Abstract
OBJECTIVE
To investigate the impact of activities of daily living (ADL) versus moderate-intensity endurance-type exercise on 24-h glycemic control in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Twenty males with type 2 diabetes participated in a randomized crossover study consisting of three experimental periods of 3 days each. Subjects were studied under sedentary control conditions, and under conditions in which prolonged sedentary time was reduced either by three 15-min bouts of ADL (postmeal strolling, ∼3 METs) or by a single 45-min bout of moderate-intensity endurance-type exercise (∼6 METs). Blood glucose concentrations were assessed by continuous glucose monitoring, and plasma insulin concentrations were determined in frequently sampled venous blood samples.
RESULTS
Hyperglycemia (glucose >10 mmol/L) was experienced for 6 h 51 min ±1 h 4 min per day during the sedentary control condition and was significantly reduced by exercise (4 h 47 min ± 1 h 2 min; P < 0.001), but not by ADL (6 h 2 min ± 1 h 16 min; P = 0.67). The cumulative glucose incremental areas under the curve (AUCs) of breakfast, lunch, and dinner were, respectively, 35 ± 5% (P < 0.001) and 17 ± 6% (P < 0.05) lower during the exercise and ADL conditions compared with the sedentary condition. The insulin incremental AUCs were, respectively, 33 ± 4% (P < 0.001) and 17 ± 5% (P < 0.05) lower during the exercise and ADL conditions compared with the sedentary condition.
CONCLUSIONS
When matched for total duration, moderate-intensity endurance-type exercise represents a more effective strategy to improve daily blood glucose homeostasis than repeated bouts of ADL. Nevertheless, the introduction of repeated bouts of ADL during prolonged sedentary behavior forms a valuable strategy to improve postprandial glucose handling in patients with type 2 diabetes.
The level of glycemia (1–3), and particularly postprandial glycemia (4–6), has been associated with an increased risk for cardiovascular complications and mortality in patients with type 2 diabetes. Therefore, proper management of blood glucose concentrations is an important goal in type 2 diabetes treatment. Despite the application of oral blood glucose–lowering medication and the consumption of a healthy diet, postprandial hyperglycemia and excessive glycemic fluctuations remain predominant features in patients with type 2 diabetes (7,8). Therefore, additional treatment strategies are warranted to improve daily blood glucose homeostasis in patients with type 2 diabetes.
Along with dietary modulation and proper medication, structured exercise is considered a cornerstone for type 2 diabetes treatment (9,10). The impact of structured exercise on long-term glycemic control (i.e., HbA1c) can be largely ascribed to the cumulative glucoregulatory effects of each successive bout of exercise (11). In line with this view, we (12–15) and others (16–18) have demonstrated that a single bout of moderate-intensity to high-intensity exercise substantially improves glycemic control throughout the subsequent 24-h period in patients with type 2 diabetes. Besides regular exercise, an accumulating body of evidence also suggests an independent role for nonexercise physical activities in restoring or maintaining optimal glycemic control. In this regard, epidemiological studies have demonstrated that light physical activity is beneficially associated with postprandial blood glucose concentrations and markers of insulin sensitivity (19,20). These observations were recently reinforced by evidence from experimental studies, showing that short bouts of slow walking during the postprandial phase effectively reduce the glycemic response to a meal (21–23). So far, these experimental studies have been restricted to a single meal and nondiabetic populations. We hypothesized that simply performing activities of daily living (ADL) improves 24-h blood glucose homeostasis in patients with type 2 diabetes. Such a low-demanding physical activity strategy would provide an attractive alternative to the application of a more structured exercise regimen, because engaging in and adhering to structured exercise programs have been proven problematic for many patients with type 2 diabetes (9). For this reason, it would be relevant to investigate whether a modest increase in ADL could equal the benefits of more intense endurance-type exercise for glycemic control.
