Abstract
OBJECTIVE
We evaluated the structural-functional relationships and the prognostic factors for renal events, cardiovascular events, and all-cause mortality in type 2 diabetic patients with biopsy-proven diabetic nephropathy.
RESEARCH DESIGN AND METHODS
Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy (n = 260) were enrolled. Patients were stratified by albuminuria (proteinuria) and estimated glomerular filtration rate (eGFR) at the time of renal biopsy. The outcomes were the first occurrence of renal events (requirement of dialysis or a 50% decline in eGFR from baseline), cardiovascular events (cardiovascular death, nonfatal myocardial infarction, coronary interventions, or nonfatal stroke), and all-cause mortality.
RESULTS
The factors associated with albuminuria (proteinuria) regardless of eGFR were hematuria, diabetic retinopathy, low hemoglobin, and glomerular lesions. The factors associated with low eGFR regardless of albuminuria (proteinuria) were age and diffuse, nodular, tubulointerstitial, and vascular lesions. The glomerular, tubulointerstitial, and vascular lesions in patients with normoalbuminuria (normal proteinuria) and low eGFR were more advanced compared to those in patients with normoalbuminuria (normal proteinuria) and maintained eGFR. In addition, compared to patients with micro-/macroalbuminuria (mild/severe proteinuria) and low eGFR, their tubulointerstitial and vascular lesions were similar or more advanced in contrast to glomerular lesions. The mean follow-up period was 8.1 years. There were 118 renal events, 62 cardiovascular events, and 45 deaths. The pathological determinants were glomerular lesions, interstitial fibrosis and tubular atrophy (IFTA), and arteriosclerosis for renal events, arteriosclerosis for cardiovascular events, and IFTA for all-cause mortality. The major clinical determinant for renal events and all-cause mortality was macroalbuminuria (severe proteinuria).
CONCLUSIONS
Our study suggests that the characteristic pathological lesions as well as macroalbuminuria (severe proteinuria) were closely related to the long-term outcomes of biopsy-proven diabetic nephropathy in type 2 diabetes.
Diabetic nephropathy occurs in 20–40% of patients with diabetes (1). The prevalence of diabetic nephropathy is increasing in proportion to the increase in prevalence of diabetes, and it has been predicted to continue to increase in future (2). Diabetes is a risk factor of cardiovascular disease and death, and diabetic nephropathy further increases these risks (3). In addition, diabetic nephropathy is the leading cause of end-stage renal disease requiring dialysis or transplantation in developed countries (4–6).
In recent years, many clinical studies have suggested strict glycemic control and blood pressure management by use of appropriate medication to suppress the onset and progression of diabetic nephropathy. Thus, it is important to identify patients at risk in the early stages to improve prognosis in patients with diabetic nephropathy (1). Albuminuria and glomerular filtration rate (GFR) are recommended for use as clinical markers of diabetic nephropathy (1,7–9). On the other hand, selection of pathological markers is complicated because a variety of renal lesions can be found in diabetic nephropathy in addition to factors such as obesity, hypertension, dyslipidemia, and aging, which are frequently complicated in type 2 diabetes, causing a wide variety of pathological changes (10).
We previously reported on the clinical factors related to the development and progression of renal lesions in diabetic nephropathy by the evaluation of serial renal biopsies or autopsy (11). In this report, we demonstrated a significant relationship between the progression of diabetic glomerulosclerosis and clinical factors such as the control of blood glucose, type of diabetes, age at onset, type of treatment, and degree of obesity.
After this study, we conducted a long-term retrospective study to evaluate the structural-functional relationships and the predictive impacts of clinicopathological parameters for renal events, cardiovascular events, and all-cause mortality among Japanese patients with biopsy-proven diabetic nephropathy in type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 260 patients who were diagnosed with diabetic nephropathy in type 2 diabetes at Kanazawa University Hospital or Kanazawa Medical Center between 1985 and 2010 were included in this study. The diagnosis of diabetes was based on the criteria of the Japanese Diabetic Society (12). The diagnosis of diabetic nephropathy was confirmed by histological characteristics, such as glomerular hypertrophy, thickened capillary basement membranes, diffuse mesangial expansion (sclerosis), nodular mesangial sclerosis, exudative lesions such as capsular drop or fibrin cap, mesangiolysis, capillary microaneurysm, or hyalinosis of afferent and efferent arterioles, using appropriate standards for renal biopsy including light microscopy, electron microscopy, and immunofluorescence examination. Patients with other glomerular diseases concomitant with diabetic nephropathy were excluded from this study. Renal biopsy was performed for precise diagnosis of renal lesions with the consent of each patient. The study protocol was approved by the medical ethics committee of Kanazawa University and Kanazawa Medical Center.
Clinical examinations
Age, sex, 24-h urinary albumin excretion, 24-h urinary protein excretion, urine dipstick test results (proteinuria and hematuria), serum creatinine, estimated GFR (eGFR), duration of diabetes, presence of diabetic retinopathy, HbA1c, BMI, systolic blood pressure, diastolic blood pressure, total cholesterol, and hemoglobin were used as baseline clinical parameters at the time of renal biopsy. eGFR for Japanese patients was calculated using the following equation: eGFR (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 (if female, ×0.739) (13). HbA1c levels were presented as National Glycohemoglobin Standardization Program values according to the recommendations of the Japanese Diabetic Society (12) and International Federation of Clinical Chemistry values.
Based on the new classification of chronic kidney disease, albuminuria at baseline was categorized as normoalbuminuria (<30 mg/day [category A1]), microalbuminuria (≥30 and <300 mg/day [category A2]), and macroalbuminuria (≥ 300 mg/day [category A3]) (7,8). We classified proteinuria among patients for whom albuminuria was not evaluated as normal proteinuria (<0.15 g/day or urine dipstick negative or trace [category A1]), mild proteinuria (≥0.15 and <0.5 g/day or urine dipstick+ [category A2]), and severe proteinuria (≥0.5 g/day or urine dipstick ≥2+ [category A3]) (7,8). When results were inconsistent, we gave priority to 24-h urinary albumin excretion, 24-h urinary protein excretion, and urine dipstick test results—in that order. In addition, eGFR at baseline was categorized as ≥60 mL/min/1.73 m2 (categories G1–2) and <60 mL/min/1.73 m2 (categories G3a-5) for categorical analyses comparing risks.
