Abstract
OBJECTIVE
Use of automated bolus advisors is associated with improved glycemic control in patients treated with insulin pump therapy. We conducted a study to assess the impact of using an insulin bolus advisor embedded in a blood glucose (BG) meter on glycemic control and treatment satisfaction in patients treated with multiple daily insulin injection (MDI) therapy. The study goal was to achieve >0.5% A1C reduction in most patients.
RESEARCH DESIGN AND METHODS
This was a 26-week, prospective, randomized, controlled, multinational study that enrolled 218 MDI-treated patients with poorly controlled diabetes (202 with type 1 diabetes, 16 with type 2 diabetes) who were 18 years of age or older. Participants had mean baseline A1C of 8.9% (SD, 1.2 [74 mmol/mol]), mean age of 42.4 years (SD, 14.0), mean BMI of 26.5 kg/m2 (SD, 4.2), and mean diabetes duration of 17.7 years (SD, 11.1). Control group (CNL) patients used a standard BG meter and manual bolus calculation; intervention group (EXP) patients used the Accu-Chek Aviva Expert meter with an integrated bolus advisor to calculate insulin dosages. Glucose data were downloaded and used for therapy parameter adjustments in both groups.
RESULTS
A total of 193 patients (CNL, n = 93; EXP, n = 100) completed the study. Significantly more EXP than CNL patients achieved >0.5% A1C reduction (56.0% vs. 34.4%; P < 0.01). Improvement in treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire scale) was significantly greater in EXP patients (11.4 [SD, 6.0] vs. 9.0 [SD, 6.3]; P < 0.01). Percentage of BG values <50 mg/dL was <2% in both groups during the study.
CONCLUSIONS
Use of an automated bolus advisor resulted in improved glycemic control and treatment satisfaction without increasing severe hypoglycemia.
Intensive insulin therapy using multiple daily insulin injections (MDI) is efficacious in achieving and maintaining optimal glycemic control (1,2). Effective use of MDI therapy requires patients to use several factors, including insulin-to-carbohydrate (CHO) ratios, insulin sensitivity factors (ISFs), target blood glucose (BG) range, current BG values, anticipated physical activity, and general health status, to accurately determine their appropriate insulin doses.
Because manual calculation of insulin boluses is complex and time-consuming, patients may rely on empirical estimates, which can limit their ability to achieve treatment goals (3). Moreover, many patients are limited in their ability to perform these calculations because of inadequate literacy and inadequate numeracy, which are common among patients with diabetes and are associated with poor glycemic control (4,5).
In addition to the potential for computation errors, manual bolus calculation does not take into account the effect of the active insulin that remains from the initial bolus (“insulin on-board”). This can create a high potential for errors when determining a correction bolus, potentially resulting in severe hypoglycemia, which can be debilitating, frightening, and socially aversive (6).
Automated bolus advisors integrated into BG meters may help overcome these challenges, improve self-care behaviors, and reduce the risk for long-term complications. Bolus advisors automatically calculate bolus insulin doses to cover CHO intake and address out-of-range BG levels based on individualized insulin parameter estimates. Several studies with patients treated with insulin pump therapy have shown that use of automated bolus advisors facilitates improvements in glycemic control and reductions in hypoglycemic events (7–10).
Although a number of small studies have shown that the use of these devices is also beneficial in improving glycemic control (11,12), treatment satisfaction (11), and overall accuracy in bolus insulin calculation (13) in MDI-treated patients, no large randomized trials have been conducted to fully assess the impact and utility of automated bolus advisor use in this population.
We hypothesized that use of an automated bolus advisor would enable more MDI-treated patients to achieve clinically significant improvements in glycemic control compared with patients who calculated their bolus insulin doses manually. To test this hypothesis, we designed a large randomized trial using a new automated bolus advisor system that integrates bolus calculations into a BG meter.
RESEARCH DESIGN AND METHODS
The Automated Bolus Advisor Control and Usability Study (ABACUS) was a 26-week, multicenter, multinational, prospective, randomized, controlled comparison between poorly controlled (>7.5% A1C [58 mmol/mol]) MDI-treated type 1 diabetic and type 2 diabetic patients. Patients in the intervention group (EXP) used a BG meter with an embedded automated bolus advisor (Accu-Chek Aviva Expert BG meter; Roche Diagnostics, Indianapolis, IN) in conjunction with enhanced usual care. Active control group (CNL) patients received enhanced usual care and manually calculated bolus insulin dosages according to individualized parameters. Enhanced care included clinic visits that focused specifically on diabetes management and patients received free BG meters and test strips.
