslo University Hospital, Regional Eating Disorder Service) for assistance with preparing the manuscript.
References
- 1.Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005;28:417–419 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S. Eating disorders in females with type 1 diabetes: An update of a meta-analysis. Eur Eat Disord Rev 2002;10:241–254 [Google Scholar]
- 3.Colton PA, Olmsted MP, Daneman D, Rydall AC, Rodin GM. Five-year prevalence and persistence of disturbed eating behavior and eating disorders in girls with type 1 diabetes. Diabetes Care 2007;30:2861–2862 [DOI] [PubMed] [Google Scholar]
- 4.Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ 2000;320:1563–1566 [PMC free article] [PubMed] [Google Scholar]
- 5.Colton P, Olmsted M, Daneman D, Rydall A, Rodin G. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case-controlled study. Diabetes Care 2004;27:1654–1659 [DOI] [PubMed] [Google Scholar]
- 6.Olmsted MP, Colton PA, Daneman D, Rydall AC, Rodin GM. Prediction of the onset of disturbed eating behavior in adolescent girls with type 1 diabetes. Diabetes Care 2008;31:1978–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svensson M, Engström I, Aman J. Higher drive for thinness in adolescent males with insulin-dependent diabetes mellitus compared with healthy controls. Acta Paediatr 2003;92:114–117 [DOI] [PubMed] [Google Scholar]
- 8.Young V, Eiser C, Johnson B, et al. Eating problems in adolescents with type 1 diabetes: a systematic review with meta-analysis. Diabet Med 2013;30:189–198 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen S, Emborg C, Mølbak AG. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care 2002;25:309–312 [DOI] [PubMed] [Google Scholar]
- 10.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 2008;31:415–419 [DOI] [PubMed] [Google Scholar]
- 11.Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HA. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care 2003;26:1052–1057 [DOI] [PubMed] [Google Scholar]
- 12.Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005;28:84–88 [DOI] [PubMed] [Google Scholar]
- 13.Wisting L, Frøisland DH, Skrivarhaug T, Dahl-Jørgensen K, Rø Ø. Psychometric properties, norms, and factor structure of the Diabetes Eating Problem Survey–Revised in a large sample of children and adolescents with type 1 diabetes. Diabetes Care 2013;36:2198–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrivarhaug T, Stene LCM, Strøm H, Drivvoll AK, Njølstad PR, Joner G, Norwegian Childhood Diabetes Study Group The incidence of childhood onset type 1 diabetes in Norway is steadily increasing for both sexes (Abstract). Pediatr Diabetes 2010;11(Suppl. 14):2420015124 [Google Scholar]
- 15.Antisdel JE LLAB: Improved detection of eating problems in women with type 1 diabetes using a newly developed survey (Abstract). Diabetes 2001;50 (Suppl. 1):A47
- 16.Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care 2010;33:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med 1982;12:871–878 [DOI] [PubMed] [Google Scholar]
- 18.Lavik NJ, Clausen SE, Pedersen W. Eating behaviour, drug use, psychopathology and parental bonding in adolescents in Norway. Acta Psychiatr Scand 1991;84:387–390 [DOI] [PubMed] [Google Scholar]
- 19.Engelsen BK, Hagtvet KA. The dimensionality of the 12-item version of the Eating Attitudes Test. Confirmatory factor analyses. Scand J Psychol 1999;40:293–300 [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;314:1–27 [PubMed] [Google Scholar]
- 21.World Health Organization. BMI classification [Internet], 2012. Available from http://www.who.int/features/factfiles/obesity/facts/en/ Accessed 27 February 2013
- 22.Cohen J. Statistical Power Analysis for the Behavioural Sciences. Hillsdale, NJ, Routledge, 1988 [Google Scholar]
- 23.Neumark-Sztainer D, Patterson J, Mellin A, et al. Weight control practices and disordered eating behaviors among adolescent females and males with type 1 diabetes: associations with sociodemographics, weight concerns, familial factors, and metabolic outcomes. Diabetes Care 2002;25:1289–1296 [DOI] [PubMed] [Google Scholar]
- 24.Howe CJ, Jawad AF, Kelly SD, Lipman TH. Weight-related concerns and behaviors in children and adolescents with type 1 diabetes. J Am Psychiatr Nurses Assoc 2008;13:376–385 [DOI] [PubMed] [Google Scholar]
- 25.Engström I, Kroon M, Arvidsson CG, Segnestam K, Snellman K, Aman J. Eating disorders in adolescent girls with insulin-dependent diabetes mellitus: a population-based case-control study. Acta Paediatr 1999;88:175–180 [DOI] [PubMed] [Google Scholar]
- 26.Meltzer LJ, Johnson SB, Prine JM, Banks RA, Desrosiers PM, Silverstein JH. Disordered eating, body mass, and glycemic control in adolescents with type 1 diabetes. Diabetes Care 2001;24:678–682 [DOI] [PubMed] [Google Scholar]
- 27.Schober E, Wagner G, Berger G, et al. Austrian Diabetic Incidence Study Group Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011;12:627–631 [DOI] [PubMed] [Google Scholar]
- 28.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care 2010;33:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Society for the Pediatric and Adolescent Diabetes. ISPAD Consensus Guidelines for the management of type 1 diabetes mellitus in children and adolescents [article online], 2000. Available from http://www.idf.org/sites/default/files/Diabetes-in-Childhood-and-Adolescence-Guidelines.pdf Accessed 14 July 2013
- 30.Kelly SD, Howe CJ, Hendler JP, Lipman TH. Disordered eating behaviors in youth with type 1 diabetes. Diabetes Educ 2005;31:572–583 [DOI] [PubMed] [Google Scholar]


