Abstract
OBJECTIVE
Diabetic nephropathy (DN) is a major cause of mortality in type 1 diabetes. Reduced insulin sensitivity is a well-documented component of type 1 diabetes. We hypothesized that baseline insulin sensitivity would predict development of DN over 6 years.
RESEARCH DESIGN AND METHODS
We assessed the relationship between insulin sensitivity at baseline and development of early phenotypes of DN—microalbuminuria (albumin-creatinine ratio [ACR] ≥30 mg/g) and rapid renal function decline (glomerular filtration rate [GFR] loss >3 mL/min/1.73 m2 per year)—with three Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations over 6 years. Subjects with diabetes (n = 449) and without diabetes (n = 565) in the Coronary Artery Calcification in Type 1 Diabetes study had an estimated insulin sensitivity index (ISI) at baseline and 6-year follow-up.
RESULTS
The ISI was lower in subjects with diabetes than in those without diabetes (P < 0.0001). A higher ISI at baseline predicted a lower odds of developing an ACR ≥30 mg/g (odds ratio 0.65 [95% CI 0.49–0.85], P = 0.003) univariately and after adjusting for HbA1c (0.69 [0.51–0.93], P = 0.01). A higher ISI at baseline conferred protection from a rapid decline of GFR as assessed by CKD-EPI cystatin C (0.77 [0.64–0.92], P = 0.004) and remained significant after adjusting for HbA1c and age (0.80 [0.67–0.97], P = 0.02). We found no relation between ISI and rapid GFR decline estimated by CKD-EPI creatinine (P = 0.38) or CKD-EPI combined cystatin C and creatinine (P = 0.50).
CONCLUSIONS
Over 6 years, a higher ISI independently predicts a lower odds of developing microalbuminuria and rapid GFR decline as estimated with cystatin C, suggesting a relationship between insulin sensitivity and early phenotypes of DN.
Diabetic nephropathy (DN) is a common and serious complication of diabetes. Its incidence is rising rapidly (1), and it is the most common cause of end-stage renal disease in the U.S. and Europe (2). The 2011 U.S. Renal Data System showed that DN accounted for 44.5% of all cases of end-stage renal disease in 2009 (3). Despite improvements in the outlook of this complication in past decades, it continues to be one of the major causes of morbidity and mortality in type 1 diabetes (4,5). DN is an important risk factor for coronary artery disease (6–8) and overall mortality (6,9). These findings highlight the need for improved methods of identifying persons at high risk for DN (10).
The role of insulin sensitivity in the development and progression of macro- (7,11,12) and microvascular complications (12,13) in type 1 diabetes is increasingly recognized. Reduced insulin sensitivity also is a plausible mechanism linking renal disease with excess mortality in type 1 diabetes. Historically, when glycemic control is poor, reduced insulin sensitivity was believed to be directly related to body weight and HbA1c (14,15), but more recent data suggest that reduced insulin sensitivity cannot simply be explained by weight or poor glycemic control. In fact, reduced insulin sensitivity has been documented in type 1 diabetic subjects with normal BMI and HbA1c compared with nondiabetic individuals (16). The Coronary Artery Calcification in Type 1 Diabetes (CACTI) longitudinal cohort study of adults with type 1 diabetes investigated the determinants of early and accelerated atherosclerosis and found that insulin sensitivity independently predicted coronary artery calcification (17,18). Reduced insulin sensitivity has also been shown to predict diabetic retinopathy, neuropathy, and nephropathy in subjects with type 1 diabetes (13).
Despite advances in the estimation of insulin sensitivity (insulin sensitivity index [ISI]) (19) and glomerular filtration rate (GFR) (20), research in the association of insulin sensitivity with DN has been limited since the Pittsburgh Epidemiology of Diabetes Complications (EDC) cohort showed more than a decade ago that the estimated glucose disposal rate (eGDR) predicts overt nephropathy (13). To readdress this relationship with contemporary data and estimating equations, we hypothesized that higher insulin sensitivity measured by ISI at baseline would be associated with decreased odds of developing two early phenotypes of DN—microalbuminuria (albumin-creatinine ratio [ACR] ≥30 mg/g) and rapid renal function decline (GFR loss >3 mL/min/1.73 m2 per year) (21–23)—calculated by the three Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations (20) over 6 years in the CACTI study.