Patients were recruited from 30 clinics in the U.K. and Germany. Patients were identified and recruited from the investigators’ established patient population or from within the patient population of other physicians within their group practice using the inclusion and exclusion criteria. Key inclusion criteria were as follows: age 18 years or older; type 1 or type 2 diabetes treated with MDI therapy for ≥6 months; A1C >7.5% (58 mmol/mol); adjustment of meal insulin doses based on CHO content of meal; and completion of CHO training within the past 2 years. Key exclusion criteria were as follows: treatment with NPH insulin, premixed insulin, noninsulin injectable antidiabetic medication or oral antidiabetic agents (except metformin); use of fixed-dose therapy; or use of sliding scale insulin doses determined exclusively by specific BG results.
The study protocol was approved by the National Research Ethics Service (Redditch, U.K.) and Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität Münster (Münster, Germany), and was in compliance with the Helsinki Declaration (14). Written informed consent was obtained from all patients.
Procedures
Patient visits occurred at screening (week minus 2), randomization (week minus 1), and bolus advisor training or therapy initiation (week 0); visits also occurred at weeks 2, 4, 11, 12, 23, and 24. A description of the study procedures and visit schedule was previously published (15).
At screening, investigators confirmed patient eligibility for the study, obtained written informed consent, recorded demographic information, collected relevant medical history and lifestyle information, documented all current medications, performed physical examinations, collected blood and urine samples, and performed pregnancy testing for women of child-bearing age. Patients completed a questionnaire that incorporated questions from standard psychometric instruments and a set of dose adjustment for normal eating (DAFNE) plates, which provide standardized photographs of meals with known CHO values (16). The specific plates selected were representative of meals common to both the U.K. and Germany and were used to assess CHO-counting skills. An additional worksheet was used to assess patient knowledge relevant to MDI therapy.
All patients received a BG meter (Accu-Chek Nano blood glucose meter; Roche Diagnostics) and were trained in its operation. Patients were instructed to generate 7-point BG profiles (preprandial and 2-h postprandial at all main meals and bedtime) over the course of 3 consecutive days and to document their results on the standardized form provided. Patients also used the form to document meals, physical exercise, basal insulin doses, prandial bolus doses, and correction bolus doses over the 3-day testing period. A subset of patients (CNL, n = 46; EXP, n = 47) used a continuous glucose monitoring (CGM) device (Dexcom Seven Plus; Dexcom, San Diego, CA). They were instructed to wear the device (“blinded” mode) until the second visit in addition to completing their BG profiles.
At the randomization visit, investigators collected the BG meters, completed 3-day glycemic profiles, and CGM devices from the patients. Meter data were uploaded via the research version of the Accu-Chek SmartPix device (Roche Diagnostics GmbH, Mannheim, Germany) to the designated secure server. Data then were downloaded to clinic software using the commercial Accu-Chek Smart Pix device or Accu-Chek 360 View Software (Roche Diagnostics GmbH). Printouts were prepared to review with patients. CGM data were uploaded to the secure server. Neither clinicians nor patients had access to the CGM data for the duration of the study. Adverse events and serious adverse events were recorded and patients’ medications were updated. Investigators conducted individualized MDI therapy and CHO-counting training to address knowledge deficits in patients as identified at the screening visit. Patients then were randomized (1:1) to the CNL for enhanced usual care or to automated bolus advisor use (EXP) with enhanced usual care.
At the bolus advisor training visit, investigators collected BG meters, uploaded BG meter data to the secure server, and downloaded meter data to clinic software. Adverse events and serious adverse events were assessed and concomitant medications were updated. Investigators completed the therapy parameter cards and instructed patients on how to use their parameters. EXP patients were given an Accu-Chek Aviva Expert blood glucose meter (Roche Diagnostics) and were instructed to discontinue use of their current BG meter. The Aviva Expert meter incorporates an automated bolus advisor, which provides prandial and correction bolus recommendations based on the current BG value, planned CHO intake, and individualized therapy parameters programmed into the meter. The meter automatically calculates the insulin bolus for the user and stores BG and meal information in an electronic diary. Investigators entered each patient’s therapy parameters into their meter and conducted 1-h training sessions regarding its use.
At subsequent visits, investigators uploaded the BG meter data, collected patient diaries, adjusted therapy parameters as needed, recorded adverse events and serious adverse events, and updated concomitant medications. CGM data were uploaded (when applicable) and patient questionnaires were administered at weeks 12 and 24. DAFNE plate assessments were administered again at week 24.
