Abstract
OBJECTIVE
It is of vital importance to elucidate the triggering factors of obesity and type 2 diabetes to improve patient care. Bariatric surgery has been shown to prevent and even cure diabetes, but the mechanism is unknown. Elevated levels of lipopolysaccharide (LPS) predict incident diabetes, but the sources of LPS are not clarified. The objective of the current study was to evaluate the potential impact of plasma LPS on abdominal obesity and glycemic control in subjects undergoing bariatric surgery.
RESEARCH DESIGN AND METHODS
This was a prospective observational study involving a consecutive sample of 49 obese subjects undergoing bariatric surgery and 17 controls. Main assessments were plasma LPS, HbA1c, adipose tissue volumes (computed tomography), and quantified bacterial DNA in adipose tissue compartments.
RESULTS
Plasma levels of LPS were elevated in obese individuals compared with controls (P < 0.001) and were reduced after bariatric surgery (P = 0.010). LPS levels were closely correlated with HbA1c (r = 0.56; P = 0.001) and intra-abdominal fat volumes (r = 0.61; P < 0.001), but only moderately correlated with subcutaneous fat volumes (r = 0.33; P = 0.038). Moreover, there was a decreasing gradient (twofold) in bacterial DNA levels going from mesenteric via omental to subcutaneous adipose tissue compartments (P = 0.041). Finally, reduced LPS levels after bariatric surgery were directly correlated with a reduction in HbA1c (r = 0.85; P < 0.001).
CONCLUSIONS
Our findings support a hypothesis of translocated gut bacteria as a potential trigger of obesity and diabetes, and suggest that the antidiabetic effects of bariatric surgery might be mechanistically linked to, and even the result of, a reduction in plasma levels of LPS.
Obesity and type 2 diabetes are rapidly emerging as major public health problems worldwide. This growing pandemic is often associated with other prevalent diseases, such as insulin resistance, metabolic syndrome, and cardiovascular disease, which are disease states that are potentially linked to chronic low-grade inflammation (1). Obesity per se facilitates a proinflammatory state, characterized by increased levels of proinflammatory cytokines, and it has been proposed that adipose tissue, particularly intra-abdominal adipose tissue, might be a major source of inflammation (2,3). However, the triggering factors of adipose tissue inflammation, obesity, and type 2 diabetes remain to be determined.
Interestingly, mesenteric fat, which is localized in close proximity to the gut wall, in mice has been shown to express higher levels of proinflammatory chemokines than other types of adipose tissue (4). The gut flora or microbiota contains 10-fold the number of cells and 150-times the number of genes compared with the human body and is currently being characterized in the Human Microbiome Project and in the MetaHIT program (5). An altered gut microbiota has been linked to several chronic disease states, including obesity (6) and type 2 diabetes (7).
Translocation of gut microbiota, particularly endotoxins or lipopolysaccharides (LPSs) on the surface of gram-negative bacteria to the systemic circulation, has been proposed to be an early trigger of inflammation and obesity (8). Endotoxins normally circulate at low levels in healthy individuals (9), and 80–97% of circulating endotoxin is bound to lipoproteins (10). LPS promotes inflammation mainly by signaling through Toll-like receptor (TLR) 4 on macrophages, monocytes, and other cells of the innate immune system, and CD14 plays a central role by transferring LPS to the TLR4 complex (11).
An increase in plasma LPS occurs in healthy individuals after a high-fat meal (12), whereas a chronic state of low-grade endotoxemia as measured by plasma LPS (8) or LPS-binding protein (13) is evident in patients with obesity and insulin resistance. Furthermore, low-grade endotoxemia predicts incident diabetes, but the sources of LPS are unknown because LPS can translocate both from the oral cavity and the gut (14). Interestingly, in a mouse model, commensal gut bacteria translocate to mesenteric adipose tissue, initiating low-grade inflammation before the onset of insulin resistance and type 2 diabetes (15). To our knowledge, these mechanisms have not been studied in humans.