Outcomes
The outcomes for this study were the first occurrence of renal events (requirement of dialysis or a 50% decline in eGFR from baseline), cardiovascular events (cardiovascular death, nonfatal myocardial infarction, coronary interventions, or nonfatal stroke), and all-cause mortality. The patients were followed up until the end of 2011 or death.
Pathological examinations
For light microscopic examination, renal biopsy specimens were fixed in 10% phosphate-buffered formalin (pH 7.2), embedded in paraffin, and sliced into sections 4 μm thick. These specimens were stained with periodic acid Schiff (PAS) reagent, periodic acid silver methenamine, hematoxylin-eosin, and Mallory-Azan and examined by light microscopy. The severity of diffuse lesions of glomeruli was graded on a scale of 0 to 4 according to the description by Gellman et al. (14) as follows: grade 0, all glomeruli appear normal; grade 1, local lesions present within each glomerulus and focal lesions present within the kidney; grade 2, mesangial thickening is diffuse within the glomerulus and generalized throughout the kidney; grade 3, capillary lumina are narrowed and obliterated only locally; and grade 4, the lumen is generally narrowed and the entire glomerulus is ischemic and appears to be hyalinized (14–17) (Supplementary Fig. 1A–D). Nodular lesions, exudative lesions, and mesangiolysis were simply shown as their presence or absence in each specimen (15–17) (Supplementary Fig. 1E–G). The severity of interstitial fibrosis and tubular atrophy (IFTA) and interstitial inflammation was scored according to the description by Tervaert et al. (18). The severity of IFTA was evaluated and graded on a scale from 0 to 3: grade 0, no IFTA; grade 1, <25%; grade 2, 25–50%; and grade 3, >50% (18). The severity of interstitial inflammation was evaluated and graded on a scale from 0 to 2: grade 0, absent; grade 1, infiltration only in relation to IFTA; and grade 2, infiltration in areas without IFTA (18). The severity of arteriolar hyalinosis was evaluated and graded on a scale from 0 to 3 according to the description by Takazakura et al. (11) as follows: grade 0, normal appearance without PAS-positive deposit; grade 1, a light PAS-positive thickening is observed but at less than half the circumference of the arteriole in many arterioles; grade 2, most vessel walls are moderately thickened with PAS-positive deposition without apparent luminal narrowing; and grade 3, a heavy thickening of the majority of the vessel walls is seen with luminal narrowing or obliteration (Supplementary Fig. 1H–J). The severity of arteriosclerosis was evaluated and graded on a scale from 0 to 2 according to the description by Tervaert et al. (18) as follows: grade 0, no intimal thickening; grade 1, intimal thickening less than thickness of media; and grade 2, intimal thickening greater than thickness of media (Supplementary Fig. 1K and L). Renal tissue specimens were examined by four nephrologists.
Statistical analysis