Outcomes
The primary end point was change in A1C from screening to 26 weeks, with an emphasis on the number of patients who achieved an A1C reduction of >0.5%. Secondary end points included changes in glycemic variability, usage of the bolus advisor (EXP) or insulin rule sets (CNL), MDI/CHO-counting accuracy, and changes in psychosocial measures.
Measures
Glucose measures.
A1C analyses were conducted by a central laboratory using high-performance liquid chromatography methodology (VII Turbo hemoglobin testing system; Bio-Rad Laboratories, Hercules, CA). Time within target range was assessed using CGM data from the subset of patients who used CGM during the study. Glucose data from 3-day glycemic profiles were derived from the uploaded self-monitoring of BG (SMBG) and CGM data (when applicable) and were used to assess mean BG, frequency of hypoglycemia (within 50–72 mg/dL) and severe hypoglycemia (<36 mg/dL and <50 mg/dL with associated symptoms), frequency of SMBG tests performed, percentage of values within fasting and preprandial and postprandial ranges, SD and coefficient of variation across the 3-day profiles, mean and SD differences between preprandial and postprandial BG values over each meal, mean amplitude of glycemic excursions (MAGE), and risk for hypoglycemia and hyperglycemia across the 3-day profiles.
Patient adherence.
Uploaded data from the bolus advisors were used to assess the frequency of bolus advisor use and subsequent adjustments based on proposed bolus amounts for EXP patients. Assessment of CNL patient adherence was derived from use of therapy parameters documented in patient diaries, which included in the 3-day glycemic profile forms.
MDI/CHO accuracy.
Changes from baseline in the ability of patients to accurately count CHOs were assessed using the DAFNE plate assessment scores from visits 1 and 9.
Psychosocial measures.
Psychosocial outcomes including treatment satisfaction, social functioning, and factors important to quality of life were assessed using the patient questionnaire, which incorporated questions from validated psychometric instruments as well as commonly used survey questions to assess depression (17), diabetes-specific distress (18), fear of hypoglycemia (19), treatment satisfaction (20), awareness of hypoglycemia (21), and health outcomes (22). A description of these measures was previously published (15).
Statistical analysis
The intention-to-treat population was defined as all eligible patients who participated in visit 1 (screening) and visit 2 (randomization); all efficacy and safety analyses were performed with this population. The demographic and performance characteristics of patients who achieved A1C reduction of >0.5% during the course of the study were considered to be clinically relevant. For analysis, the study population was divided into a subgroup of patients with an absolute A1C reduction of >0.5% from screening to study end and a subgroup of patients with a reduction of ≤0.5% or no reduction. The characteristics and behaviors relevant to the study were reported. The study was designed to have 90% power to detect a mean difference of 0.5% change in A1C levels between CNL and EXP from baseline to study end in favor of the EXP group. This was determined using a one-sided, two-sample t test (α = 0.05) assuming a common SD of 0.9% for the intention-to-treat population.
Pearson correlation coefficients were computed for the A1C values (weeks −1, 11, and 24), BG measures from SMBG and CGM scores for each collection visit, other laboratory parameters, frequencies of hypoglycemia, CHO scores, summary scores from psychosocial questionnaires, and all other variables of interest. For all of these covariables, the correlation coefficients for their changes from baseline compared with the A1C parameters also were computed. The correlation coefficients for the A1C parameters compared with bolus advisor parameters were computed for EXP participants.
Other secondary outcome variables were compared descriptively by study groups for each scheduled visit and for changes between visits. Continuous variables, including scores from the questionnaires, were summarized using N, mean, SD, median, lower quartiles, upper quartiles, minimums, and maximums; categorical variables were summarized using counts and percentages of patients in each category. Two-sample t tests in the case of continuous variables and χ2 tests in the case of categorical variables were performed for group comparisons.
We assessed the effect of bolus advisor use on glycemic variability as measured by MAGE in the subset of patients who used CGM. Bolus advisor usage was assessed by calculating the average number of times per day patients sought and accepted bolus advice and the percentage of time patients modified the advice, adjusting dosage higher or lower than recommended. The number of possible bolus opportunities was derived from internally “flagged” events presented in the downloaded meter data. Usage of prescribed MDI rule sets by CNL patients was assessed by examining actual use of MDI insulin-to-CHO ratios and ISFs as reported in the 3-day profiles.
Competency in CHO estimation was calculated using the mean of meal errors (MME), which indicates accuracy comparing the true and estimated CHO values (i.e., average of estimated minus true CHO contents in grams) of the selected DAFNE plates. Variability was calculated using the mean of meal absolute errors (MMAE) between true and estimated values (i.e., average of absolute values of the differences between estimated and true CHO contents in grams).