RESEARCH DESIGN AND METHODS
The CACTI study enrolled subjects 19–56 years of age with and without type 1 diabetes who were asymptomatic for cardiovascular disease at the baseline visit in 2000–2002 and then were reexamined 3 and 6 years later as previously described (24). Subjects with type 1 diabetes had to have a disease duration of at least 10 years at enrollment, with the exception of 109 subjects who had taken part in a pilot study in 1997–1998 and had 2–48 years of diabetes duration. Subjects with serum creatinine levels >2 mg/dL were excluded at baseline unless they were participants in the pilot study. All subjects with the data needed to calculate ISI and GFR at the baseline and 6-year visits were included in this analysis (n = 449 with type 1 diabetes, n = 565 without diabetes). Among subjects with type 1 diabetes, those with missing data had slightly worse baseline renal function (e.g., CKD-EPI cystatin C eGFR 108 ± 18 vs. 100 ± 30 mL/min/1.73 m2) and fasting lipid profile than those with complete data. In contrast, nondiabetic control subjects with missing data were younger (35 ± 9 vs. 40 ± 9 years) and had better renal function (CKD-EPI cystatin C eGFR 111 ± 13 vs. 108 ± 12 mL/min/1.73 m2). The study was approved by the Colorado Multiple Institutional Review Board, and all subjects provided informed consent.
We measured height and weight and calculated BMI as kilograms per meter squared. Resting systolic blood pressure (SBP) and fifth phase diastolic blood pressure (DBP) were measured three times while the subject was seated, and the second and third measurements were averaged. Hypertension was defined as current antihypertensive therapy or untreated hypertension (blood pressure ≥140/90 mmHg) at the time of the study visit. Antihypertension medication use was determined by a medication inventory as previously described (24), and use of an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) was combined for these analyses.
After an overnight fast, blood was collected, centrifuged, and separated. Plasma was stored at 4°C until assayed. Total plasma cholesterol and triglyceride levels were measured by standard enzymatic methods, HDL cholesterol was separated with the use of dextran sulfate, and LDL cholesterol was calculated by the Friedewald equation. High-performance liquid chromatography (Bio-Rad variant) was used to measure HbA1c.
Overnight urine samples were collected in duplicate, and urine creatinine and albumin levels were measured by radioimmunoassay (Diagnostic Products) and averaged. In subjects (n = 35) who did not have two timed overnight urine samples, only one timed overnight urine sample was collected. Two subjects did not provide a timed overnight urine sample, and instead, spot urine was collected.
Early DN was defined as microalbuminuria (ACR ≥30 mg/g) and rapid GFR decline (>3 mL/min/1.73 m2 per year) (23,25) by all three CKD-EPI equations. Twenty-five type 1 diabetic subjects had an ACR <30 mg/g at the first visit (V1), and ACR ≥30 mg/g developed in them by the third visit (V3). In contrast, 284 subjects had ACR <30 mg/g at both V1 and V3. The analysis compared these two groups of subjects. Microalbuminuria developed in only five control subjects, so we did not have sufficient power to run analyses on this outcome.
GFR was determined with the use of the CKD-EPI serum creatinine, serum cystatin C, and combined equations recently published by Inker et al. (20) for the CKD-EPI Investigators Group. Cystatin C was measured in the University of Colorado Hospital clinical laboratory according to the package insert instructions of a commercially available assay (Dade-Behring) on a BN II or Prospec nephelometer. The coefficient of variation was 3.3%. Intraassay precision is 2.3–4.1%, and interassay precision is 2.6–3.3% according to the package insert. Results are reported in milligrams per liter, with a sensitivity cutoff of 0.23 mg/L. Because of a systematic shift in the Dade-Behring cystatin C assay over the period of the study, cystatin C levels were standardized to V3 levels by Deming regression equations, as previously described (26).