Bariatric surgery has been shown to prevent and potentially cure diabetes (16–18), but the mechanism is unknown. We hypothesized that adipose tissue in proximity to the gut would have a relatively higher quantity of bacterial DNA than other types of adipose tissue, that circulating plasma LPS would be associated with the amount of intra-abdominal adipose tissue and HbA1c, and that a reduction of LPS after bariatric surgery would correlate with improved glycemic control.
RESEARCH DESIGN AND METHODS
Patients and controls
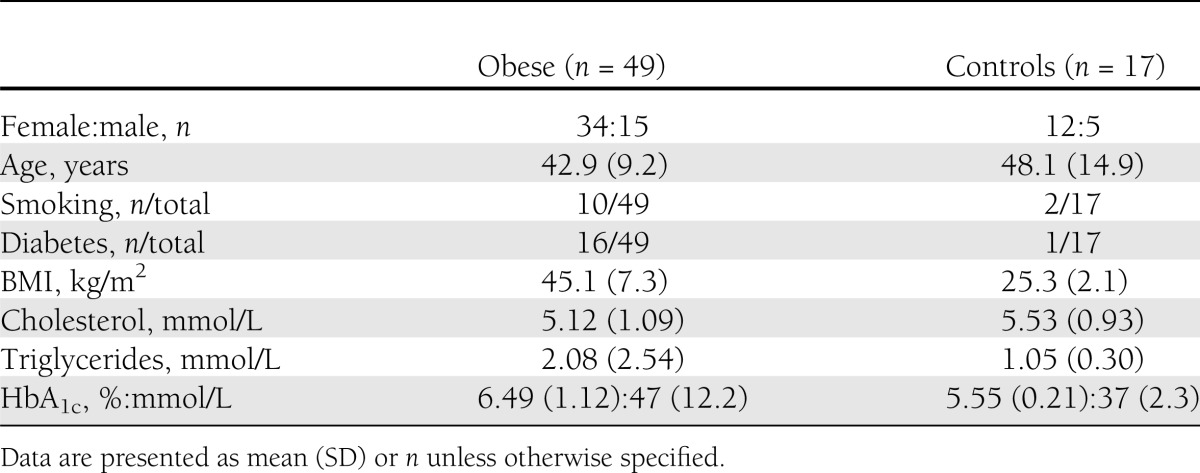
Forty-nine obese individuals were included, and all the patients underwent conservative and subsequent surgical treatment at the Regional Centre for Treatment of Morbid Obesity at Nordland Hospital in Norway. The patients were evaluated at three time points: at baseline, preoperatively (after 3 months of lifestyle intervention), and 1 year after bariatric surgery. In addition, 17 individuals with BMI ≤28 kg/m2 undergoing elective laparoscopic procedures (cholecystectomy or fundoplication) were included as controls at baseline. Baseline characteristics are given in Table 1.
Table 1.
Baseline characteristics of obese subjects and controls
Patients with abdominal diameter >50 cm were excluded from attending computed tomography (CT) scan because of the diameter of the CT scanner. Hence, 24 obese patients and 17 controls were evaluated with CT scan and were included in the correlation analyses at baseline (Supplementary Fig. 1). The study was performed in accordance with the Helsinki Declaration and approved by the ethics committee (Regional Ethics Committee of Northern Norway), and written informed consent was obtained of all participants.
Preoperative lifestyle intervention
All patients had to undergo lifestyle changes, resulting in a minimum of 10% excess weight loss preoperatively. The patients received repeated personal guidance by telephone consultations concerning eating habits and were encouraged to perform physical activity according to the Norwegian guidelines for treatment of morbidly obese patients. The average time period from baseline to operation was 12 weeks.