Data are expressed as means ± SD. Comparisons of continuous variables among groups were performed using the Mann-Whitney U test for nonparametric data. Comparisons of categorical variables among groups were performed using χ2 test. The survival curves were obtained using the Kaplan-Meier method and compared by log-rank test. The influence of different categories of albuminuria (proteinuria) and eGFR on each outcome was evaluated with the use of the Cox proportional hazards model after adjustment for age and sex. The results are presented as hazard ratios (HRs) and 95% CI. Patients with normoalbuminuria (normal proteinuria) and eGFR ≥60 mL/min/1.73 m2 served as the reference group in the analyses. A multivariate Cox proportional hazards regression model was used to select factors that significantly affected the incidence of each outcome and to estimate the risks. The following variables were incorporated as covariates: age, sex, microalbuminuria (mild proteinuria), macroalbuminuria (severe proteinuria), eGFR, duration of diabetes, presence of diabetic retinopathy, HbA1c, BMI, systolic blood pressure, total cholesterol, and hemoglobin as clinical covariates or diffuse lesions, nodular lesions, exudative lesions, mesangiolysis, IFTA, interstitial inflammation, arteriolar hyalinosis, and arteriosclerosis as pathological covariates. All analyses were carried out using SPSS, version 19 (SPSS, Tokyo, Japan). Two-sided P < 0.05 was considered indicative of statistical significance.
RESULTS
Baseline characteristics
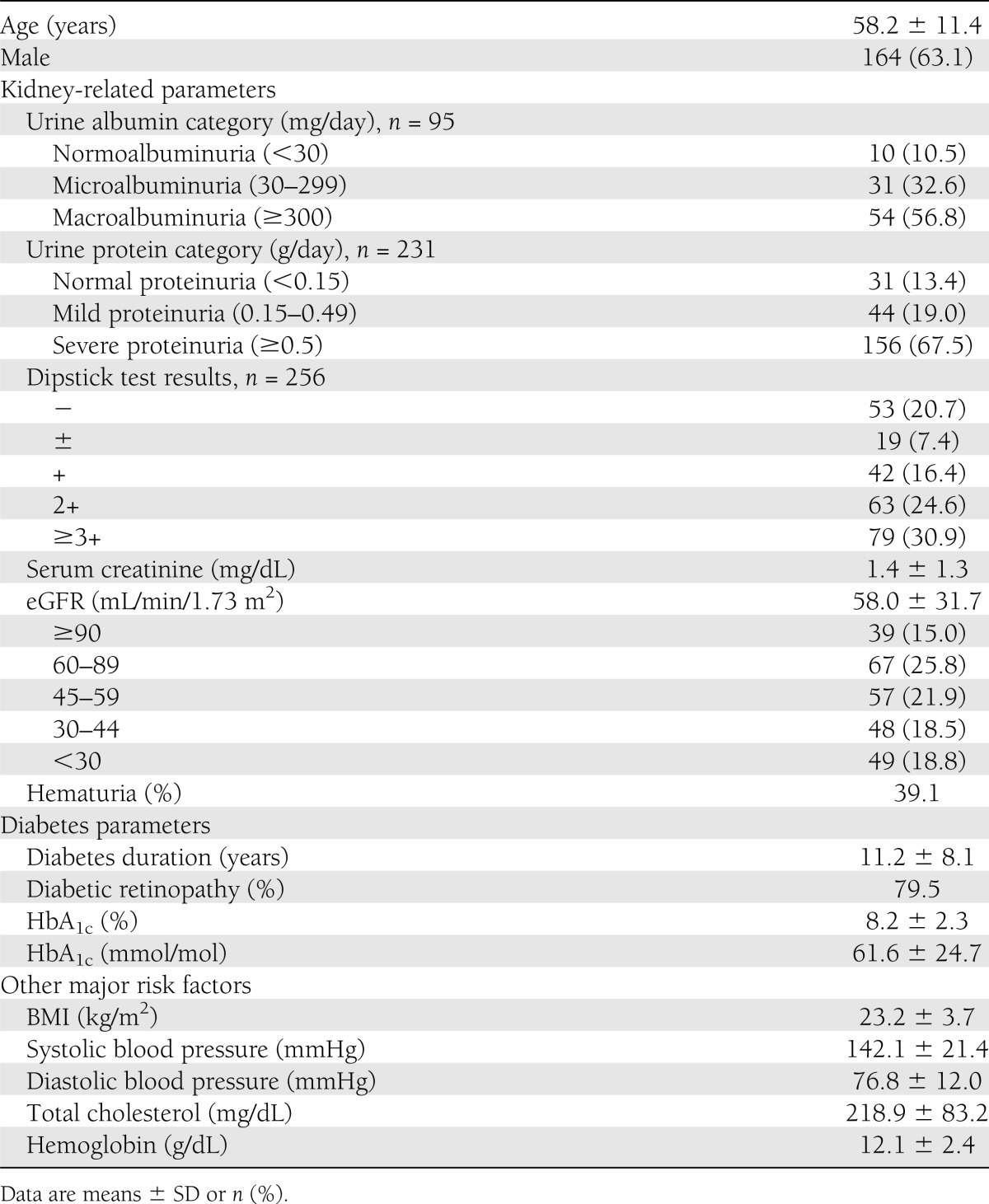
The baseline characteristics of 260 patients are shown in Table 1. In the clinical parameters, the mean age was 58.2 years, and 63.1% of the patients were male. Among the 95 patients for whom daily urinary albumin excretion measurements were available, 10 (10.5%) showed normoalbuminuria (category A1), 31 (32.6%) showed microalbuminuria (category A2), and 54 (56.8%) showed macroalbuminuria (category A3). Among the 231 patients for whom daily urinary protein excretion measurements were available, 31 (13.4%) showed normal proteinuria (A1), 44 (19.0%) showed mild proteinuria (A2), and 156 (67.5%) showed severe proteinuria (A3). Among the 256 patients for whom urinary dipstick protein test results were available, 53 (20.7%) showed negative (A1), 19 (7.4%) showed trace (A1), 42 (16.4%) showed + (A2), 63 (26.4%) showed 2+ (A3), and 79 (30.9%) showed ≥3+ (A3). The mean serum creatinine was 1.4 mg/dL, and the mean eGFR was 58.0 mL/min/1.73 m2. The proportions with eGFR ≥90 (G1), 60–89 (G2), 45–59 (G3a), 30–44 (G3b), 15–29 (G4), and <15 (G5) mL/min/1.73 m2 were 15.0%, 25.8%, 21.9%, 18.5%, 12.7%, and 6.2%, respectively.
Table 1.
Clinical characteristics of patients at the time of renal biopsy (n = 260)
The proportions of patients stratified by albuminuria (proteinuria) and eGFR categories are demonstrated in Supplementary Table 1. The proportions of patients with normoalbuminuria (normal proteinuria), microalbuminuria (mild proteinuria), and macroalbuminuria (severe proteinuria) were 16.5% (43 of 260), 21.2% (55 of 260), and 62.3% (162 of 260), respectively. The proportions of patients with eGFR ≥60 and <60 mL/min/1.73 m2 were 40.8% (106 of 260) and 59.2% (154 of 260), respectively. The proportions of patients with normoalbuminuria (normal proteinuria), microalbuminuria (mild proteinuria), and macroalbuminuria (severe proteinuria) among those with eGFR ≥60 mL/min/1.73 m2 were 26.4% (28 of 106), 29.2% (31 of 106), and 44.3% (47 of 106), respectively. The proportions of patients with normoalbuminuria (normal proteinuria), microalbuminuria (mild proteinuria), and macroalbuminuria (severe proteinuria) among those with eGFR <60 mL/min/1.73 m2 were 9.7% (15 of 154), 15.6% (24 of 154), and 74.7% (115 of 154), respectively.
Clinical and pathological features associated with albuminuria (proteinuria) and low eGFR
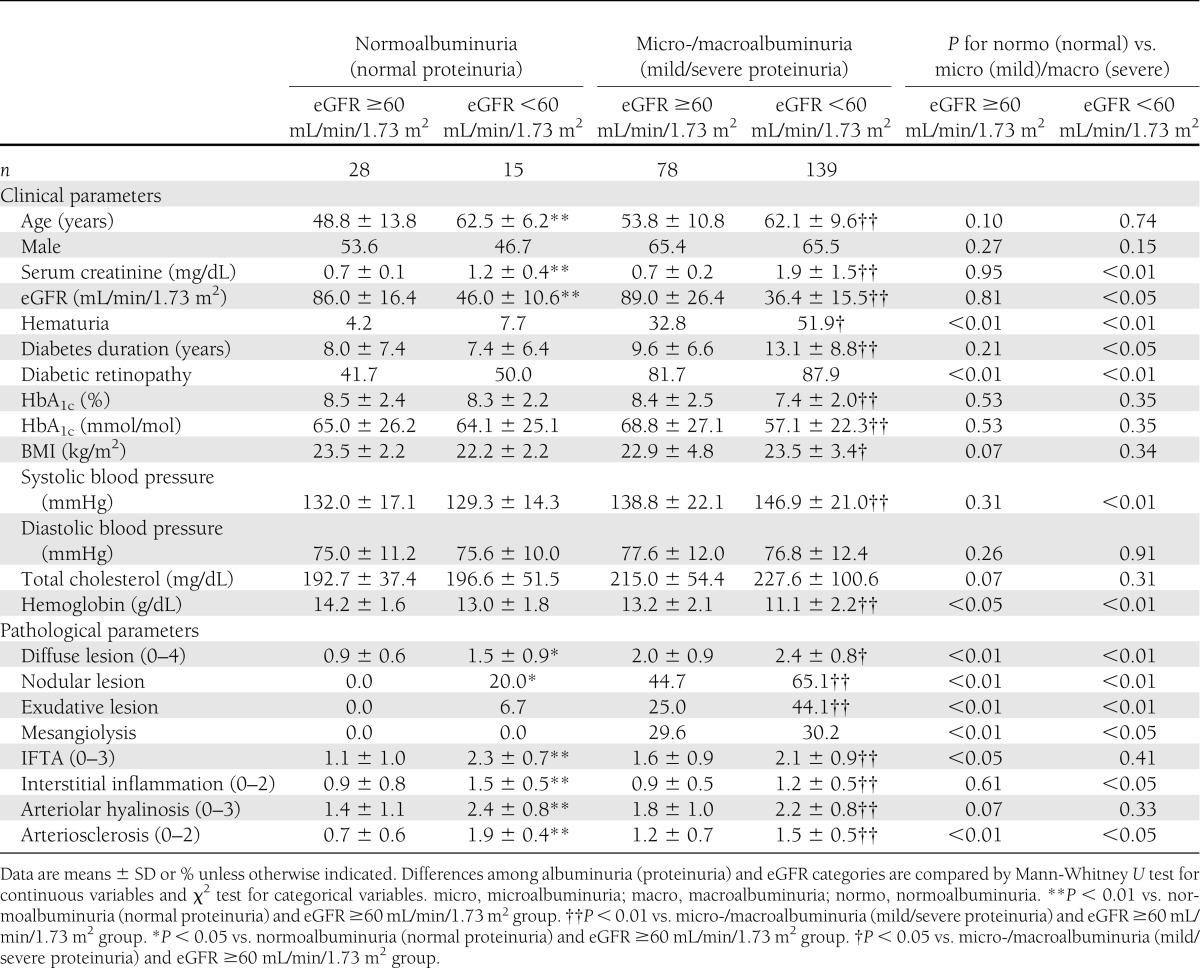