The Patient Health Questionnaire-8 severity score was calculated as the sum of all items of the questionnaire's depression scale. The major depressive disorder was derived from the Patient Health Questionnaire-8 score as an ordinal scale variable with five categories ranging from “no significant symptoms” to “severe symptoms.” The Problem Areas in Diabetes (PAID) overall score was calculated as the sum of all the items of the PAID scale multiplied by 1.25, resulting in a score between 0 and 100. The total Hypoglycemia Fear Survey (HFS) behavior score was calculated as the sum of the 15 items of the HFS-II regarding behavior, and the total HFS worry score was calculated as the sum of the 18 items of the HFS-II regarding worry. The total HFS-II score was calculated as the sum of these two scores. The total Diabetes Treatment Satisfaction Questionnaire status score was calculated as the sum of the items (except items 2 and 3) of the Diabetes Treatment Satisfaction Questionnaire at baseline, and the total change score at the end of the study was equivalently calculated from the total change score at the end of the study items collected at study end. Impaired awareness of hypoglycemia was derived as a binary variable from the Gold scale questionnaire. From the EQ-5D questionnaire, the EQ-5D health score (0 = worst health and 100 = best health) and the EQ-5D-5L index scores (values between 0 and 1 in general) were used. All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
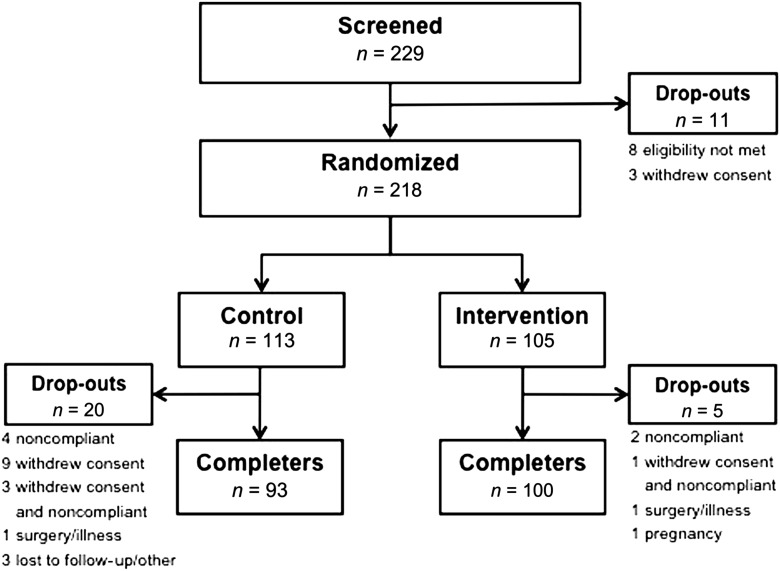
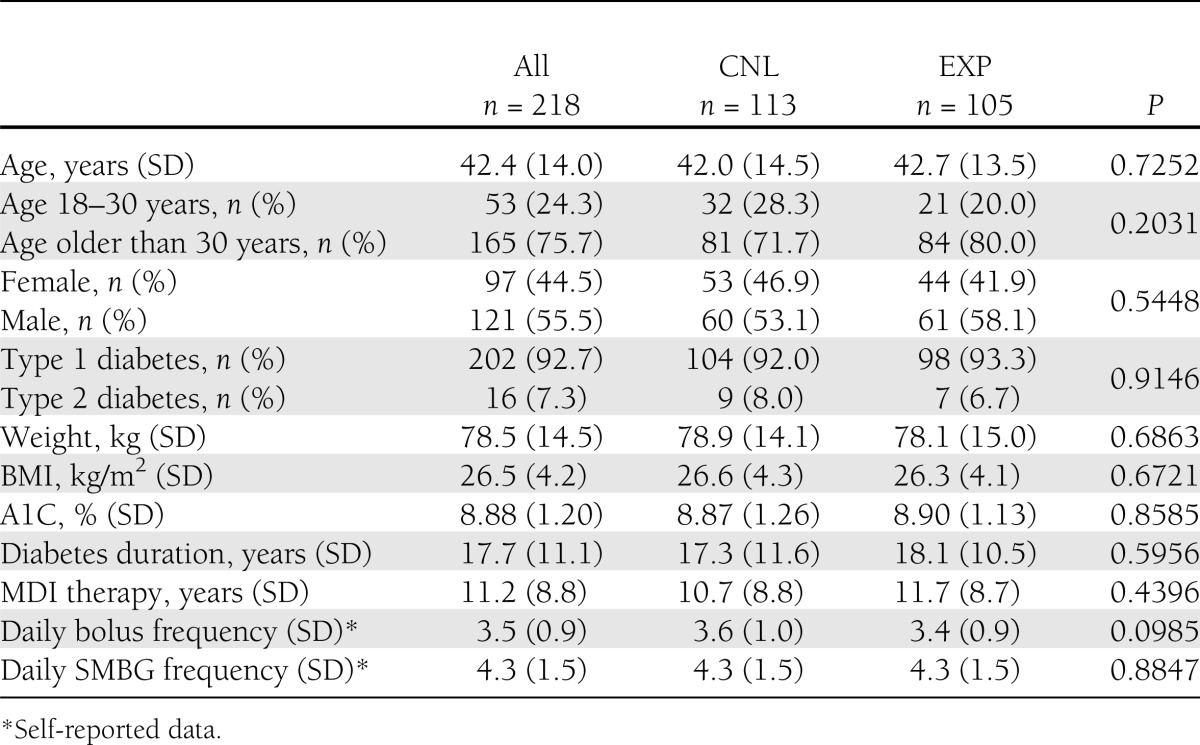
We screened 229 patients for the study; 218 were randomized and included in the intention-to-treat analysis (CNL, n = 113; EXP, n = 105). A total of 193 patients (CNL, n = 93; EXP, n = 100) completed the study (Fig. 1). Of the 11 patients not randomized, eight did not meet inclusion or exclusion