In the current study we investigated the impact of repeated short bouts of ADL as opposed to a single session of moderate-intensity endurance-type exercise on daily blood glucose homeostasis in patients with type 2 diabetes. For this purpose, 24-h glycemic profiles of patients were assessed by continuous glucose monitoring under sedentary control conditions, and under conditions in which sedentary time was reduced either by three 15-min bouts of ADL (postmeal strolling; ∼3 METs) or by a single 45-min bout of cycling exercise (∼6 METs).
RESEARCH DESIGN AND METHODS
Subjects
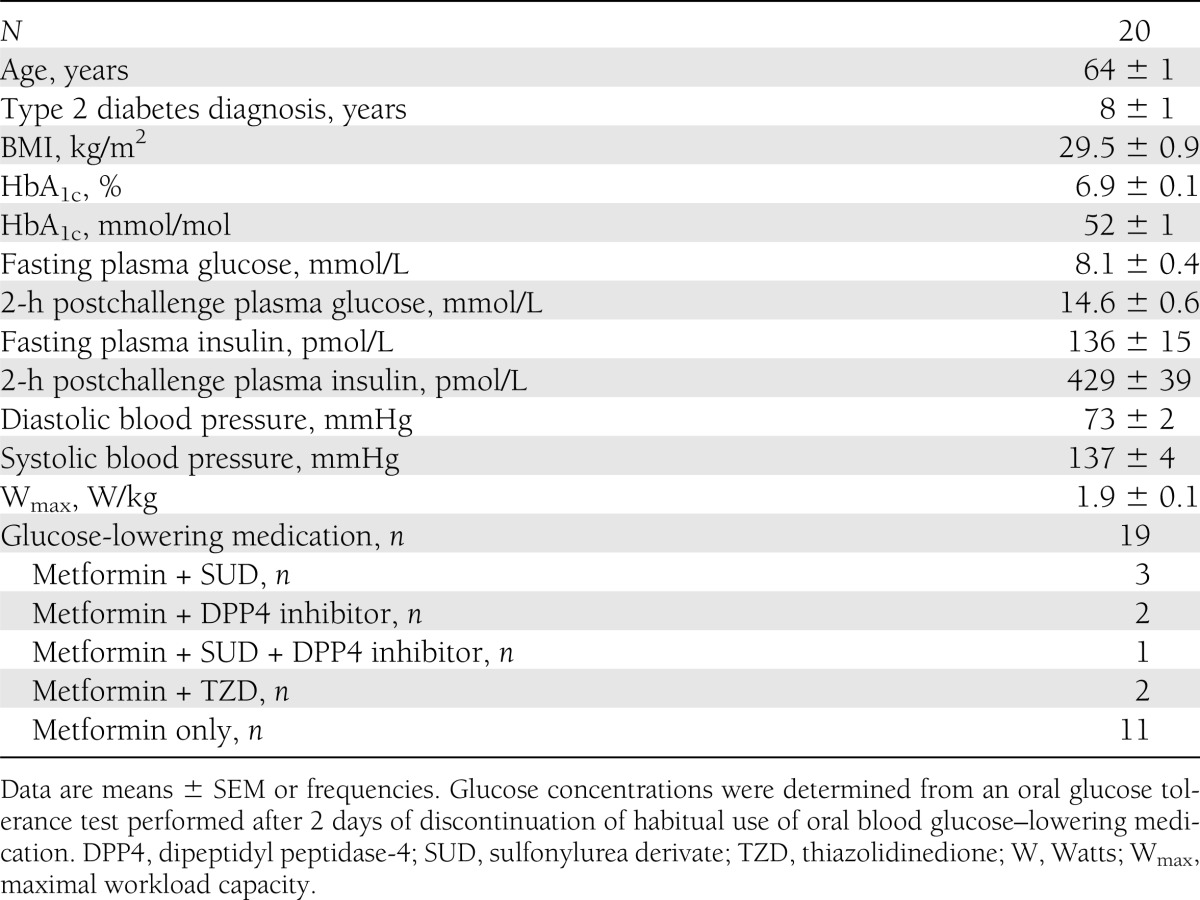
A total of 20 male type 2 diabetic patients treated with oral glucose–lowering medication (n = 19) or diet only (n = 1) participated in the current study. Exclusion criteria were self-reported renal failure and liver disease (hepatitis and cirrhosis), morbid obesity (BMI >40 kg/m2), uncontrolled hypertension (>160 mmHg systolic or >100 mmHg diastolic or both), and a history of severe cardiovascular problems (myocardial infarction in the past year or stroke). All subjects were informed about the nature and the risks of the experimental procedures before their written informed consent was obtained. The Medical Ethical Committee of the Maastricht University Medical Centre approved the study.