Surgical methods
Two surgical methods were used for the patients undergoing bariatric surgery. Laparoscopic Roux-en-Y gastric bypass was performed by a standardized procedure and used for patients with BMI <50 kg/m2. This procedure included a small ventricular pouch of 30 mL and a bileopancreatic limb of 50 cm, with an alimentary limb of 100 cm. For patients with a BMI >50 kg/m2, duodenal switch was performed by a standardized procedure that included a gastric sleeve using a 32-French probe to measure the diameter, an alimentary limb of 150 cm, and a common channel of 100 cm. All operations were performed by two experienced bariatric surgeons (19).
Adipose tissue biopsies were performed during the surgical procedure using laparoscopic technique from the mesenteric, omental, and subcutaneous adipose tissue compartments. The biopsy specimens were immediately transferred to pyrogen-free limulus amebocyte lysate–tested tubes (Nunc), stored on ice, and snap-frozen at −80°C until analysis.
Quantification of adipose tissue volumes
For both the obese patients (if eligible) and the control group, preoperative CT scans were performed on a Siemens Sensation 4 Slice CT scanner (Siemens AG) using validated methods (20). The scans were performed at 120 kV and 250 mA, and the subjects were examined in the supine position with their arms stretched above their head.
To minimize the amount of radiation, a CT volume scan with a slice thickness of 10 mm was performed at only one site in women at the level 5 cm above the L4/L5 intervertebral space and in men 10 cm above the L4/L5 level. The attenuation interval for adipose tissue calculations was −30 to −190 Hounsfield units.
Subcutaneous and intra-abdominal fat compartments were calculated using a Leonardo work station. The subcutaneous and intra-abdominal (including mesenteric and omental) adipose tissue compartments were manually traced, and the inner abdominal muscular wall separated the compartments. All CT scans were examined by the same radiologist at the end of the study.
Analysis of circulating LPS and soluble CD14
Fasting blood samples were collected with standard venipuncture. Serum levels of triglycerides and HDL cholesterol were analyzed in the hospital routine laboratory on the day the serum was obtained. LPS and soluble CD14 were determined in EDTA plasma, which was stored at −70°C until analysis in batch.
LPS was analyzed by limulus amebocyte lysate colorimetric assay (Lonza, Walkersville, MD) according to the manufacturer’s instructions, with the following modifications: samples were diluted 10-fold to avoid interference with background color and preheated to 68°C for 12 min before analysis to dissolve immune complexes as previously described (21). Soluble CD14 (R&D, Minneapolis, MN) was analyzed by ELISA according to the manufacturer’s instructions.
To minimize the interassay and intra-assay variability, samples from baseline, preoperative, and postoperative evaluations as well as from controls were analyzed in the same run, and all samples were run in duplicates. Our intra-assay coefficient of variation was <5% and the interassay coefficient of variation was <10% for LPS and soluble CD14, respectively.
Quantification of bacterial load in adipose tissue
Approximately 0.5 g adipose tissue was excised from the biopsy samples after thawing in a Petri dish and transferred to MagNA Lyser Green Beads tubes (Roche). Each tube was homogenized with 600 µL STAR Buffer (Roche), processed twice at 6,000 rpm for 40 s, and rested on ice for 40 s. The lysates were centrifuged at 3,500 rpm for 5 min.
From the recovered supernatant in each tube, 250 µL was further lysed in 250 µL lysis buffer and 20 µL proteinase, and then incubated for 10 min at 55°C. Finally, 400 µL proteinase lysate from each well were transferred to a KingFisher 96-deep-well plate. DNA was subsequently purified on the KingFisher Flex Robot using the LGC Genomics mag maxi DNA extraction kit.
The quantification of the bacterial load was achieved using quantitative PCR targeting generally conserved regions of the gene encoding small subunit ribosomal RNA, as previously described (22), using primers and probe modified from Nadkarni et al. (23). The ratio between bacterial and human DNA was used as a measure of the bacterial load. Each sample was amplified using 1, 2, and 5 µL templates, respectively. A high DNA yield and low levels of inhibition were obtained from the fat tissue samples, ensuring reliable quantification of bacterial DNA.