criteria and three withdrew consent before randomization. After randomization, 19 patients (CNL, n = 16; EXP, n = 3) did not comply or withdrew consent (or both), two patients (CNL, n = 1; EXP, n = 1) discontinued because of surgery or illness, one patient (EXP) discontinued because of pregnancy, and three patients (CNL) were lost to follow-up or discontinued for other reasons. Demographic characteristics of the dropouts were not significantly different between the two study groups. Baseline patient characteristics of all randomized patients are summarized in Table 1. There were no significant between-group differences.
Figure 1.
Patient disposition.
Table 1.
Patient demographic characteristics at baseline
Changes in glycemic measures
A1C.
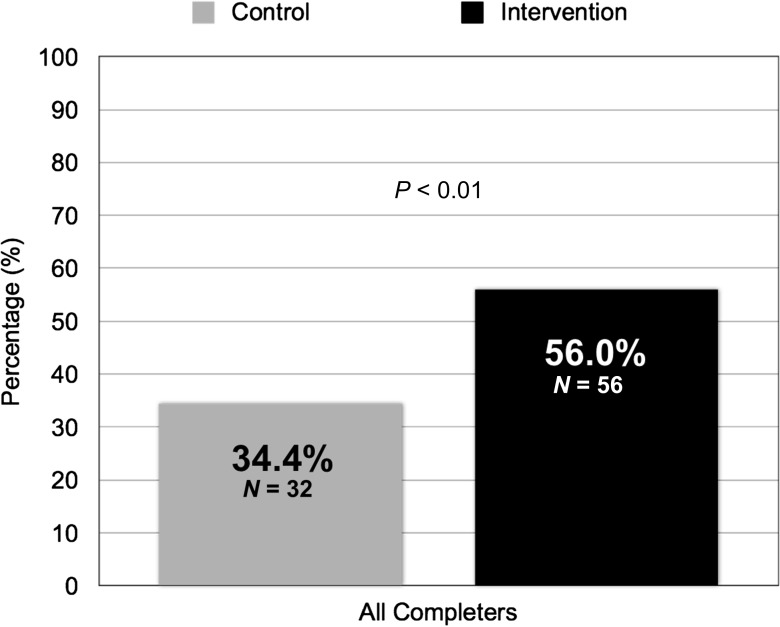
As a group, EXP patients experienced a slightly greater reduction in mean A1C from baseline compared with CNL patients (−0.7% [SD, 0.7] vs. −0.5% [SD, 0.7]; Δ0.2%; one-sided P < 0.05). However, significantly more EXP patients achieved the study goal of >0.5% A1C reduction than CNL patients (56.0% vs. 34.4%; P < 0.01) (Fig. 2). The average A1C reduction among EXP and CNL patients (n = 88) who achieved the study goal was −1.2% (SD, 0.5).
Figure 2.
Percentage of patients who achieved >0.5% A1C reduction.
Although older patients (older than 30 years) in both groups were more likely to achieve the primary outcome than younger patients (49% vs. 33%), more younger EXP patients achieved >0.5% A1C reductions than did younger CNL patients (53% vs. 15%; P < 0.05). Additionally, the mean age of EXP patients who achieved the goal was younger than that of those EXP patients who did not (42.4 [12.8] years vs. 44.3 [14.2] years). Conversely, the age of CNL patients who achieved the A1C goal was older than that of those who did not (49.4 [13.7] years vs. 41.4 [13.9] years; P < 0.05).
