Abstract
The cost of diabetes, driven primarily by the cost of preventable diabetes complications, will continue to increase with the epidemic rise in its prevalence in the U.S. The Diabetes Working Group (DWG), a consortium of professional organizations and individuals, was created to examine the barriers to better diabetes care and to recommend mitigating solutions. We consolidated three sets of guidelines promulgated by national professional organizations into 29 standards of optimal care and empanelled independent groups of diabetes care professionals to estimate the minimum and maximum time needed to achieve those standards of care for each of six clinical vignettes representing typical patients seen by diabetes care providers. We used a standards-of-care economic model to compare provider costs with reimbursement and calculated “reimbursement gaps.” The reimbursement gap was calculated using the maximum and minimum provider cost estimate (reflecting the baseline- and best-case provider time estimates from the panels). The cost of guideline-driven care greatly exceeded reimbursement in almost all vignettes, resulting in estimated provider “losses” of 470,000–750,000 USD/year depending on the case mix. Such “losses” dissuade providers of diabetes care from using best practices as recommended by national diabetes organizations. The DWG recommendations include enhancements in care management, workforce supply, and payment reform.
The prevalence of diabetes in the U.S. has more than tripled from 5.6 to 19.7 million people between 1980 and 2009 and is anticipated to reach ~42 million people by 2034 (1,2). This high and growing prevalence of diabetes translates into high current (245 billion USD as of 2010) and projected (334 USD billion by 2034) costs, most of which are for treatment of diabetes complications (3,4). Previous large randomized controlled trials have demonstrated that the rate at which diabetes complications develop can be reduced in cost-effective ways, but translating these findings into clinical practice has been only modestly successful because of the existence of several barriers to providing guideline-directed care (5–10). The medical community must find methods to overcome these barriers in the hope that it will reverse these ominous trends. Many interconnected obstacles to achieving optimal diabetes care exist, including patient barriers (behavioral, psychosocial, and socioeconomic), structural and technological hurdles, and provider and delivery system concerns. DWG, primarily a consortium of professional societies, was formed in 2009 to study crucial aspects of this problem and recommend solutions.
METHODS
For determination of the demographics and practice patterns of diabetes care providers, an Internet-based survey was performed of the membership of the American Diabetes Association, the Pediatric Endocrine Society, the American Academy of Pediatrics, The Endocrine Society, and the American Association of Clinical Endocrinologists using software provided by SurveyGizmo 3.0. Participants who indicated that they do not currently treat patients with diabetes were excluded from the analysis. In addition, the American Association of Diabetes Educators posted a link to the survey on its Web site. The members of each organization had up to 3 months to complete the survey. One e-mail reminder notice was sent to members in each of the five organizations that communicated directly with their members. Since many providers of diabetes care belong to multiple organizations, the survey was set with a browser cookie to prevent duplicate responses. There were a total of 1,267 responses to the survey. Owing to survey distribution to multiple diabetes-focused organizations with overlapping membership, the total number of surveys sent out and, thus, the total response rate are unknown.
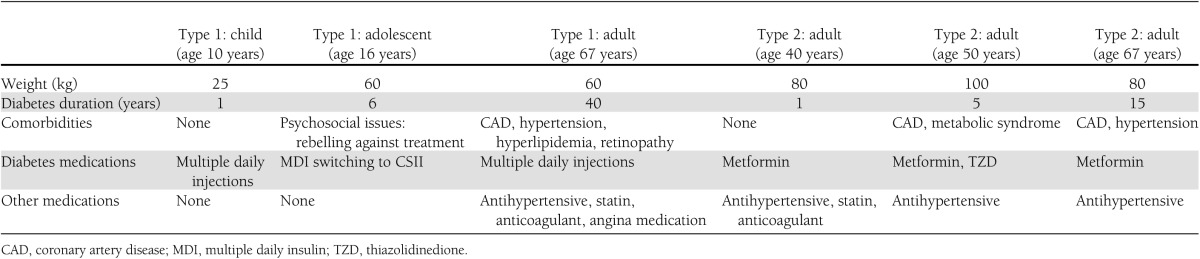