Screening and pretesting
All patients underwent an oral glucose tolerance test. Blood glucose–lowering medication was withheld 2 days before the oral glucose tolerance test. After an overnight fast, subjects arrived at the laboratory at 0800 h by car or public transportation. A fasting blood sample was obtained, after which a standard 75-g oral glucose tolerance test was performed to confirm type 2 diabetes according to the American Diabetes Association criteria (24). After blood sampling, all subjects performed an incremental exercise test on a cycle ergometer (Lode Excalibur) to determine their maximal workload capacity. Cardiac function was monitored at rest and during exercise using a 12-lead electrocardiogram.
Study design
Subjects participated in a randomized crossover study consisting of three intervention periods separated by at least 1 week. Each intervention period consisted of 3 days, during which blood glucose homeostasis was assessed under standardized dietary conditions (Supplementary Fig. 1). During one experimental period, subjects were monitored under sedentary control conditions. During the other two experimental periods, subjects reduced their sedentary behavior either by three 15-min bouts of ADL (postmeal strolling; ∼3 METs) or by a single 45-min bout of moderate-intensity cycling exercise (∼6 METs).
Study protocol
On day 1 of each intervention period, participants arrived at the laboratory during the afternoon and received a short period of training in the use of the capillary blood sampling method (Glucocard X Meter; Arkray, Kyoto, Japan). Subsequently, a continuous glucose-monitoring device (GlucoDay S; A. Menarini Diagnostics, Florence, Italy) was attached as described previously (7) and participants returned home. On day 2, participants arrived at the laboratory by car at 0730 h after an overnight fast. An intravenous catheter was inserted into an antecubital vein for blood sampling purposes, and participants were equipped with a heart rate monitor (Polar RS300). Participants received breakfast, lunch, and dinner at 0830, 1230, and 1700 h, respectively. Blood samples were collected in prechilled EDTA-coated tubes 5 min before each meal, and 90 and 150 min after each meal (total of 9 samples). After the last blood sample at 1930 h subjects went home. On day 3, subjects reported back to the laboratory during the morning for removal of the continuous glucose-monitoring device.
The three intervention periods were identical with the exception of the three 15-min bouts of ADL or the single 45-min bout of endurance-type exercise, which were performed on the second day of the experimental period (Supplementary Fig. 1). During the sedentary control condition, participants were restricted to a sedentary laboratory environment and spent the day seated in a chair or couch while reading, talking, viewing television, or working on a laptop computer. During the ADL condition, sedentary behavior of the patients was interrupted by three 15-min bouts of slow-paced strolling (∼3 MET) performed after each main meal (0915, 1315, and 1745 h). The bouts of strolling were chosen to reflect light ADL, such as walking the dog, household tasks, light gardening work, and so on. During each bout, a distance of 800–1,000 m was covered and two stairs were climbed. The bouts were supervised by a physical therapist. During the exercise condition, participants completed a single 45-min bout of moderate-intensity endurance-type exercise after breakfast (0915 to 1000 h) and were sedentary throughout the remainder of the day. The moderate-intensity exercise bout consisted of continuous cycling performed on a cycle ergometer at 50% of individual maximal workload capacity of the patients, translating to an average absolute workload of 85 ± 4 W (∼6 MET). The MET values for both the ADL and moderate-intensity exercise intervention were estimated based on the Compendium of Physical Activities (25).