The baseline clinical and pathological features were compared among subgroups stratified by albuminuria (proteinuria) and eGFR categories (Table 2). Clinical and pathological factors associated with micro-/macroalbuminuria (mild/severe proteinuria) regardless of eGFR categories were hematuria, diabetic retinopathy, low hemoglobin, and glomerular lesions. On the other hand, clinical and pathological factors associated with low eGFR regardless of albuminuria (proteinuria) categories were age, diffuse lesions, nodular lesions, tubulointerstitial lesions, and vascular lesions. Glomerular lesions in patients with normoalbuminuria (normal proteinuria) were less advanced for both eGFR ≥60 and eGFR <60 mL/min/1.73 m2 categories. On the other hand, as to tubulointerstitial and vascular lesions in patients with normoalbuminuria (normal proteinuria), there were different trends between eGFR ≥60 and eGFR <60 mL/min/1.73 m2 categories. In the eGFR ≥60 mL/min/1.73 m2 category, tubulointerstitial and vascular lesions in patients with normoalbuminuria (normal proteinuria) were less advanced compared with those in patients with micro-/macroalbuminuria (mild/severe proteinuria). However, in the eGFR <60 mL/min/1.73 m2 category, tubulointerstitial and vascular lesions in patients with normoalbuminuria (normal proteinuria) were similar or more advanced compared with those in patients with micro-/macroalbuminuria (mild/severe proteinuria) in contrast to glomerular lesions (Supplementary Fig. 2A–C).
Table 2.
Baseline clinical and pathological features of patients stratified by albuminuria (proteinuria) and eGFR categories
Prognosis of renal events, cardiovascular events, and all-cause mortality
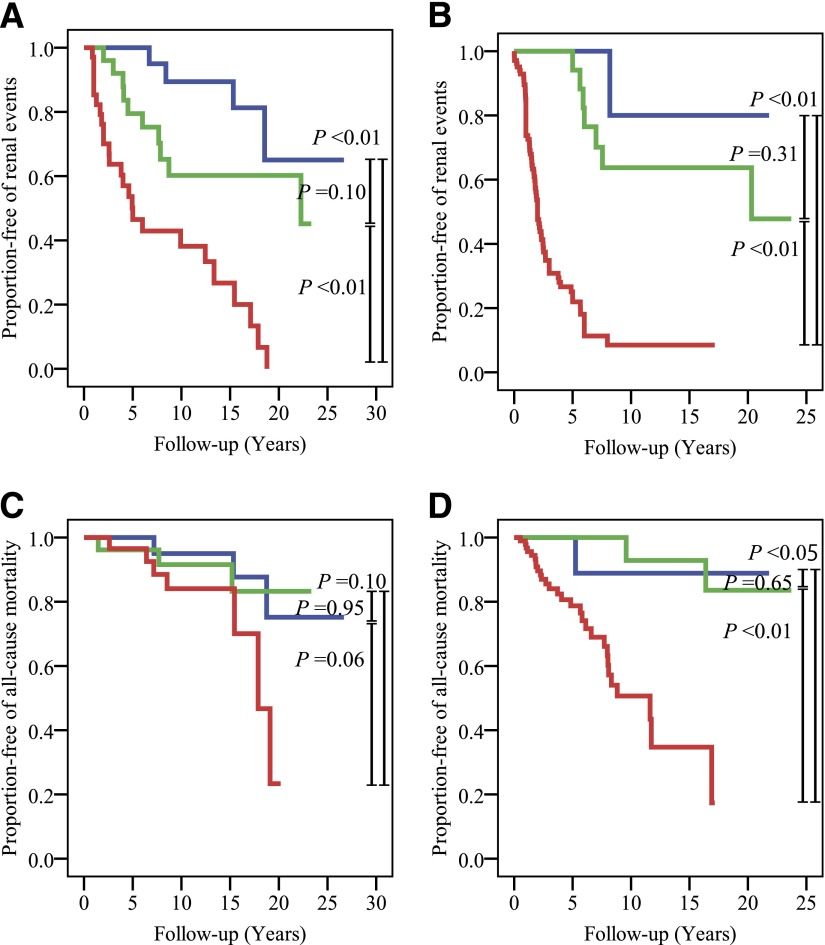
Follow-up data were available for renal events in 229 patients and for cardiovascular events and all-cause mortality in 233 patients. The mean duration of follow-up was 8.1 years (range 5–9,739 days) during 1985–2011. There were a total of 118 renal events, 62 cardiovascular events, and 45 deaths (Supplementary Table 2). Event-free rate of renal events in patients with macroalbuminuria (severe proteinuria) was significantly lower than in those with normoalbuminuria (normal proteinuria) or microalbuminuria (mild proteinuria) for both eGFR ≥60 and <60 mL/min/1.73 m2 categories (vs. normoalbuminuria [normal proteinuria] and eGFR ≥60 mL/min/1.73 m2, P < 0.01; vs. microalbuminuria [mild proteinuria] and eGFR ≥60 mL/min/1.73 m2, P < 0.01; vs. normoalbuminuria [normal proteinuria] and eGFR <60 mL/min/1.73 m2, P < 0.01; and vs. microalbuminuria [mild proteinuria] and eGFR <60 mL/min/1.73 m2, P < 0.01) (Fig. 1A and B). Event-free rate of cardiovascular events showed no significant differences between albuminuria (proteinuria) categories for both eGFR ≥60 and <60 mL/min/1.73 m2 categories. Event-free rate of all-cause mortality in patients with macroalbuminuria (severe proteinuria) was significantly lower than in those with normoalbuminuria (normal proteinuria) or microalbuminuria (mild proteinuria) in the eGFR <60 mL/min/1.73 m2 category (vs. normoalbuminuria [normal proteinuria] and eGFR <60 mL/min/1.73 m2, P < 0.05; vs. microalbuminuria [mild proteinuria] and eGFR <60 mL/min/1.73 m2, P < 0.01) (Fig. 1C and D). Event-free rates of renal events, cardiovascular events, and all-cause mortality in patients with eGFR <60 mL/min/1.73 m2 were significantly lower than in those with eGFR ≥60 mL/min/1.73 m2 only among patients with macroalbuminuria (severe proteinuria) (renal events P < 0.01, cardiovascular events P < 0.05, all-cause mortality P < 0.01).