We used the standards-of-care economic model to build a theoretical model to estimate the resources necessary for providers to consistently deliver the current standards of care to diabetic patients in the U.S.; the objectives were also to evaluate provider costs to meet those standards and to assess patient outcomes specified in diabetes care guidelines relative to current reimbursement for these services. Three sets of national standards (American Diabetes Association, American Association of Clinical Endocrinologists, and The Endocrine Society) for diabetes care were integrated to produce the consolidated set of 28 standards of optimal care (11–13) (Table 1). A matrix of providers rendering the care needed to achieve the standards was developed, including physicians (adult and pediatric, general, and specialty care), certified diabetes educators (CDEs), registered dietitians (RDs), registered nurses, physician assistants, medical assistants, eye care professionals, mental health professionals, podiatrists, clinical laboratory personnel, and smoking cessation professionals. Six clinical vignettes—three patients with type 1 diabetes and three with type 2 diabetes (Table 2)—were developed representing a broad spectrum of patients. Three independent panels of four to seven diabetes care professionals (physicians, dietitians, nurses, and diabetes educators) were convened, and with a facilitator’s guidance, the minimum and maximum time (in minutes) needed to achieve that standard of care for patients with those specific characteristics over 1 year was estimated. A separate expert panel was convened to estimate the time needed to start or continue to follow patients using continuous subcutaneous insulin infusion (CSII) or the subset of type 1 diabetic patients using a continuous glucose-monitoring system (CGM) throughout 1 year. Panel meetings were conducted by a facilitator, who gained group consensus for the inputs and assured that the panel had not overestimated total time.
Table 1.
Consolidated standards of optimal diabetes care from the American Association of Clinical Endocrinologists, the American Diabetes Association, and The Endocrine Society

Table 2.
Diabetes vignettes
Time estimates based on baseline case and best case were determined for each activity, where baseline case was the amount of time required assuming a mix of patient complications, nonoptimal patient/caretaker adherence, and possible administrative delays (e.g., delays in scheduling, paperwork, etc.) and best case was the amount of time required to provide standard-based care assuming optimal patient/caretaker adherence, no patient complications, and no administrative delays. The two primary components of the model were provider costs and provider reimbursement. Provider costs were calculated by multiplying provider time estimates by the average wage and overhead amounts for each provider type. Salary and indirect (overhead) rates were based on data from nationally representative sources (14,15). Provider cost calculations were repeated for each provider type involved in each activity. Sums of total provider costs were used to determine cost per activity, and total activity costs were used to determine a per-visit cost.
Next, the expert panels’ timing estimates were mapped to Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System billing codes and the associated Medicare national average payment rate (adjusted as necessary for non-Medicare-applicable vignettes) to compute the reimbursement (based on the expected payer mix for the patient characteristics) that would be collected by providers as a result of activity performance. Total reimbursement was finalized by repeating these revenue calculations in a fashion similar to that used for the provider costs.
The total reimbursement amount was compared with the total provider costs and any differences noted as a “reimbursement gap.” Two reimbursement gap amounts were calculated using baseline case and best case estimates. Provider cost, reimbursement, and gap amounts were calculated on a per-patient, per-year basis using the median number of diabetic patients seen per week for adult and pediatric practices (as found in the provider survey), multiplied by the average number of patients seen per year based on national estimates from the Medical Group Management Association (14).
RESULTS
The survey showed that practice or employment arrangements were as follows: group practice in 32.2% (2–4 physicians 15%; 5 or more physicians 17.2%); solo practice office, 13.9%; and hospital setting, 41.4% (university teaching hospital, 30.3%; community teaching hospital, 7.1%; and community nonteaching hospital, 4.0%). The remaining 12.5% reported working as staff at an HMO or other private plan, being employed by a diabetes manufacturer, working at an accredited or recognized diabetes education program, or working as staff at a community nonteaching hospital.