Serum creatinine level was measured according to package insert instructions on a Roche Mira Plus II analyzer until 2006 and then an Olympus AU400e (r = 0.9999 between methodologies) traceable to the National Institute of Standards and Technology standard reference material at the University of Colorado Clinical Translational Research Laboratory. Intra- and interassay precision are 1.6 and 3.3%, respectively. Because of the longitudinal design of CACTI and to assess for any potential change over time in the serum creatinine assay, 100 samples each from all three visits were remeasured as a single batch. Deming regression equations were used to standardize serum creatinine level by the same methodology as previously described for cystatin C (26).
With the use of the CKD-EPI creatinine equation to estimate GFR, 122 subjects with type 1 diabetes experienced a GFR loss >3 mL/min/1.73 m2 per year compared with 327 who had a stable renal function (GFR loss <3 mL/min/1.73 m2 per year). In contrast, by CKD-EPI cystatin C equation, rapid GFR decline developed in only 58 subjects with type 1 diabetes compared with 391 with stable renal function. By combined CKD-EPI creatinine and cystatin C equation, rapid GFR decline developed in 64 subjects with type 1 diabetes compared with 385 with stable renal function on follow-up. We compared subjects with rapid GFR decline with those with stable renal function over the 6 years of the study. Rapid GFR decline developed in only 29 control subjects per CKD-EPI cystatin C equation compared with 135 and 47 control subjects per CKD-EPI creatinine and combined creatinine and cystatin C equations, respectively.
CACTI clamp cohort: ISI
The ISI is derived from previous euglycemic-hyperinsulinemic clamp studies in a subset of 87 subjects (40 with and 47 without diabetes frequency matched for age, sex, and weight) of the CACTI cohort, as previously described in detail (18). The best-fit ISI model for subjects with type 1 diabetes comprises waist circumference, daily insulin dose per kilogram body weight, triglyceride levels, and DBP and explained 63% of the variance in GDR. The equation to calculate the ISI is as follows: exp(4.1075 – 0.01299 × waist [cm] – 1.05819 × insulin dose [daily units/kg] – 0.00354 × triglyceride level [mg/dL] – 0.00802 × DBP [mmHg]) (19).
Statistical analysis
Analyses were performed with SAS version 9.2 for Windows (SAS Institute, Cary, NC) software. Differences between type 1 diabetic subjects and control subjects at baseline and follow-up were assessed by χ2 test for categorical variables and t test for continuous variables. Nonparametric variables (e.g., ACR and triglyceride level) were log transformed to compute geometric means. Univariate logistic regression was performed to evaluate the associations between variables at baseline and the development of microalbuminuria and rapid renal function decline calculated by the CKD-EPI creatinine, cystatin C, and combined creatinine and cystatin C equations. The following variables were tested for univariate associations with the dependent outcomes: LDL cholesterol, HDL cholesterol, total cholesterol, SBP, HbA1c, type 1 diabetes duration, and age. DBP and triglyceride level were not included because they are part of the ISI. Only variables significantly associated with the dependent variables in univariate analyses were eligible for selection in the multivariable models, with a significance level of P < 0.05 for inclusion. Stepwise logistic regression was used to determine which variables remained in multivariable models predicting microalbuminuria and rapid GFR decline. Only variables with P < 0.1 in stepwise selection were included in the models. We further evaluated the independence of the association of ISI to renal outcomes by adjusting the multivariable models for the dichotomous variables of sex, smoking status, and ACEi/ARB use. All analyses were stratified by type 1 diabetes status, and significance was based on an α of 0.05.