Figure 1.
Event-free rate stratified by albuminuria (proteinuria) and eGFR categories. A: Event-free rate of renal events stratified by albuminuria (proteinuria) in the eGFR ≥60 mL/min/1.73 m2 category according to the Kaplan-Meier method. Blue line, normoalbuminuria (normal proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 24); green line, microalbuminuria (mild proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 27); red line, macroalbuminuria (severe proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 37). Differences between groups were compared by a log-rank test. B: Event-free rate of renal events stratified by albuminuria (proteinuria) in the eGFR <60 mL/min/1.73 m2 category according to the Kaplan-Meier method. Blue line, normoalbuminuria (normal proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 14); green line, microalbuminuria (mild proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 21); red line, macroalbuminuria (severe proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 106). Differences between groups were compared by a log-rank test. C: Event-free rate of all-cause mortality stratified by albuminuria (proteinuria) in the eGFR ≥60 mL/min/1.73 m2 category according to the Kaplan-Meier method. Blue line, normoalbuminuria (normal proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 25); green line, microalbuminuria (mild proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 27); red line, macroalbuminuria (severe proteinuria) and eGFR ≥60 mL/min/1.73 m2 group (n = 38). Differences between groups were compared by a log-rank test. D: Event-free rate of all-cause mortality stratified by albuminuria (proteinuria) in the eGFR <60 mL/min/1.73 m2 category according to Kaplan-Meier method. Blue line, normoalbuminuria (normal proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 14); green line, microalbuminuria (mild proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 22); red line, macroalbuminuria (severe proteinuria) and eGFR <60 mL/min/1.73 m2 group (n = 107). Differences between groups were compared by a log-rank test.
Risks of renal events, cardiovascular events, and all-cause mortality stratified by albuminuria (proteinuria) and eGFR categories
HRs of renal events, cardiovascular events, and all-cause mortality were calculated in subgroups of patients stratified by albuminuria (proteinuria) and eGFR categories after adjustment for age and sex (Supplementary Table 2). The group of patients with normoalbuminuria (normal proteinuria) and eGFR ≥60 mL/min/1.73 m2 served as a reference group. HRs of renal events were 8.99-fold higher risk (95% CI 3.07–26.37) in patients with macroalbuminuria (severe proteinuria) and eGFR ≥60 mL/min/1.73 m2 and 20.82-fold higher risk (95% CI 7.12–60.85) in patients with macroalbuminuria (severe proteinuria) and eGFR <60 mL/min/1.73 m2. HRs of cardiovascular events was 3.11-fold higher risk (95% CI 1.15–8.39) in patients with macroalbuminuria (severe proteinuria) and eGFR <60 mL/min/1.73 m2. HRs of all-cause mortality was 5.87-fold higher risk (95% CI 1.62–21.25) in patients with macroalbuminuria (severe proteinuria) and eGFR <60 mL/min/1.73 m2. Reduced eGFR was not predictive of renal events, cardiovascular events, and all-cause mortality except in patients with macroalbuminuria (severe proteinuria).