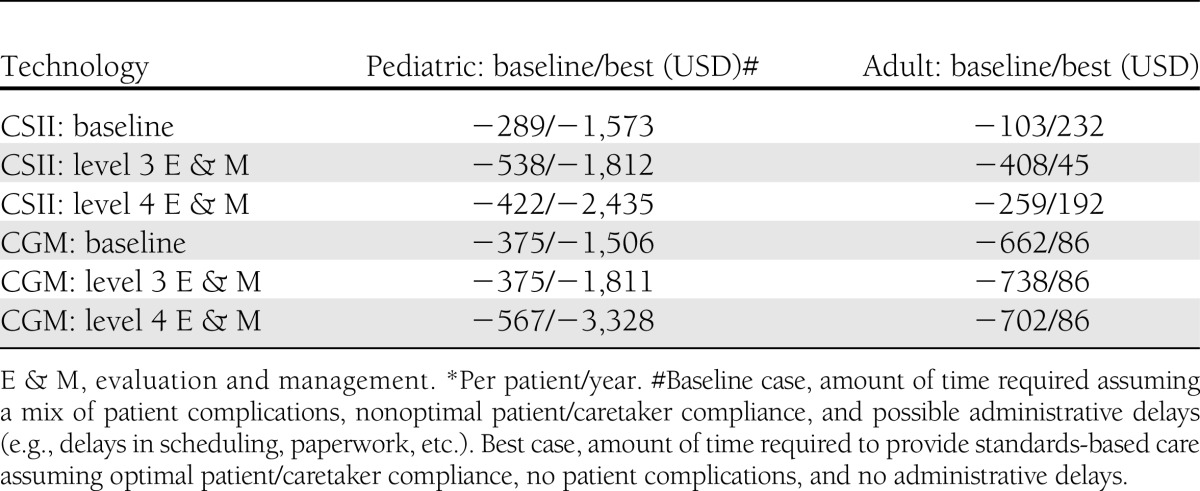
The results of the baseline model of the standards-of-care economic model show that provider costs exceed reimbursements for all scenarios (Table 3), whereas best case provider time estimates reimbursement exceeds costs in five of the six scenarios. Sensitivity analyses showed that the model is highly sensitive to assumptions about provider reimbursement, particularly assumptions regarding the level of office visit code reimbursed. Payers often limit the amount of diabetes education and nutrition therapy allowed, and two of the type 1 diabetic patient vignettes are sensitive to assumptions about the amount of diabetes education and nutrition therapy reimbursed. Provider costs exceed reimbursement for CSII and CGM services for both adult and pediatric patients when baseline case time estimates are used (Table 4). CGM but not CSII model results were sensitive to assumptions about provider reimbursement.
Table 3.
Baseline and best case reimbursement gaps for evaluation and management services with different reimbursement assumptions*
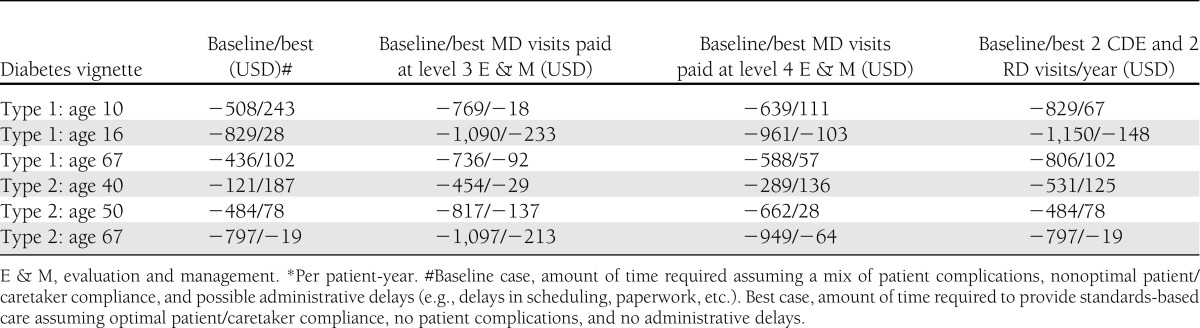
Table 4.
Baseline case and best case reimbursement gaps for initiation of CSII or CGM with different reimbursement assumptions*
The annual gap between provider cost and reimbursement for a typical adult and pediatric diabetes practice was calculated based on the number of diabetic patients seen per year. This estimate is based both on the median number of diabetic patients seen per week for adult and pediatric practices and the mean number of patients seen per year based on the provider survey and data obtained from the Medical Group Management Association. Depending on the number and case mix of patients seen by diabetes care providers, the costs of treating diabetic patients in an adult practice would exceed reimbursement by >750,000 USD/year. For a pediatric practice, costs would exceed reimbursement by >471,000 USD/year. These gaps are increased for patients using intensive management technologies such as CSII and CGM. An adult diabetologist’s practice would require a 19% increase in overall reimbursement in order to break even in the baseline case scenario; the individual diabetologist would require a 63% increase. The reason for the higher increase in individual reimbursement compared with the overall practice is the significant disparity between the cost versus reimbursement for evaluation and management services of providing cognitive services to patients with diabetes. This disparity is much smaller for such services such as ophthalmologic evaluation, podiatric care, diabetes education, and nutritional services, which are all considered part of the practice costs.