RESULTS
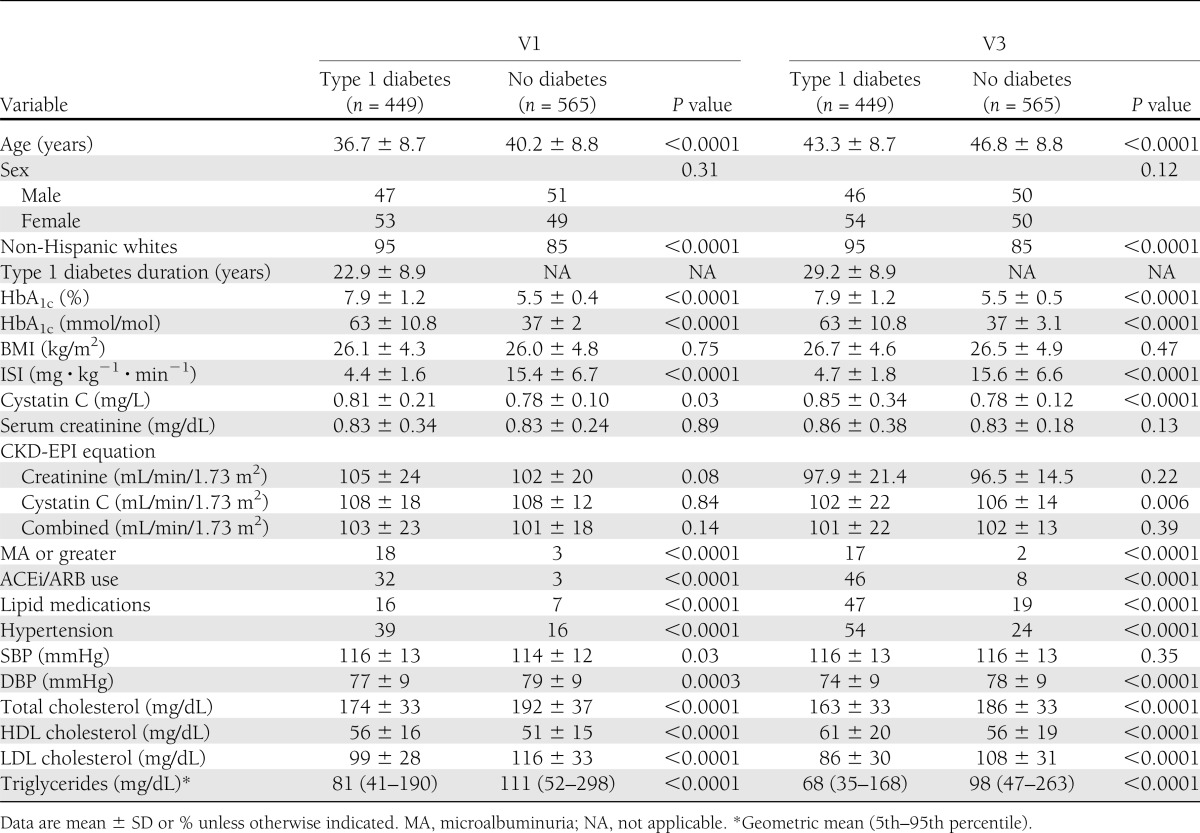
Data, including ISI, were available for 449 subjects with type 1 diabetes and 565 control subjects at baseline (V1) and follow-up (V3). Among type 1 diabetic subjects, 76 had an ACR ≥30 mg/g, and 15–21 (depending on which eGFR calculation was used) had a GFR <60 mL/min/1.73 m2 at V1; all were excluded from the analyses. Twenty-five subjects progressed from normoalbuminuria at V1 to microalbuminuria at V3, and there were 12–14 new cases of GFR <60 mL/min/1.73 m2. Conversely, among control subjects, ACR ≥30 mg/g developed in only six, and GFR <60 mL/min/1.73 m2 developed in only two to four at V3.
The mean ISI at V1 among type 1 diabetic subjects was 4.4 ± 1.6 mg ⋅ kg−1 ⋅ min−1 vs. 15.4 ± 6.7 mg ⋅ kg−1 ⋅ min−1 in control subjects (P < 0.0001). Similarly at V3, the mean was 4.7 ± 1.8 and 15.6 ± 6.6 mg ⋅ kg−1 ⋅ min−1 among type 1 diabetic and control subjects, respectively (P < 0.0001). In addition, among both subject groups, the mean differences in HbA1c; age; total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels; SBP and DBP; and the proportions of hypertension, ACEi/ARB use, and non-Hispanic whites were all statistically significantly different (Table 1). There was a significant overall decline in eGFR over 6 years in the entire cohort as calculated by CKD-EPI creatinine (P < 0.0001), cystatin C (P < 0.0001), and combined creatinine and cystatin C (P < 0.0001).
Table 1.