Hypoglycemia.
Significantly more EXP than CNL patients reported glucose levels <70 mg/dL (43 vs. 31; P < 0.05). Among these patients, 11 EXP and seven CNL patients reported glucose levels <36 mg/dL or required third-party assistance (or both), which was our defined measure of severe hypoglycemia; however, the between-group difference was not significant. There were no significant differences in hypoglycemia (<70 mg/dL or <36 mg/dL) between patients who achieved >0.5% A1C reductions and those who did not achieve the goal in either study group.
We also assessed severe hypoglycemia using a cut point of <50 mg/dL. In this analysis, 28 EXP and 22 CNL patients reported glucose levels <50 mg/dL with no statistical between-group differences. Achievement of >0.5% A1C reduction was not associated with incidence of <50 mg/dL in either study group. Overall, the percentage of BG values <50 mg/dL remained at <2% in both groups throughout the study.
Glycemic variability.
Analysis of CGM data showed that EXP but not CNL patients experienced significant reductions in mean MAGE (−20.2 mg/dL [SD, 41.1; P < 0.01] vs. −2.9 mg/dL [SD, 32.1; P = not significant]). Even among patients who achieved ≤0.5% A1C improvement, EXP patients showed significant reductions in MAGE compared with CNL patients (−15.3 mg/dL [SD, 29.3; P < 0.05] vs. 1.1 mg/dL [SD, 35.8; P = not significant]). Similar improvements in MAGE were seen in EXP patients who achieved >0.5%, but the between-group differences (EXP vs. CNL) did not achieve statistical significance. Within the EXP group, older patients experienced significant MAGE reductions but younger patients did not (−23.8 mg/dL [SD, 42.4; P < 0.01] vs. 0.6 mg/dL [SD, 27.8; P = NS]). Changes in MAGE correlated with changes in SD of glucose values (r = 0.87) and were independent of education level, duration of diabetes, duration of MDI, or sex.
Bolus advisor usage
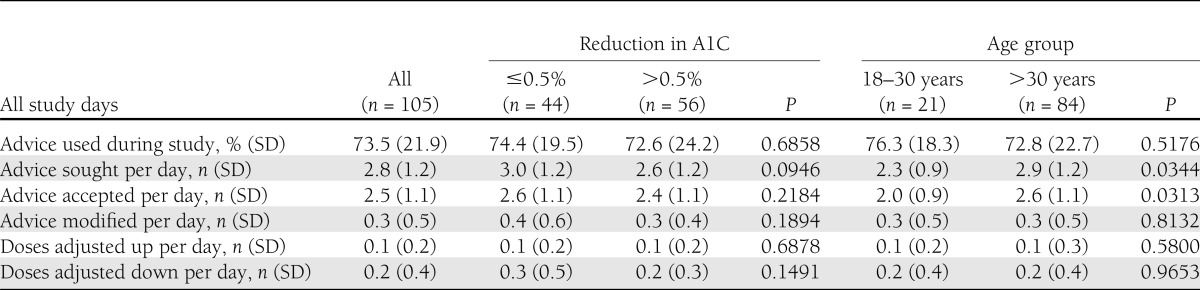
EXP patients used their bolus advisor for a significant percentage of all possible bolus opportunities (Table 2). Patients sought bolus advice an average of 2.9 times (SD, 1.2) per day at the beginning of the study; however, daily frequency of advice sought decreased significantly (−0.2 per day; P < 0.01) during the course of the study. Younger patients sought and accepted bolus advice less often than older patients did. There was a slight, but not statistically significant, difference in frequency of bolus advice sought by patients who achieved >0.5% A1C reductions compared with patients who achieved ≤0.5% A1C reduction (3.0 [SD, 1.2] vs. 2.6 [SD, 1.2] per day; P = not significant). However, during the course of the study, there was a significant reduction in daily bolus advisor use frequency among patients who achieved >0.5% A1C compared with those who did not (−0.4 [SD, 0.5] vs. 0.1 [SD, 0.4] per day; P < 0.01). When modifications to bolus advice were made, patients were two-times more likely to reduce their insulin than they were to increase the dose, with no difference between age groups. Neither frequency of bolus advisor use nor acceptance of advice was associated with sex or type of diabetes.
Table 2.