CONCLUSIONS AND RECOMMENDATIONS
This modeling study demonstrates that compensation for optimal diabetes care is inadequate and inconsistent with national standards. Even using conservative assumptions regarding reimbursement, the model results suggest that provider costs greatly exceed reimbursement for most patients for standards-based care. Indeed, the three scenarios for type 2 diabetes while representing a spectrum of age, weight, and comorbidities are relatively straightforward cases commonly seen in a primary care setting, suggesting that the reimbursement gap would be even larger for more complex cases. There are several limitations of our modeling study. First, it uses time estimates based on input from experienced diabetes providers, which might be substantially different with another panel of experts or of primary care providers (PCPs). PCP organizations were invited to join the DWG but chose not to do so. The vast majority of people with diabetes receive their health care from PCPs and not diabetes specialists; yet, their care is no less governed by the standards of care than is care from specialists. While magnitude of the misalignment of incentives to provide guideline-driven care is likely to be less for a PCP because of the diversity of illness that they see, it will still be substantial given the prevalence of diabetes in the U.S. In addition, the model assumes that all standards-based services included in the model will be covered by all payers. Finally, it assumes that providers will receive full reimbursement for the services provided and that they can collect the full patient copayment/coinsurance amounts from all patients. Indeed, the sensitivity analyses show that the model is sensitive to assumptions about coverage of services and level of reimbursement, and the gap between provider costs and reimbursement would increase significantly under differing assumptions. The modeling study confirms general perceptions of an untenable situation in which providers are financially unable to meet the established standards of diabetes care that would prevent or delay costly diabetes complications. The panels’ time estimates were high and were guided by the stated goal of what it would take to produce guideline-driven care. We believe that these time estimates are likely to represent the large amount of unreimbursed care that is generally provided to patients with diabetes.
The DWG recommends the following changes, which are arrayed across three areas of provider engagement: care management, payment reform, and workforce supply. We recognize that some of the care management and payment reform options may not be appropriate or possible in some practice settings at this time.
Care management
Diabetes is unique among chronic diseases in that, by definition, it requires a high level of engagement and never-ending self-management by patients and (often) their family members. Improved glycemic control and thereby reduced complications can be enhanced with greater provider focus on care management. In fact, enhanced provider/patient communication leads to greater adherence among patients with diabetes (16). Among the strategies to accomplish this are the following:
Increasing the use of shared decision making with providers discussing the standards of care, the treatment options, and their recommendations with patients to maximize patient engagement in self-management of diabetes. This heightened understanding increases a patient’s motivation and sense of empowerment to reach treatment goals that are their own rather than those of their providers (17,18).
Creating strong teams to implement the shared decision-making approach and promoting the use of the core team explicitly to patients. The core team includes a physician (or nurse practitioner or physicians assistant), a nurse, a dietitian, and a CDE. Other team members that can assist with care include a podiatrist, a pharmacist, and a psychologist or social worker. Each of these provider types manages aspects of the standards of care such as glucose monitoring, diabetes self-management education, nutrition therapy, and psychosocial assessment and care (19).
Leveraging existing health information technology more fully to assist patients in diabetes self-management and track blood glucose values and overall performance (20–25).
Prescribing electronically to improve monitoring of medication adherence. The use of electronic prescribing has increased dramatically in recent years. By mid-2012, 48% of physicians in the U.S. are using e-prescribing systems—an increase from 7% in 2008 (26–28);
Participating in patient registries or locally based databases to track and trend goal achievement. Recent emphasis on coordinated care models that are currently being piloted and adopted provides an opportunity for registries to be designed and implemented in a more coordinated and comprehensive fashion. Accountable Care Organizations and patient-centered medical homes (PCMHs) require the collection and sharing of data on their patient populations (29–31).