The bacterial load was estimated as the amount of bacteria relative to fat tissue, as determined by the amount of DNA. We used the following formula in the estimation: bacterial load = −ct value bacterial DNA * (log10 of amplification efficiency) − log10 (nanogram total DNA).
Statistics
Differences between obese patients and controls were evaluated with a paired t test or Mann-Whitney U test, as appropriate. Wilcoxon test for paired observations was used to evaluate changes from baseline compared with preoperative and postoperative evaluations. Correlation analyses were performed using Spearman ρ or the Pearson correlation, as appropriate. ANOVA was used for multiple comparisons of bacterial load in the various strata of adipose tissue. A two-tailed significance level of 0.05 was used. The statistical analyses were performed with SPSS software (version 19.0; SPSS, Chicago, IL).
RESULTS
Baseline characteristics
Baseline characteristics of the patients are provided in Table 1.
Plasma levels of LPS and soluble CD14 at baseline and effect of bariatric surgery
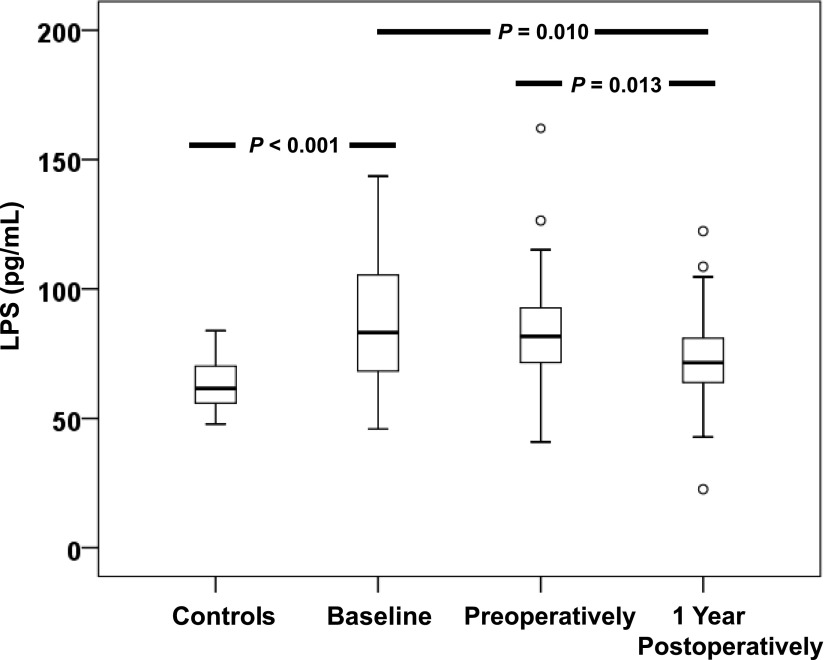
At baseline, plasma levels of LPS (Fig. 1) and soluble CD14 (Supplementary Fig. 2) were significantly elevated in obese subjects compared with controls.
Figure 1.
Reduced LPS levels after bariatric surgery. Plasma levels of LPS in controls (n = 17), obese subjects (n = 49) at baseline, obese subjects preoperatively, and obese subjects at 1 year postoperatively. Data are presented as medians (25–75 percentiles). Outliers (○) were defined by SPSS software.
Plasma LPS, but not soluble CD14, was reduced 1 year after bariatric surgery compared with baseline (P = 0.010) and preoperative evaluations (P = 0.013). However, no reduction in LPS levels was observed from baseline to preoperative evaluation after a period of lifestyle intervention (Fig. 1).