Subject characteristics
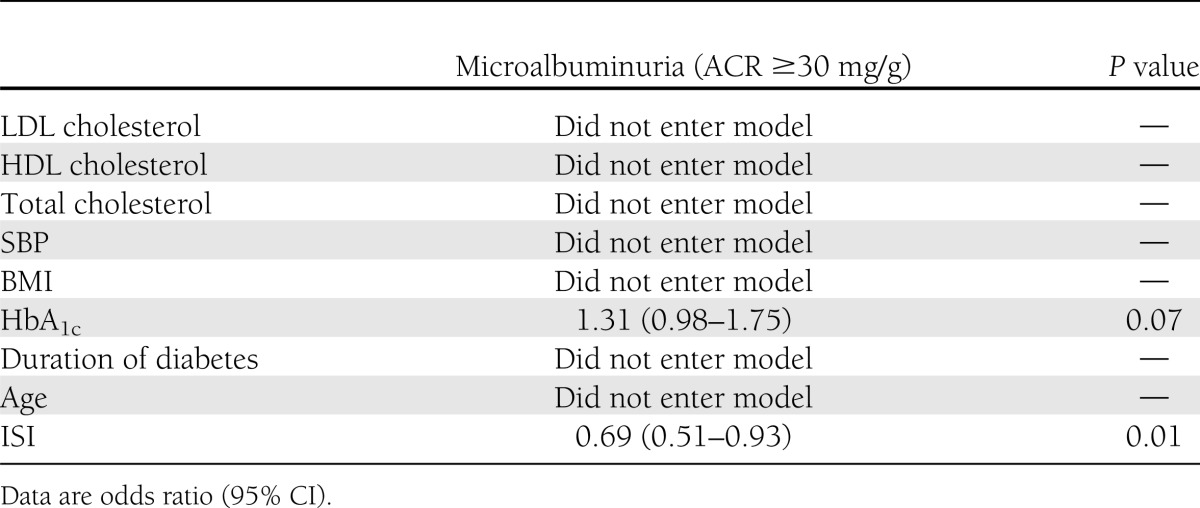
In univariate analysis, only ISI at baseline predicted a lower odds of developing ACR ≥30 mg/g (odds ratio 0.65 [95% CI 0.49–0.85], P = 0.003), and HbA1c predicted a higher odds of developing ACR ≥30 mg/g (1.45 [1.10–1.92], P = 0.009). ISI remained in the model after adjusting for HbA1c in a multivariable logistic regression (0.69 [0.51–0.93], P = 0.013) (Table 2). Moreover, the association remained significant with additional adjustments for sex, smoking status, and ACEi/ARB use (P = 0.018).
Table 2.
Multivariable analysis predicting development of microalbuminuria
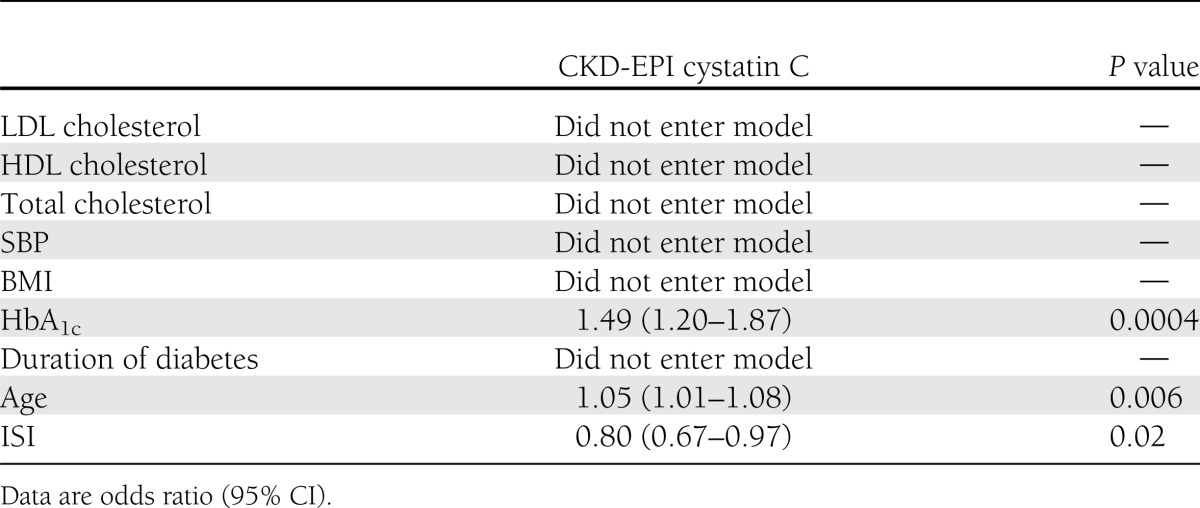
In univariate analysis, ISI was associated with a decreased odds (0.77 [0.64–0.92], P = 0.004) and HbA1c (P < 0.0001), age (P = 0.04), SBP (P = 0.02), and BMI (P = 0.01) were associated with an increased odds of developing rapid GFR decline as calculated by CKD-EPI cystatin C. In the stepwise model, ISI remained significant after adjusting for HbA1c and age (0.80 [0.67–0.97], P = 0.02) (Table 3). Moreover, the association remained unchanged with additional adjustments for both sex and smoking status, but adjusting for ACEi/ARB use at baseline attenuated the significance of the association (0.831 [0.686–1.006], P = 0.058). We found no associations between ISI or HbA1c and rapid GFR decline when calculated by CKD-EPI creatinine (P = 0.38) or combined creatinine and cystatin C (P = 0.50).
Table 3.
Multivariable analysis predicting development of rapid GFR decline (>3 mL/min/1.73 m2/year)
CONCLUSIONS
A major challenge in preventing DN is the difficulty in accurately identifying high-risk patients and the need for additional therapeutic targets. The aim of this study was to evaluate the association of insulin sensitivity and DN. The data show that greater insulin sensitivity at baseline independently predicts lower risk of developing ACR ≥30 mg/g and confers protection from a rapid decline of GFR as calculated by CKD-EPI cystatin C.