Payment reform
Consideration of a broad spectrum of payment solutions is necessary to fully address provider barriers. The problem of inadequate reimbursement is twofold. First, much of the care delivered to diabetes patients is not described by existing CPT codes that determine coverage and payment, is considered by payers to be included or “bundled” into existing CPT codes, or is described by existing CPT codes that are not covered or reimbursed by payers. Second, in cases where appropriate codes exist, the associated payments are often insufficient. Both of these problems are exacerbated by the large amount of non–face-to-face care delivered to patients with diabetes. Better aligning payment with desired patient outcomes can both improve outcomes and lower costs through decreasing emergency department and inpatient hospital use. For example, contracting with third-party payers for pediatric and adolescent diabetes intensive case-management services has been an effective strategy, since it allows for intensive education and immediate access to the diabetes care team for crisis management (32).
The solutions include the following:
Reviewing and revising billing codes in the current fee-for-service system to more appropriately describe the work being performed and ensuring that the coding results in adequate payment.
Testing and implementing new payment models that reward providers for supplying optimal care to patients with diabetes. Payment models that hold promise for diabetes include the following:
The episode-of-care payment model that allows for reimbursement of multiple services at one time covering different providers and different types of care. Unlike fee-for-service models, it creates efficiencies by encouraging provider teams to work with a set amount of funding for each care episode and to tailor that experience to the patient’s needs. Several pilot programs of diabetes episode-of-care payments, including the PROMETHEUS payment model for diabetes, are under way, but there are no published results at this time (33).
The patient-management-fee model that provides a monthly per-patient payment for all care. It would facilitate extensive care coordination, education, and training services and cover the various patient-management activities required to achieve optimal patient outcomes, including between-visit care via phone or e-mail and excluding acute services such as episodes of ketoacidosis. The payment would account for the multidisciplinary team required for optimal care, allowing for education services from diabetes educators, nutritionists, and dietitians as appropriate. This model could include a pay-for-performance adjustment.
The diabetes-focused PCMH option that both encourages care coordination and aligns reimbursement incentives while incorporating guideline-directed quality measures that benefit the patient. Initial evidence suggests that such a model has potential benefits for patients, providers, and payers (30,34,35). It would place value on the services necessary to provide optimal diabetes care (e.g., between-visit care and patient education).
These payment models could be applied to a system whereby there are shared (i.e., group) medical appointments. Some studies of shared medical appointments have shown improvement surrogate end points of A1C and cardiovascular risk in patients with diabetes (36,37). Such shared medical appointments may be used as an integral part of the PCMH.
Workforce supply
The current supply of diabetes specialists, including both physician and nonphysician providers, is inadequate to meet the demands of today and certainly will fall short of future needs, including many of the care management and innovative payment recommendations. Wait times for appointments range from 3 to 9 months, and many practices are closed to accepting new diabetic patients (38). Expanding the workforce is a necessary investment if we are to have a chance at resolving the barriers to optimal diabetes care. The solutions include the following:
Forgiving educational loans to make diabetes care an attractive choice for new medical professionals. Such programs, similar to the National Health Service Corps and state-supported programs, could be implemented by state or federal agencies, private sector organizations, and educational institutions through funding from nonprofit foundations and trusts (39–41). This could counteract the reputation of diabetes care as an underpaid professional endeavor that dissuades providers from seeking to enter it as they face looming debt repayment. For physicians, these programs could operate in a manner similar to existing, successful loan-forgiveness programs for PCPs and physicians working in rural and underserved areas (42). Offering similar types of loan-forgiveness programs and other financial assistance to potential CDEs and to RDs specializing in diabetes patient care is equally critical (43).
Realigning financial incentives to allow for more time with diabetic patients and for the provision of non–face-to-face care. This will project a more positive image for those who work to keep diabetic patients healthy.
Encouraging diabetes-centric professional societies to promote the positive attributes of working with diabetic patients to medical, nursing, pharmacy, and nutrition students.
Better educating PCPs including nurse practitioners and physicians assistants on the current standards of care, the principles of proactive management, and the need for timely referral to specialist. This training approach acts as a “force multiplier,” thereby mitigating some of the specialist work force supply issues.
In summary, the DWG has found that delivering high-quality, guideline-based diabetes care is unrealistic given the current care and payment paradigms and proposes alternative approaches that may mitigate the increasing medical and financial burdens of this epidemic chronic illness.