Clinical and pathological parameters associated with renal events, cardiovascular events, and all-cause mortality
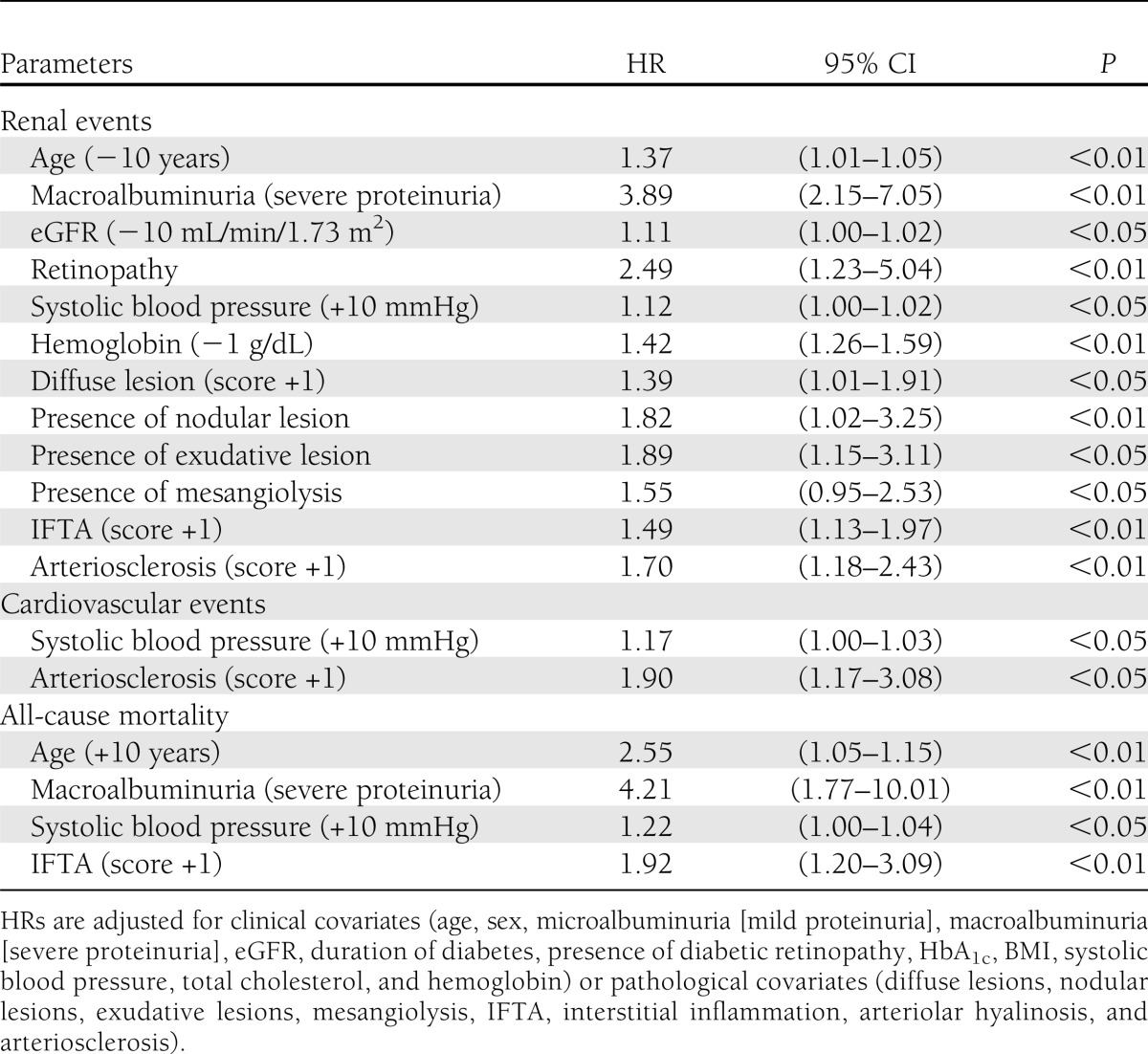
The results of multivariate Cox proportional hazards regression analysis are shown in Table 3. Young age, macroalbuminuria (severe proteinuria), low eGFR, presence of diabetic retinopathy, high systolic blood pressure, low hemoglobin, advanced diffuse lesions, presence of nodular lesions, presence of exudative lesions, presence of mesangiolysis, advanced IFTA, and advanced arteriosclerosis were the independent risk factors for renal events. High systolic blood pressure and advanced arteriosclerosis were the independent risk factors for cardiovascular events. High age, macroalbuminuria (severe proteinuria), high systolic blood pressure, and advanced IFTA were the independent risk factors for all-cause mortality.
Table 3.
Parameters identified by multivariate Cox proportional hazards regression analysis associated with renal events, cardiovascular events, and all-cause mortality
CONCLUSIONS
The present retrospective study is the first report to describe the pathological features with accompanying long-term clinical outcomes among the patients with normoalbuminuria (normal proteinuria) and low eGFR in type 2 diabetes. The glomerular, tubulointerstitial, and vascular lesions in patients with normoalbuminuria (normal proteinuria) and low eGFR were more advanced compared to those in patients with normoalbuminuria (normal proteinuria) and maintained eGFR. In addition, compared to patients with micro-/macroalbuminuria (mild/severe proteinuria) and low eGFR, their tubulointerstitial and vascular lesions were similar or more advanced in contrast to glomerular lesions.
Furthermore, we showed that the evaluation of renal pathology provides practical information concerning overall management including renal events and cardiovascular events of diabetic nephropathy in type 2 diabetes. Glomerular lesions, IFTA, and arteriosclerosis were identified as the pathological determinants for renal events. In addition, arteriosclerosis was identified as the pathological determinant for cardiovascular events, and IFTA was identified as the pathological determinant for all-cause mortality.
Clinically, we revealed that macroalbuminuria (severe proteinuria) has a higher impact for renal events and all-cause mortality than low eGFR, whereas the impact of low eGFR on clinical outcomes was observed only in patients with macroalbuminuria (severe proteinuria).
First, we evaluated the structural-functional relationships of diabetic nephropathy in type 2 diabetes. As to the renal lesions related to albuminuria (proteinuria), our results showed that hematuria, diabetic retinopathy, low hemoglobin, and glomerular lesions were increased and more advanced with progression of albuminuria (proteinuria) categories regardless of eGFR. Previous studies in type 1 and type 2 diabetes have shown that the major renal pathological changes of diabetic nephropathy associated with increasing urinary albumin (protein) excretion are mesangial expansion and glomerular basement membrane thickening (19). Further, previous reports in type 2 diabetes have found that nodular lesions and mesangiolysis are correlated with urinary albumin (protein) excretion consistently with our results (15,20–23). Although the presence of hematuria has been considered one of the atypical features indicating the presence of nondiabetic renal disease, several studies have suggested a positive association between the severity of albuminuria and the development of hematuria in patients with diabetic nephropathy, in accordance with our results (24). Our results suggest that the presence of hematuria is associated with more advanced histological alterations in diabetic nephropathy. However, previous studies of biopsy-proven diabetic nephropathy did not correlate the pathological changes with the presence of hematuria (24).
As to the renal lesions related to low eGFR with micro-/macroalbuminuria (mild/severe proteinuria), our results showed that more advanced diffuse, nodular, exudative, tubulointerstitial, and vascular lesions compared to those related to maintained eGFR with micro-/macroalbuminuria (mild/severe proteinuria). In type 1 diabetes, previous studies evaluating structural-functional relationships in diabetic nephropathy among patients ranging from normoalbuminuria to proteinuria demonstrated that the main lesions that determine low GFR shift from glomerular lesions to interstitial lesions (19,25). Our results demonstrate similarities to those in patients with type 1 diabetes and more severe vascular lesions, perhaps reflecting older age and hypertension.