The association between reduced insulin sensitivity and diabetes complications is increasingly recognized, but it is not a recent discovery. In 1968, Martin and Stocks (27) showed that microvascular complications were associated with reduced insulin sensitivity in long-standing type 1 diabetes. In 1993, Yip et al. (28) explored insulin resistance as an underlying factor in type 1 diabetes and found reduced insulin sensitivity, measured by GDR, in a small group with microalbuminuria, whereas Orchard et al. (13) later demonstrated that eGDR (a marker of insulin sensitivity) predicted overt nephropathy in type 1 diabetic subjects in the Pittsburgh EDC cohort. The utility of eGDR as a marker of microvascular complications in type 1 diabetes was also confirmed by Chillarón et al. (29) and Girgis et al. (30) in two smaller cross-sectional studies in 91 and 61 subjects, respectively.
The exact mechanism of reduced insulin sensitivity in type 1 diabetes is poorly understood. One hypothesis suggests that reduced insulin sensitivity is secondary to prolonged exposure to supraphysiologic levels of exogenous insulin, which has been shown to be associated with increased ectopic fat accumulation in the liver and skeletal muscles, increased oxidative stress, and decreased mitochondrial biogenesis (31,32). One possible mechanistic pathway linking reduced insulin sensitivity to DN in type 1 diabetes is through reduced insulin sensitivity effects on overall nonessential fatty acid exposure and lipotoxicity in the development of macro- and microangiopathy.
The present study is similar to that of Orchard et al. (13) but with a larger sample and contemporary equations for estimating GFR and insulin sensitivity and the inclusion of nondiabetic subjects in the analyses. The present study population was also 10 years older than the Pittsburgh EDC study population and had a lower mean HbA1c of 7.9%. In addition, the present study uses the ISI, an equation derived from the study of Snell-Bergeon et al. (19), which is the largest euglycemic-hyperinsulinemic clamp study in adults with and without type 1 diabetes and improves on the adjusted R2 increase from the Pittsburgh EDC study (from 0.57–0.63). Microalbuminuria is still recognized as the earliest stage of DN in type 1 diabetes (33), but evidence shows that ACR ≥30 mg/g does not inexorably lead to progression of DN (34). For that reason, we decided to add a second recognized phenotype of early DN: rapid GFR decline, which some believe to be a better marker of trajectory toward impaired renal function (25). Annual screening for albuminuria and eGFR is recommended by the American Diabetes Association (35).