Acknowledgments
This work was funded by unrestricted educational grants from Abbott Diabetes Care; Animas Corporation, a Johnson & Johnson Company; Bayer HealthCare, Diabetes Care; Dexcom, Inc.; Johnson & Johnson; LifeScan, Inc., a Johnson & Johnson Company; Medtronic Diabetes; Roche Diagnostics; and Sanofi. R.A.V. has received investigator-initiated research grants from Dexcom and Novo Nordisk. He has served on medical advisory boards of Bayer, Medtronic, and Sanofi. J.L. is an employee of Avelere Health, which was contracted to perform the modeling study. Avalere Health receives consulting fees from a range of companies that manufacture diabetes products, including Abbott, Bayer, Johnson & Johnson, Medtronic, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
The companies listed had no role in the design and conduct of the study, the interpretation of the data, or the manuscript preparation, review, or approval.
R.A.V. was the co-chair of the DWG, led the group in the design of the study and facilitated its implementation, analyzed and interpreted data, and wrote the manuscript. K.F. was the co-chair of the DWG, helped design the study, and reviewed and edited the manuscript. J.L. applied data to the model, interpreted data, performed a literature search, and assisted in writing and editing the manuscript.
Appendix
DWG members represented the following seven professional societies, who have endorsed this work: the American Academy of Pediatrics, American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, The Endocrine Society, the JDRF, and the Pediatric Endocrine Society. Members of the DWG are as follows: R. Bergenstal, MD; B. Bode, MD; S. Brink, MD; K. Close; D. Einhorn, MD; K.F., PhD (co-chair); I. Hirsch, MD; D. Kendall, MD; A. Kowalski, PhD; L.L. Levitsky, MD; E. Moghissi, MD; M. Rinker, JD; W. Tamborlane, MD; R.A.V., MD (co-chair); and F. Zageneh, MD.
Footnotes
A full list of members of the Diabetes Working Group can be found in the APPENDIX.
The opinions expressed in this article reflect the personal views of the authors and not the official views of the U.S. Army or the Department of Defense.
References
- 1.2011 National Diabetes Fact Sheet [Internet], 2011. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed 28 December 2011
- 2.Huang, ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care 2009;32:2225–2229 [DOI] [PMC free article] [PubMed]
- 3.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study Group Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. BMJ 1998;317:720–726 [PMC free article] [PubMed] [Google Scholar]
- 8.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 9.CDC Diabetes Cost-effectiveness Group Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 10.Choe HM, Townsend KA, Blount G, Lo CH, Sadowski L, Standiford CJ. Treatment and control of blood pressure in patients with diabetes mellitus. Am J Health Syst Pharm 2007;64:97–103 [DOI] [PubMed] [Google Scholar]
- 11.American Association of Clinical Endocrinologists Diabetes mellitus guideline. Endocr Pract 2007;13(Suppl. 1) [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryer PE, Axelrod L, Grossman AB, et al. Endocrine Society Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709–728 [DOI] [PubMed] [Google Scholar]
- 14.Medical Group Management Association. Cost Survey: 2009 Report Based on 2008 Data Englewood, CO, 2010 [Google Scholar]
- 15.U.S. Bureau of Labor Statistics. Occupational employment statistics, healthcare practitioner and technical occupations [Internet], May 2012. Available from http://www.bls.gov/oes/current/oes_nat.htm#29-0000 Accessed 10 June 2013
- 16.Ratanawongasa N, Karter AJ, Parker MM, et al. Communication and medication refill adherence: the Diabetes Study of Northern California. JAMA Intern Med 2013;173:210–218 [DOI] [PMC free article] [PubMed]
- 17.Kurtz SMS. Adherence to diabetes regimens: empirical status and clinical applications. Diabetes Educ 1990;16:50–59 [DOI] [PubMed] [Google Scholar]
- 18.Funnell MM, Anderson RM. MSJAMA: the problem with compliance in diabetes. JAMA 2000;284:1709. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Team Care: Comprehensive Lifetime Management for Diabetes Atlanta, GA, U.S. Department of health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 2001 [Google Scholar]
- 20.Tao D, Or CK. Effects of self-management health information technology on glycaemic control for patients with diabetes: a meta-analysis of randomized controlled trials. J Telemed Telecare. 5 April 2013 [Epub ahead of print] [DOI] [PubMed]