Correlations between LPS levels, HbA1c, adipose tissue volumes, and cardiometabolic risk factors
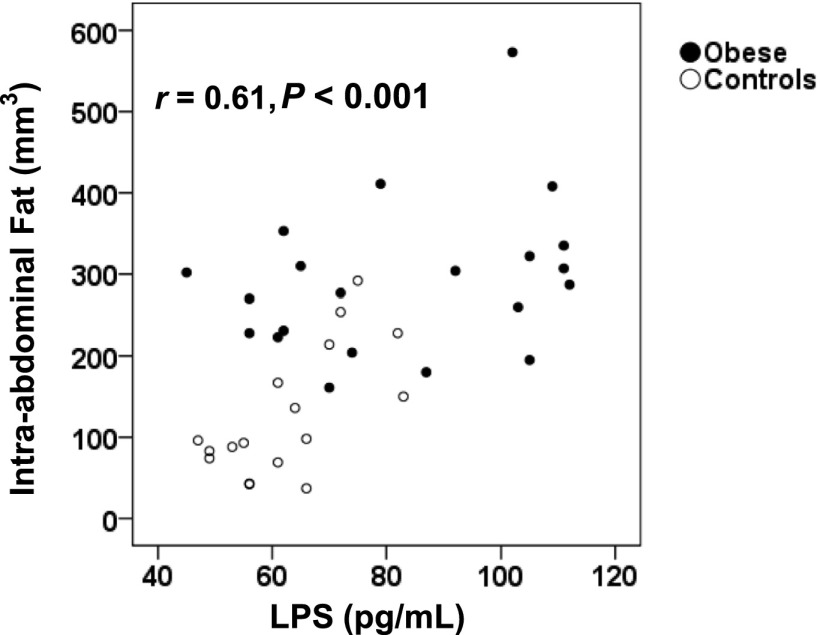
At baseline, plasma levels of LPS were closely correlated with HbA1c (r = 0.56; P = 0.001). There was a close correlation between LPS levels and intra-abdominal fat volumes (r = 0.61; P < 0.001) (Fig. 2), whereas the correlation with subcutaneous fat was moderate (r = 0.33; P = 0.038). Furthermore, plasma LPS was positively correlated with several cardiometabolic risk factors, including fasting triglycerides (r = 0.52; P = 0.001), systolic blood pressure (r = 0.40; P = 0.009), and BMI (r = 0.37; P = 0.017), and was negatively correlated with HDL cholesterol (r = −0.43; P = 0.006).
Figure 2.
Correlation between LPS levels and intra-abdominal fat. Spearman correlation between plasma LPS and the volume of intra-abdominal fat at baseline. ●, obese; ○, controls.
Correlation between reduced LPS levels and HbA1c after bariatric surgery
When comparing changes in LPS levels from baseline to 1-year follow-up after bariatric surgery, there was a direct correlation between reduced levels (δ values) of LPS and improved glycemic control as measured by HbA1c (r = 0.85; P < 0.001). There also was a close correlation between a reduction in LPS levels and fasting triglycerides (r = 0.76; P < 0.001), but not with the other cardiometabolic risk factors (BMI, HDL cholesterol, blood pressure).