We calculated a rapid decline in kidney function with all three of the CKD-EPI equations (20). Concordance between CKD-EPI cystatin C and creatinine eGFR has been reported to be as low as 62% (36), and CKD-EPI cystatin C eGFR is considered to be less biased by age and weight than creatinine-based measurements (22). Consistent with the present data, cystatin C has been shown to be more accurate in detecting rapid GFR decline than creatinine-based measurements in type 1 diabetic subjects with normal GFR levels (37). Furthermore, rapid GFR decline estimated by cystatin C has also been shown to be associated with a higher risk for cardiovascular complications and mortality than GFR estimated by creatinine (21,36). This is also consistent with our findings from the CACTI cohort, where eGFR calculated by cystatin C predicted coronary artery calcification progression better than eGFR calculated by the CKD-EPI creatinine or combined equations (8). These findings agree with the present data, where only rapid GFR decline calculated by CKD-EPI cystatin C eGFR was associated with ISI in subjects with type 1 diabetes.
There are limitations to the current study, including the observational design and no direct measurements of GFR or insulin sensitivity because of the large cohort size. However, we used all three of the recently published CKD-EPI equations. It should be noted that eGFR at higher levels is associated with greater variability; however, these equations are state of the art and used by such studies as the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) (38). Moreover, cystatin C–based equations have been shown to estimate GFR well in diabetic subjects with normal and elevated renal function (37,39). In addition, the ISI has strong agreement with GDR measured by the gold standard method in the CACTI clamp study (R2 = 0.63), thereby supporting its role as a true reflection of insulin sensitivity. There was insufficient power to assess the ability of ISI to predict risk of developing microalbuminuria in control subjects and the categorical development of eGFR <60 mL/min/1.73 m2 in both groups because these end points developed in very few subjects in the cohort. The subjects with type 1 diabetes without complete data at baseline had worse renal function and lipid panel findings, which may bias the results to the null because less healthy subjects with type 1 diabetes were not included in the analyses.
In summary, greater insulin sensitivity as measured by ISI at baseline appears to be protective against the development of microalbuminuria and rapid eGFR decline as estimated by cystatin C. Despite the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study (40) showing no benefit of an insulin sensitizing strategy on nephropathy in subjects with type 2 diabetes, modification of insulin sensitivity holds promise as a therapeutic target to reduce vascular complications in type 1 diabetes because both lifestyle changes (diet and exercise) and medications, such as metformin, can improve insulin sensitivity. Further research is required to better assess the role of insulin sensitivity in DN in type 1 diabetes, especially given the increased incidence of obesity in people with type 1 diabetes.
Acknowledgments
Support for this study was provided by National Heart, Lung, and Blood Institute grant R01 HL113029, Diabetes Endocrinology Research Center Clinical Investigation Core grant P30-DK-57516, and JDRF grant 17-2013-313. The study was performed at the Adult Clinical and Translational Research Center at the University of Colorado Denver with support from National Institutes of Health grant M01-RR-00051, at the Barbara Davis Center for Childhood Diabetes, and at the Colorado Heart Imaging Center in Denver. J.K.S.-B. was supported by an American Diabetes Association Junior Faculty Award (1-10-JF-50). D.M.M. was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-075360).
R.J.J. has patent applications related to the lowering of uric acid and blocking fructose metabolism as a means for slowing DN or improving insulin resistance and has shares with XORT Therapeutics related to these patents. No other potential conflicts of interest relevant to this article were reported.