Bolus advisor use for EXP patients
Use of MDI rule sets
Data from 3-day profiles of 96 CNL patients (87 with type 1 diabetes, 9 with type 2 diabetes) were analyzed. Patient-recorded bolus calculations were checked to determine if their stated insulin-to-CHO ratios and ISF rules were used in a mathematically correct manner for each bolus calculation. Patients calculated an average of 4.0 (SD, 1.0) boluses per day during the study with no change in frequency from study start to study end. Patients correctly used their insulin-to-CHO ratios and ISF rules 1.6 times (SD, 1.2) and 0.8 times (SD, 0.7) per day, respectively. There was no change in correct or incorrect usage of either parameter from study start to study end.
Changes in CHO counting competency
At study end, EXP patients showed significant improvements in variability (MMAE) from 15.2 g (SD, 9.0) to 12.4 g (SD, 7.3; P < 0.01), and a trend toward improvement in accuracy (MME) from 1.0 g (SD, 10.1) to 0.3 g (SD, 7.1; P = NS). Small but insignificant changes in MMAE or MME were seen in CNL patients.
Changes in psychosocial measures
EXP patients reported significantly greater improvement in treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire scale) than CNL patients (11.4 [SD, 6.0] vs. 9.0 [SD, 6.3]; P < 0.01). Changes in depression, diabetes-related distress, fear of hypoglycemia, hypoglycemia awareness, and health outcomes will be presented in subsequent study reports.
CONCLUSIONS
We investigated the impact and utility of automated bolus advisor use in diabetic patients treated with MDI therapy. Considerably more EXP patients were able to achieve clinically significant improvements in glycemic control, as measured by reductions in A1C and glycemic variability, compared with manual bolus insulin dose calculation with no increase in severe hypoglycemia. These findings are consistent with previous studies of bolus advisor use in MDI-treated patients (11–13). Although bolus advisor use was beneficial in both age groups studied, it was especially useful in younger adults, a population that often finds it difficult to achieve optimal glycemic control (23).
It is noteworthy that use of the Expert meter did not appear burdensome; patients frequently sought bolus advice and seldom modified the recommendations provided, which suggests that patients placed a high level of trust in the bolus recommendations they received. The significant increase in treatment satisfaction combined with the low dropout rate in the intervention arm contributes additional support indicating that patients perceived the meter to be both useful and user-friendly, a finding that is consistent with previous research (24). It is interesting that A1C levels continued to improve during the course of the study despite the small decrease in bolus advisor use over time. This suggests that patients not only trusted the bolus advice provided but also learned from their insulin-to-meal responses and, thus, required slightly less frequent bolus advice over time. Conversely, most CNL patients did not use their prescribed MDI rule sets often.
Additionally, our results suggest that use of a bolus advisor may be associated with improvement in competency in CHO counting by providing frequent feedback regarding accuracy of the patients in estimating their CHO intake. Patients were able to continually verify the accuracy of their CHO calculations through improved postprandial glucose control as indicated by both SMBG data and fewer correction boluses, which can reduce the risk of insulin “stacking.” Thus, automated bolus advisors may have an additional benefit as educational tools that can improve the effectiveness of diabetes self-management in patients treated with MDI. It is noteworthy, however, that MMAE (variability) appears to have a greater impact on clinical outcomes than MME (accuracy).
A key strength of the study was our focus on the achievement of a specific glycemic goal rather than group differences in A1C levels at the end of the study. This approach allowed us to more readily identify and describe those patient subpopulations that are most likely to benefit from bolus advisor use and, therefore, lead to more personalized treatment regimens. Moreover, the study design facilitated the gathering of clinically relevant information about patient behaviors and adherence to therapy. For example, as reported, CNL patients correctly applied their prescribed MDI rule set in <35% of their daily bolus calculations. Although incorrect (or lack of) use may be attributable to mathematical errors, it is possible that a portion of these patients may have been distrustful of their parameters and, in response, may have developed unknown “compensatory mechanisms” for dose calculation. This may explain improvements in those CNL patients who achieved the A1C goal; however, this behavior could have a negative impact on therapy adjustment if clinicians are unaware of these discrepancies.
A potential limitation of our study design was the intensity of diabetes care provided to both groups, which may explain why significant improvements were seen in both study groups. A third “pure” control arm with no enhanced care would have allowed a more realistic comparison of bolus advisor versus manual bolus calculation in real-world clinical care. Additionally, use of the 3-day, 7-point profiles in both groups may have impacted changes in A1C in both groups. Also, we did not capture insulin parameters at baseline; instead, we conducted analyses based on visit 3 measures, which may underestimate the significance of the parameters entered at visit 3 compared with baseline parameters.