Bacterial load in adipose tissue compartments
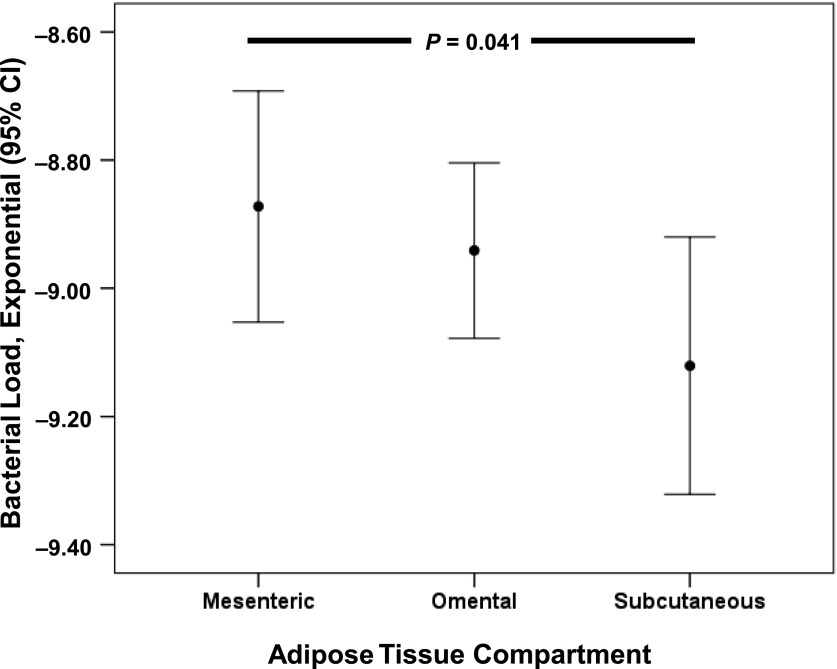
Based on the higher correlation between circulating LPS and intra-abdominal fat volumes as compared with subcutaneous adipose tissue volumes, we hypothesized that adipose tissue in proximity to the gut would have a relatively higher quantity of bacterial DNA. There was a statistically significant decreasing trend in bacterial loads when comparing bacterial DNA in mesenteric, omental, and subcutaneous adipose tissue biopsy specimens, respectively (Fig. 3). The relative difference in bacterial load between mesenteric and subcutaneous adipose tissue was ∼0.3 log, or twofold.
Figure 3.
Bacterial load in adipose tissue compartments. Relative amount of bacterial DNA (bacterial load = −ct value bacterial DNA * (log10 of amplification efficiency) − log10(nanogram total DNA)) in mesenteric, omental, and subcutaneous adipose tissue in obese patients at baseline. Data are presented as mean (95% CI).
CONCLUSIONS
In this study, we investigated the potential impact of translocated commensal gut bacteria on abdominal obesity, glycemic control, and the antidiabetic effects of bariatric surgery. The significant associations between adipose tissue volumes and low-grade endotoxemia are novel findings. Although firm conclusions about possible causation cannot be drawn, the close correlation between intra-abdominal adipose tissue and plasma LPS supports a model of translocated gut microbiota to the surrounding adipose tissue with subsequent trigging of macrophage infiltration, inflammation, and development of obesity (8,24).
Conversely, the association between endotoxemia and subcutaneous adipose tissue was only moderate. Hence, we hypothesized the presence of a “microbial gradient” from the gut lumen to intra-abdominal and subcutaneous fat that could explain the increased proinflammatory activity in the adipose tissue surrounding the gut (4). The decreasing bacterial loads when comparing mesenteric, omental, and subcutaneous fat are novel findings in humans and are well in line with recent results from a mouse model in which gut bacteria were found in mesenteric adipose tissue before the onset of insulin resistance and type 2 diabetes (15). However, our data give no direct evidence that these bacteria are translocated from the gut or contribute to the obese phenotype of the patients. Hence, future studies including fecal microbiota analyses or functional intestinal permeability tests (25) are warranted to determine whether translocation of commensal gut bacteria contributes to abdominal obesity in humans.
Bariatric surgery promotes remission of diabetes mellitus and improved glycemic control compared with medical intervention (17), but the mechanisms are unknown. Based on recent findings of LPS as a potential trigger of diabetes (14), we hypothesized that reduced LPS levels would be correlated with improved glycemic control. Our findings are in line with a recent report of reduced LPS and inflammatory stress after Roux-en-Y gastric bypass surgery (26). The close correlation between reduced LPS and HbA1c in our study even suggests that reduced microbial translocation might be mechanistically linked to the antidiabetic effect of bariatric surgery.