P.B. contributed to the research and discussion and wrote, reviewed, and edited the manuscript. J.K.S.-B. formulated the ISI model, contributed to the research and discussion, and reviewed and edited the manuscript. M.R. designed the CACTI study, contributed to the research and discussion, and reviewed and edited the manuscript. D.J., M.B.C., and R.J.J. contributed to the discussion and reviewed and edited the manuscript. D.M.M. contributed to the research and discussion and reviewed and edited the manuscript. D.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
References
- 1.Guilbert JJ. The World Health Report 2006: working together for health. Educ Health (Abingdon) 2006;19:385–387 [DOI] [PubMed]
- 2.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. Diabetic nephropathy. Diabetes Care 2003;26(Suppl. 1):S94–S98 [DOI] [PubMed]
- 3.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 annual data report. Am J Kidney Dis 2011;57(1 Suppl. 1):A8, e1–e526 [DOI] [PubMed]
- 4.Maahs DM, Rewers M. Editorial: mortality and renal disease in type 1 diabetes mellitus–progress made, more to be done. J Clin Endocrinol Metab 2006;91:3757–3759 [DOI] [PubMed]
- 5.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319 [DOI] [PMC free article] [PubMed]
- 6.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374–1379 [DOI] [PubMed]
- 8.Maahs D, Jalal D, Chonchol M, Johnson R, Rewers M, Snell-Bergeon JK. Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: the CACTI study. Diabetes Care 2013;36:2607–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diabetes Epidemiology Research International Mortality Study Group. International evaluation of cause-specific mortality and IDDM. Diabetes Care 1991;14:55–60 [DOI] [PubMed]
- 10.Maahs DM. Cardiovascular disease (CVD) limbo: how soon and low should we go to prevent CVD in diabetes? Diabetes Technol Ther 2012;14:449–452 [DOI] [PubMed]
- 11.Soedamah-Muthu SS, Chaturvedi N, Toeller M, et al. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 2004;27:530–537 [DOI] [PubMed]
- 12.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed]
- 13.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int 2002;62:963–970 [DOI] [PubMed]
- 14.Yki-Jarvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. New Engl J Med 1986;315:224–230 [DOI] [PubMed]
- 15.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. New Engl J Med 1986;315:215–219 [DOI] [PubMed]
- 16.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed]
- 17.Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther 2011;13:991–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes 2011;60:306–314 [DOI] [PMC free article] [PubMed]
- 19.Snell-Bergeon JK, Maahs DM, Schauer IE, Bergman BC, Rewers M. A method for estimating insulin sensitivity in adults with type 1 diabetes. Presented at the 70th Annual Meeting of the American Diabetes Association, 25–29 June 2010, Orlando, Florida [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, et al.; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed]
- 21.Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 2009;20:2625–2630 [DOI] [PMC free article] [PubMed]
- 22.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol 2009;30:171–178 [DOI] [PMC free article] [PubMed]
- 23.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008;168:2212–2218 [DOI] [PMC free article] [PubMed]
- 24.Maahs DM, Kinney GL, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care 2005;28:301–306 [DOI] [PubMed]
- 25.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed]
- 26.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol 2011;6:1952–1955 [DOI] [PMC free article] [PubMed]
- 27.Martin FI, Stocks AE. Insulin sensitivity and vascular disease in insulin-dependent diabetics. Br Med J 1968;2:81–82 [DOI] [PMC free article] [PubMed]
- 28.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet 1993;342:883–887 [DOI] [PubMed]
- 29.Chillarón JJ, Goday A, Flores-Le-Roux JA, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab 2009;94:3530–3534 [DOI] [PubMed]
- 30.Girgis CM, Scalley BD, Park KE. Utility of the estimated glucose disposal rate as a marker of microvascular complications in young adults with type 1 diabetes. Diabetes Res Clin Pract 2012;96:e70–e72 [DOI] [PubMed]
- 31.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 2003;285:E1174–E1181 [DOI] [PubMed] [Google Scholar]
- 32.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948 [DOI] [PubMed]
- 33.Standards of medical care in diabetes–2013. Diabetes Care 2013;36 (Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed]
- 34.de Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed]
- 35.Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012;61:2993–2997 [DOI] [PMC free article] [PubMed]
- 36.Krolewski AS, Warram JH, Forsblom C, et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care 2012;35:2311–2316 [DOI] [PMC free article] [PubMed]
- 37.Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care 2008;31:971–973 [DOI] [PubMed]
- 38.de Boer IH, Sun W, Cleary PA, et al.; DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed]
- 39.Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia 2006;49:1686–1689 [DOI] [PubMed]
- 40.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515 [DOI] [PMC free article] [PubMed]