Remarkably, we confirmed the pathological features related to low eGFR without albuminuria (proteinuria). In our study, 9.7% of patients with low eGFR (<60 mL/min/1.73 m2) were not associated with albuminuria (proteinuria). The frequencies of normoalbuminuria observed in patients with low GFR have been reported to be 22–24% in type 1 diabetes and 32–71% in type 2 diabetes (25,26). These results suggest that normoalbuminuric renal insufficiency is not uncommon among diabetic patients, especially in type 2 diabetes. This study revealed that the glomerular, tubulointerstitial, and vascular lesions in patients with normoalbuminuria (normal proteinuria) and low eGFR were more advanced compared to those in patients with normoalbuminuria (normal proteinuria) and maintained eGFR. In addition, compared to patients with micro-/macroalbuminuria (mild/severe proteinuria) and low eGFR, the tubulointerstitial and vascular lesions in patients with normoalbuminuria (normal proteinuria) and low eGFR in type 2 diabetes were similar or more advanced in contrast to glomerular lesions. Our results suggest that tubulointerstitial lesions observed among patients with normoalbuminuria (normal proteinuria) and low eGFR are strongly affected by vascular lesions rather than by glomerular lesions. A previous study in type 1 diabetes showed that the pathological features among the patients with normoalbuminuria and low GFR included more advanced diabetic glomerular lesions compared to those among the patients with normoalbuminuria and maintained GFR (25). An animal model of type 2 diabetes, the Cohen diabetic rat, which shows progressive depression of renal function without proteinuria, was also reported to show typical diabetic glomerulosclerosis (27). Therefore, further examinations are required to determine the pathophysiological conditions of patients with normoalbuminuria and low GFR in type 2 diabetes.
Next, we evaluated the pathological impact of glomeruli, tubulointerstitium, and vessels on renal events, cardiovascular events, and all-cause mortality. As to glomerular lesions related to renal events, diffuse lesions, nodular lesions, exudative lesions, and mesangiolysis were identified as the pathological determinants in this study. Previous reports have found that diffuse lesions, nodular lesions, and mesangiolysis are associated with renal outcome in accordance with our results (15,21–23).
In addition to glomerular lesions, IFTA was identified as the pathological determinant for renal events and all-cause mortality in this study. There are numerous studies suggesting that tubulointerstitial damage, as well as glomerular damage, contributes to a decline in renal function (21,28). However, this is the first report identifying IFTA as the predictor of all-cause mortality in diabetic nephropathy. In IgA nephropathy, a Japanese scoring system consisting of clinical findings and histological grades has been reported to predict 10-year risk of end-stage renal disease as well as all-cause mortality risk (29). Even though the histological evaluation is not commonly applied in patients with diabetic nephropathy and we are unable to assess sufficiently how confounding factors influenced our results, the evaluation of renal lesions in addition to clinical findings may improve mortality risk prediction in diabetic nephropathy.
Furthermore, arteriosclerosis in renal biopsy specimens was identified as the pathological determinant for renal events and cardiovascular events in this study. Arteriosclerosis included in the evaluation proposed by the Renal Pathology Society in the U.S. has been shown to worsen glomerular lesions in diabetic nephropathy (18,28). In addition, several autopsy-based studies have shown that intimal thickness of small renal arteries and renal arteriolar hyalinization are strongly associated with atherosclerotic lesions in the coronary arteries, aorta, and major cerebral vessels (30–32). These data support our results.
Considering these findings, various pathological lesions in glomeruli, tubulointerstitium, and vessels were orchestrated to promote and escalate diabetic kidney injuries, resulting in renal failure. It is important to determine whether pathological information from renal biopsy improves the predictive power when added to albuminuria and renal dysfunction. Based on our results, it is reasonable to predict renal prognosis of diabetic nephropathy by combination of clinical and pathological parameters. Prospective studies to develop a prognostic model by research biopsy may be useful for addressing this issue. Furthermore, we speculate that the evaluation of renal pathology provides a key for overall management including renal events and cardiovascular events in patients with diabetic nephropathy.
Finally, our study highlighted the impact of albuminuria (proteinuria) on clinical outcomes of patients with biopsy-proven diabetic nephropathy in type 2 diabetes. Patients with macroalbuminuria (severe proteinuria) had higher incidence of renal events and all-cause mortality than patients with normoalbuminuria (normal proteinuria) or microalbuminuria (mild proteinuria). In addition, macroalbuminuria (severe proteinuria) was a major clinical determinant of renal events and all-cause mortality in this study. Supporting our notion, previous studies have found that the renal outcome of patients with normoalbuminuria and low GFR is better than that of patients with albuminuria, even with maintained GFR (33–35). These results suggest that albuminuria has a greater impact than low GFR on predicting the development and progression of diabetic nephropathy. However, recent studies showed that the higher levels of urinary albumin excretion within the normal range predict faster decline in GFR and higher incidence of cardiovascular disease in type 2 diabetic patients (36,37). In addition, our study shows histological alterations even in the normoalbuminuria (normal proteinuria) category, although it is possible that including negative proteinuria as well as trace proteinuria in the normoalbuminuria (normal proteinuria) category affected the results. Further, some previous studies have found that albuminuria and renal function independently predict renal events, cardiovascular events, and death in diabetic patients (38–40). Therefore, further studies on clinical impacts of low GFR with or without albuminuria and new biomarkers for early and definitive diagnosis of diabetic nephropathy are required.
There are some limitations in this study. First, this study had a retrospective design that was dependent on collectable data. Second, there was likely an influence of bias through limitation of subjects to patients with renal biopsy. Third, there was the lack of quantitative structural measurements. Fourth, the data for proteinuria including dipstick test results were used when data for albuminuria were not available. Finally, treatment contents were not evaluated. These limitations may have placed significant constraints on the interpretation of the results, particularly related to differences in renal and cardiovascular outcomes. However, clinical examination by long-term observation in 260 patients with biopsy-proven diabetic nephropathy is of importance for understanding the pathophysiology of diabetic kidney lesions and clinical outcomes.
In conclusion, the current study of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy suggest that the characteristic pathological lesions and macroalbuminuria (severe proteinuria) are closely related to the long-term outcomes of diabetic nephropathy in type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported in part by a grant-in-aid for Diabetic Nephropathy Research and for Diabetic Nephropathy and Nephrosclerosis Research from the Ministry of Health, Labor and Welfare of Japan.
No potential conflicts of interest relevant to this article were reported.