Notably, there was no reduction in plasma LPS levels after the period of lifestyle intervention before surgery, suggesting that bariatric surgery is needed to reduce endotoxemia in this patient group. The mechanism by which bariatric surgery reduces LPS levels is unknown. Because the reduction in LPS was not correlated with a reduction in BMI, factors beyond weight loss per se are likely to be involved. It recently was shown that patients who lost weight after Roux-en-Y gastric bypass experienced changes in gut microbiota that were negatively correlated with energy intake and obesity (27). Interestingly, transplantation of healthy microbiota from lean patients improved insulin sensitivity in subjects with the metabolic syndrome (28).
Two hypotheses have been proposed to explain the increased systemic LPS levels associated with obesity (29). First, LPS reaches the circulation because the gut is more permeable in obesity (25). A recent study showed that increased gut permeability is associated with mesenteric fat inflammation and metabolic dysfunction in obese mice (30). Second, LPS can be cotransported over the gut wall together with dietary fat by incorporation in triglyceride-rich chylomicrons (31).
The latter explanation fits well with the close correlation between fasting triglycerides and plasma LPS found in our study. Elevated plasma LPS levels recently have been reported to be closely associated with components of the metabolic syndrome, particularly elevated fasting triglycerides (14). Of note, high-fat meals increase circulating endotoxins in parallel with increased postprandial hypertriglyceridemia (12). Moreover, a high-fat, high-carbohydrate meal increased plasma levels of not only LPS but also LPS-binding protein, TLR4 expression, reactive oxygen species generation, and proinflammatory markers (32), an effect that was prevented by intake of fresh orange juice (33).
Although association studies do not prove causation, several mechanistic studies from animal models provide evidence that an altered gut microbiota and translocation of LPS could be early triggers of obesity, insulin resistance, and diabetes. First, long-term infusion of endotoxin is sufficient to initiate obesity and insulin resistance in mice, an effect that is almost abolished in CD14 knockout mice (8). This metabolic endotoxemia is likely to be associated with alterations of the gut microbiota, because antibiotic treatment reduced cecal and systemic LPS levels in parallel with reduced glucose intolerance and fat mass development (34). Furthermore, translocation of LPS might promote obesity by cross talk with the liver via the portal vein (35), or by interference with the endocannabinoid system to promote adipogenesis (36). Moreover, colonization of germ-free mice with Escherichia coli is sufficient to augment adipose tissue inflammation (37), and gut bacteria are present in mesenteric adipose tissue before the onset of insulin resistance and type 2 diabetes (15). Interestingly, LPS-producing Enterobacter cloacae isolated from a morbidly obese patient recently was shown to trigger obesity and insulin resistance in germ-free mice (38).
Our study has several limitations. The lack of biopsy specimens and lack of quantification of adipose tissue volumes during postoperative evaluation limit our ability to link a reduction in intra-abdominal fat volume or bacterial load to a reduction in endotoxemia. Moreover, the imbalance between female and male participants could be of significance, because the gut microbiota differs between diabetic mice of different sexes (39). Finally, it should be acknowledged that the median BMI of the control group is overweight per definition (>25 kg/m2), and that cholecystolitiasis has been proposed to be associated with altered gut barrier (40). Thus, the difference in plasma LPS between obese and controls in our study is likely to be an underestimate. It also should be noted that the limited sample size increases the risk of statistical type II errors, whereas type I errors are less likely. Hence, the strong and significant correlations between plasma LPS and intra-abdominal fat volumes as well as HbA1c are likely to be reliable. Our study also has several strengths, including the prospective longitudinal design, the presence of a control group, and quantification of adipose tissue volumes, as well as availability of biopsy samples from relevant adipose tissue compartments in a human model.