- 21.Misono AS, Cutrona SL, Choudhry NK, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. Am J Manag Care 2010;16(Suppl. HIT):SP82–SP92 [PubMed] [Google Scholar]
- 22.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34:1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med 2006;23:1332–1338 [DOI] [PubMed] [Google Scholar]
- 24.Kim HS. A randomized controlled trial of a nurse short-message service by cellular phone for people with diabetes. Int J Nurs Stud 2007;44:687–692 [DOI] [PubMed] [Google Scholar]
- 25.Bell AM, Fonda SJ, Walker MS, Schmidt V, Vigersky RA. Mobile phone-based video messages for diabetes self-care support. J Diabetes Sci Tech 2012;6:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hufstader M, Swain M, Furukawa MF. State variation in e-prescribing trends in the United States [article online], 2012. Washington, DC, Office of the National Coordinator for Health Information Technology. Available from http://www.healthit.gov/sites/default/files/us_e-prescribingtrends_onc_brief_4_nov2012.pdf Accessed 23 January 2013
- 27.Johnson KB, Lehmann CU; Council on Clinical Information Technology of the American Academy of Pediatrics. Electronic prescribing in pediatrics: toward safer and more effective medication management. Pediatrics 2013;131e1350–e1367
- 28.Warren J, Kennelly J, Warren D, et al. Using the general practice EMR for improving blood pressure medication adherence. Stud Health Technol Inform 2012;178:228–234 [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services. FQHC advanced primary care practice demonstration. Available from http://innovation.cms.gov/initiatives/fqhcs/ Accessed 9 August 2013
- 30.Grumbach K, Grundy P. Outcomes of implementing patient centered medical home interventions: a review of the evidence from prospective evaluation studies in the United States [article online], 2010. Patient-Centered Primary Care Collaborative. Available from http://dhhs.nv.gov/HealthCare/Docs/MedicalHomes/Outcomes%20of%20Implementing%20Medical%20Home%20Interventions%20by%20the%20PCPCC%20-%20Nov%202010.pdf Accessed 10 June 2013
- 31.McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff (Millwood) 2010;29:982–990 [DOI] [PubMed] [Google Scholar]
- 32.Beck JK, Logan KJ, Hamm RM, et al. Reimbursement for pediatric diabetes intensive case management: a model for chronic diseases? Pediatrics 2004;113:e47–e50 [DOI] [PubMed] [Google Scholar]
- 33.De Brantes F, Rosenthal MB, Painter M. Building a bridge from fragmentation to accountability—the Prometheus payment model. N Eng J Med 2009;361:1033–1036 [DOI] [PubMed] [Google Scholar]
- 34.Carroll J. Lessons learned in building the patient-centered medical home [article online], 2010. Available from http://www.managedcaremag.com/archives/1008/1008.medicalhome.html Accessed 10 June 2013 [PubMed]
- 35.Patient-Centered Primary Care Collaborative. Practices in the spotlight: the medical home and diabetes care [article online]. http://www.pcpcc.org/guide/practices-spotlight Accessed 29 December 2011
- 36.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med 2011;171:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med 2010;152:689–696 [DOI] [PubMed] [Google Scholar]
- 38.Stewart AF. The United States endocrinology workforce: a supply-demand mismatch. J Clin Endocrinol Metab 2008;93:1164–1166 [DOI] [PubMed] [Google Scholar]
- 39.National Health Service Corps. Pay off your student loans while serving communities in need [article online]. Available from http://nhsc.hrsa.gov/loanrepayment/index.html Accessed 2 May 2013
- 40.Pathman DE, Taylor DH, Jr, Konrad TR, et al. State scholarship, loan forgiveness, and related programs: the unheralded safety net. JAMA 2000;284:2084–2092 [DOI] [PubMed] [Google Scholar]
- 41.Pathman DE, Konrad TR. Growth and changes in the National Health Service Corps (NHSC) workforce with the American Recovery and Reinvestment Act. J Am Board Fam Med 2012;25:723–733 [DOI] [PubMed] [Google Scholar]
- 42.Bärnighausen T, Bloom DE. Financial incentives for return of service in underserved areas: a systematic review. BMC Health Serv Res 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thaker SI, Pathman DE, Mark BA, Ricketts TC., 3rd Service-linked scholarships, loans, and loan repayment programs for nurses in the Southeast. J Prof Nurs 2008;24:122–130 [DOI] [PubMed] [Google Scholar]