M.S. and T.W. designed the study protocol, researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. K.F. and T.To. designed the study protocol, researched data, contributed to discussion, and reviewed and edited the manuscript. S.Ki., A.H., K.K., Y.I., and N.S. researched data and contributed to discussion. T.Ta., M.Y., H.Y., and S.Ka. researched data, contributed to discussion, and reviewed and edited the manuscript. All authors approved the final version. T.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Dr. Hiroshi Kida (National Hospital Organization Kanazawa Medical Center) for his helpful comments on this study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0298/-/DC1.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 [DOI] [PubMed] [Google Scholar]
- 4.Nakai S, Iseki K, Itami N, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2010). Ther Apher Dial 2012;16:483–521 [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System USRDS 2012 Annual Data Report. Am J Kidney Dis 2013;61(Suppl. 1):e165–e192 [Google Scholar]
- 6.Steenkamp R, Castledine C, Feest T, Fogarty D. UK Renal Registry 13th Annual Report (December 2010): Chapter 2: UK RRT prevalence in 2009: national and centre-specific analyses. Nephron Clin Pract 2011;119(Suppl. 2):c27–c52 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28 [DOI] [PubMed] [Google Scholar]
- 8.Japanese Society of Nephrology. In Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease 2012 Tokyo, Japan, Tokyo-Igakusya, 2012, p. 1–4 [Google Scholar]
- 9.Wada T, Shimizu M, Toyama T, Hara A, Kaneko S, Furuichi K. Clinical impact of albuminuria in diabetic nephropathy. Clin Exp Nephrol 2012;16:96–101 [DOI] [PubMed] [Google Scholar]
- 10.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 1996;39:1569–1576 [DOI] [PubMed] [Google Scholar]
- 11.Takazakura E, Nakamoto Y, Hayakawa H, Kawai K, Muramoto S. Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes 1975;24:1–9 [DOI] [PubMed] [Google Scholar]
- 12.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010;1:212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992 [DOI] [PubMed] [Google Scholar]
- 14.Gellman DD, Pirani CL, Soothill JF, Muehrcke RC, Kark RM. Diabetic nephropathy: a clinical and pathologic study based on renal biopsies. Medicine (Baltimore) 1959;38:321–367 [PubMed] [Google Scholar]
- 15.Saito Y, Kida H, Takeda S, et al. Mesangiolysis in diabetic glomeruli: its role in the formation of nodular lesions. Kidney Int 1988;34:389–396 [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 2000;58:1492–1499 [DOI] [PubMed] [Google Scholar]
- 17.Sakai N, Wada T, Furuichi K, et al. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis 2005;45:54–65 [DOI] [PubMed] [Google Scholar]
- 18.Tervaert TW, Mooyaart AL, Amann K, et al. Renal Pathology Society Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556–563 [DOI] [PubMed] [Google Scholar]
- 19.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stout LC, Kumar S, Whorton EB. Focal mesangiolysis and the pathogenesis of the Kimmelstiel-Wilson nodule. Hum Pathol 1993;24:77–89 [DOI] [PubMed] [Google Scholar]
- 21.Oh SW, Kim S, Na KY, et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract 2012;97:418–424 [DOI] [PubMed] [Google Scholar]
- 22.Furuichi K, Hisada Y, Shimizu M, et al. Matrix metalloproteinase-2 (MMP-2) and membrane-type 1 MMP (MT1-MMP) affect the remodeling of glomerulosclerosis in diabetic OLETF rats. Nephrol Dial Transplant 2011;26:3124–3131 [DOI] [PubMed] [Google Scholar]
- 23.Wada T, Shimizu M, Yokoyama H, et al. Nodular lesions and mesangiolysis in diabetic nephropathy. Clin Exp Nephrol 2013;17:3–9 [DOI] [PubMed] [Google Scholar]
- 24.Akimoto T, Ito C, Saito O, et al. Microscopic hematuria and diabetic glomerulosclerosis—clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron Clin Pract 2008;109:c119–c126 [DOI] [PubMed] [Google Scholar]
- 25.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 2003;52:1036–1040 [DOI] [PubMed] [Google Scholar]
- 26.Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011;20:246–257 [DOI] [PubMed] [Google Scholar]
- 27.Yagil C, Barak A, Ben-Dor D, et al. Nonproteinuric diabetes-associated nephropathy in the Cohen rat model of type 2 diabetes. Diabetes 2005;54:1487–1496 [DOI] [PubMed] [Google Scholar]
- 28.Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 1991;187:251–259 [DOI] [PubMed] [Google Scholar]
- 29.Bjørneklett R, Vikse BE, Bostad L, Leivestad T, Iversen BM. Long-term risk of ESRD in IgAN; validation of Japanese prognostic model in a Norwegian cohort. Nephrol Dial Transplant 2012;27:1485–1491 [DOI] [PubMed] [Google Scholar]
- 30.Tracy RE, MacLean CJ, Reed DM, Hayashi T, Gandia M, Strong JP. Blood pressure, nephrosclerosis, and age autopsy findings from the Honolulu Heart Program. Mod Pathol 1988;1:420–427 [PubMed] [Google Scholar]
- 31.McGill HC, Jr, Strong JP, Tracy RE, McMahan CA, Oalmann MC, The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Relation of a postmortem renal index of hypertension to atherosclerosis in youth. Arterioscler Thromb Vasc Biol 1995;15:2222–2228 [DOI] [PubMed] [Google Scholar]
- 32.Burchfiel CM, Tracy RE, Chyou PH, Strong JP. Cardiovascular risk factors and hyalinization of renal arterioles at autopsy. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol 1997;17:760–768 [DOI] [PubMed] [Google Scholar]
- 33.Kramer CK, Leitão CB, Pinto LC, Silveiro SP, Gross JL, Canani LH. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care 2007;30:1998–2000 [DOI] [PubMed] [Google Scholar]
- 34.Hoefield RA, Kalra PA, Baker PG, et al. The use of eGFR and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant 2011;26:887–892 [DOI] [PubMed] [Google Scholar]
- 35.Bruno G, Merletti F, Bargero G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 2007;50:941–948 [DOI] [PubMed] [Google Scholar]
- 36.Babazono T, Nyumura I, Toya K, et al. Higher levels of urinary albumin excretion within the normal range predict faster decline in glomerular filtration rate in diabetic patients. Diabetes Care 2009;32:1518–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Porrini E, Motterlini N, et al. BENEDICT Study Investigators Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012;23:1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama H, Oishi M, Kawai K, Sone H, Japan Diabetes Clinical Data Management Study Group Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in Type 2 diabetes: JDDM study 16. Diabet Med 2008;25:1426–1432 [DOI] [PubMed] [Google Scholar]
- 39.Ninomiya T, Perkovic V, de Galan BE, et al. ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011;54:32–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.