In conclusion, plasma levels of LPS were closely correlated with HbA1c and intra-abdominal adipose tissue volume, there was an increasing bacterial content in adipose tissue compartments with increasing proximity to the gut, and reduced LPS levels after bariatric surgery were associated with improved glycemic control. Our findings support a hypothesis of low-grade endotoxemia as a potential trigger of obesity-related complications and diabetes. Furthermore, our results suggest that the antidiabetic effects of bariatric surgery might be mechanistically linked to, and may even be a result of, a reduction in systemic LPS levels. Future studies should investigate strategies to decrease microbial translocation to prevent obesity-related complications and type 2 diabetes.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.T. researched data and drafted the manuscript. T.K.N. enrolled the patients, researched data, and reviewed and edited the manuscript. K.R. and H.T. researched data and reviewed the manuscript. E.W.N. reviewed the manuscript and contributed to the discussion. K.T.L. reviewed and edited the manuscript, contributed to the discussion, and supervised the research project. M.T., T.K.N., and K.T.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the nurses at the Regional Centre for Treatment of Morbid Obesity, Jeanette Andersen of Department of Radiology at Nordland Hospital, and Hilde Fure at the Somatic Research Laboratory at Nordland Hospital for their skillful assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0451/-/DC1.
References
- 1.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev 2007;65:S152–S156 [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You T, Yang R, Lyles MF, et al. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab 2005;288:E741–E747 [DOI] [PubMed] [Google Scholar]
- 4.Yu R, Kim CS, Kwon BS, et al. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–1362 [DOI] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 7.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010;5:e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 9.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:2227–2236 [DOI] [PubMed] [Google Scholar]
- 10.Kallio KA, Buhlin K, Jauhiainen M, et al. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun 2008;14:247–253 [DOI] [PubMed] [Google Scholar]
- 11.Latz E, Visintin A, Lien E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 2002;277:47834–47843 [DOI] [PubMed] [Google Scholar]
- 12.Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286–1292 [DOI] [PubMed] [Google Scholar]
- 13.Sun L, Yu Z, Ye X, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 2010;33:1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pussinen PJ, Havulinna AS, Lehto M, et al. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011;34:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar J, Chabo C, Waget A, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 2011;3:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 17.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 18.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis 2008;4(Suppl.):S109–S184 [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr 2004;80:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trøseid M, Nowak P, Nyström J, et al. Elevated plasma levels of lipopolysaccharide and high mobility group box-1 protein are associated with high viral load in HIV-1 infection: reduction by 2-year antiretroviral therapy. AIDS 2010;24:1733–1737 [DOI] [PubMed] [Google Scholar]
- 22.Milinovich GJ, Burrell PC, Pollitt CC, et al. Microbial ecology of the equine hindgut during oligofructose-induced laminitis. ISME J 2008;2:1089–1100 [DOI] [PubMed] [Google Scholar]
- 23.Nadkarni MA, Martin FE, Jacques NA, et al. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002;148:257–266 [DOI] [PubMed] [Google Scholar]
- 24.Nakarai H, Yamashita A, Nagayasu S, et al. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immun 2012;18:164–170 [DOI] [PubMed] [Google Scholar]
- 25.Gummesson A, Carlsson LM, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring) 2011;19:2280–2282 [DOI] [PubMed] [Google Scholar]
- 26.Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 2012;151:587–593 [DOI] [PubMed] [Google Scholar]
- 27.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010;59:3049–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–916.e7 [DOI] [PubMed] [Google Scholar]
- 29.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–249 [DOI] [PubMed] [Google Scholar]
- 30.Lam YY, Ha CW, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012;7:e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghoshal S, Witta J, Zhong J, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009;50:90–97 [DOI] [PubMed] [Google Scholar]
- 32.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression [corrected in Am J Clin Nutr 2011;93:674]. Am J Clin Nutr 2010;91:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–1481 [DOI] [PubMed] [Google Scholar]
- 35.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muccioli GG, Naslain D, Bäckhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 2010;6:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caesar R, Reigstad CS, Bäckhed HK, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012;61:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013;7:880–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013;339:1084–1088 [DOI] [PubMed] [Google Scholar]
- 40.Su Y, Wu S, Fan Y, et al. The preliminary experimental and clinical study of the relationship between the pigment gallstone and intestinal mucosal barrier. J Gastroenterol Hepatol 2009;24:1451–1456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.