Manual calculation of insulin boluses is complex and time-consuming, and it often results in dosing errors that can limit the ability of patients to achieve their treatment goals (3). Our findings demonstrate that use of an automated bolus advisor can be efficacious and clinically meaningful in MDI therapy, and that most patients are willing and able to use this technology appropriately when adequate clinical support is provided.
Acknowledgments
Funding for the study was provided by Roche Diagnostics (Indianapolis, IN).
R.Z., D.A.C., I.C., K.B., J.R., C.V., C.G.P., and W.K. received consulting fees for their involvement in the study design, study implementation, and preparation of the manuscript. C.G.P. has received consulting fees from Roche Diagnostics and Dexcom, Inc. (San Diego, CA). I.V., B.P., M.A.S., and R.S.W. are employees of Roche Diagnostics. No other potential conflicts of interest relevant to this article were reported.
R.Z., D.A.C., I.C., and R.S.W. had the original idea for the study, wrote the study proposal and protocol, developed study measures and intervention, and developed the manuscript. K.B. had the original idea for the study, wrote the study proposal and protocol, and developed the manuscript. J.R. and C.V. developed study measures and intervention, developed patient instructional materials, and provided training. C.G.P. developed patient instructional materials and provided training. W.K. provided statistical analysis support. I.V. developed study measures and intervention, reviewed the manuscript, and was the study site coordinator. B.P. developed study measures and intervention and reviewed the manuscript. M.A.S. developed the manuscript. All authors read and approved the final manuscript and take full responsibility for the accuracy of its content. R.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Key results of this study were presented at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012; at the 6th Annual Meeting of the Advanced Technologies and Treatments for Diabetes, Paris, France, 28 February 2013; and in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank the patients, physicians, and clinic staff for participating in this study.
Footnotes
Clinical trial reg. no. NCT01460446, clinicaltrials.gov.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther 2003;5:365–369 [DOI] [PubMed] [Google Scholar]
- 4.Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA 2002;288:475–482 [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med 2008;148:737–746 [DOI] [PubMed] [Google Scholar]
- 6.Anderbro T, Amsberg S, Adamson U, et al. Fear of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 2010;27:1151–1158 [DOI] [PubMed] [Google Scholar]
- 7.Klupa T, Benbenek-Klupa T, Malecki M, Szalecki M, Sieradzki J. Clinical usefulness of a bolus calculator in maintaining normoglycaemia in active professional patients with type 1 diabetes treated with continuous subcutaneous insulin infusion. J Int Med Res 2008;36:1112–1116 [DOI] [PubMed] [Google Scholar]
- 8.Zisser H, Wagner R, Pleus S, et al. Clinical performance of three bolus calculators in subjects with type 1 diabetes mellitus: a head-to-head-to-head comparison. Diabetes Technol Ther 2010;12:955–961 [DOI] [PubMed] [Google Scholar]
- 9.Lepore G, Dodesini AR, Nosari I, Scaranna C, Corsi A, Trevisan R. Bolus calculator improves long-term metabolic control and reduces glucose variability in pump-treated patients with Type 1 diabetes. Nutr Metab Cardiovasc Dis 2012;22:e15–e16 [DOI] [PubMed] [Google Scholar]
- 10.Enander R, Gundevall C, Strömgren A, Chaplin J, Hanas R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatr Diabetes 2012;13:545–551 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt S, Meldgaard M, Serifovski N, et al. Use of an automated bolus calculator in MDI-treated type 1 diabetes: the BolusCal Study, a randomized controlled pilot study. Diabetes Care 2012;35:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurizi AR, Lauria A, Maggi D, et al. A novel insulin unit calculator for the management of type 1 diabetes. Diabetes Technol Ther 2011;13:425–428 [DOI] [PubMed] [Google Scholar]
- 13.Sussman A, Taylor EJ, Patel M, et al. Performance of a glucose meter with a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabetes Sci Tech 2012;6:339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association Declaration of Helsinki World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 15.Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 19.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 20.Bradley C. The Diabetes Treatment Satisfaction Questionnaire (DTSQ). In Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Bradley C, Ed. Chur, Switzerland, Hardwood Academic, 1994, p. 111–132 [Google Scholar]
- 21.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 22.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340–349 [DOI] [PubMed] [Google Scholar]
- 23.Wolpert HA, Anderson BJ. Young adults with diabetes: need for a new treatment paradigm. Diabetes Care 2001;24:1513–1514 [DOI] [PubMed] [Google Scholar]
- 24.Cukierman-Yaffe T, Konvalina N, Cohen O. Key elements for successful intensive insulin pump therapy in individuals with type 1 diabetes. Diabetes Res Clin Pract 2011;92:69–73 [DOI] [PubMed] [Google Scholar]