Medication, habitual physical activity, and food intake
Glucose-lowering medications used by patients are listed in Table 1. Except for the screening, intake of all medication was continued throughout the entire study, including the days that subjects visited the laboratory for testing. All subjects were asked to maintain their normal physical activity patterns throughout the study but to refrain from exhaustive physical labor and exercise training for 2 days before each experimental period. On day 1 of each intervention period, subjects were provided with a standardized dinner and evening snack. On day 2 of each intervention period, the actual test day, participants were served a standardized breakfast (0830 h), lunch (1230 h), and dinner (1700 h) in the laboratory, and they consumed a standardized evening snack at home (2030 h). On day 3 of each intervention period, participants remained fasted until 0830 h (end of data collection), after which they resumed their regular diets.
Table 1.
Participant characteristics
Breakfast and lunch were identical and both meals consisted of bread, margarine, salami slices, cheese, jam, and semi-skim (1.5% fat) milk. Dinner comprised macaroni with Bolognese sauce and fruit yogurt as dessert. The evening snack included an apple and whole grain cookies. The quantity of the diet (energy content) was based on individual daily energy requirements of the patients as calculated with the Harris and Benedict equation multiplied by a physical activity level value of 1.4. The resulting diet provided 9.8 ± 0.1 MJ/day, consisting of 50% of energy from carbohydrate, 15% from protein, and 35% from fat.
Blood sample analysis
Venous blood samples were collected in prechilled EDTA-coated tubes and centrifuged at 1,000g and 4°C for 10 min. Aliquots of plasma were immediately frozen in liquid nitrogen and stored at −80°C until analyses. Plasma insulin concentrations were determined by a radioimmunoassay for human insulin (HI-14K; Millipore, MA). Whole blood HbA1c content was determined in 3 mL venous blood samples by high-performance liquid chromatography (Bio-Rad Diamat, Munich, Germany).
Statistics and data analysis
The data obtained by the continuous glucose monitor were downloaded to a personal computer with GlucoDay software (version 3.2.2). Values reported by the continuous glucose-monitoring device were converted into glucose values using the self-monitored capillary blood glucose values, which were obtained at least before each main meal and before night time. The 24-h glycemic control was derived from the glycemic profiles obtained between 8:30 and 8:30 h on days 2 and 3 of each experimental period and was defined as the average 24-h blood glucose concentration and the prevalence of hyperglycemia over the course of 24 h. Based on the American Diabetes Association/European Association for the Study of Diabetes guidelines for glycemic control (26,27), the prevalence of hyperglycemia was defined as total time during which glucose concentrations exceeded 10 mmol/L. The continuously monitored glycemic profiles obtained on day 2 of each experimental period also were used to assess postprandial glycemic control. Postprandial glycemic control was defined as the incremental area under the curve (iAUC) above fasting glucose assessed over the 3.5-h postprandial periods after each main meal (3.5-h glucose iAUC), and as the cumulative glucose iAUC of all main meals (10.5-h glucose iAUC). Plasma insulin concentrations obtained on day 2 of each experimental period (from 0830 to 1930 h) were used to calculate the iAUC above fasting plasma insulin by the trapezoidal rule (11-h insulin iAUC).
Differences between nontime-dependent variables (prevalence of hyperglycemia, 24-h glucose concentration, iAUC) were assessed by one-way repeated-measures ANOVA, with treatment as the within-subject factor. In case of a significant main effect, pairwise comparisons with Bonferroni correction were applied to locate differences between treatments. A two-way repeated-measures ANOVA with time and treatment as within-subject factors was used to compare differences between treatments over time (plasma insulin concentrations). In case of interaction between significant time and treatment, pairwise comparisons with Bonferroni correction were applied for separate time points to locate differences between treatments.
Statistical comparisons were considered significant at P < 0.05. All statistical calculations were performed using the SPSS 15.0.1.1 software package. Unless otherwise indicated, data are reported as means ± SEM.
RESULTS
Heart rate
Average heart rates of subjects that were measured between 0830 and 1930 h were 72 ± 2 bpm during the sedentary control condition and 78 ± 2 and 82 ± 2 bpm during the ADL (P < 0.001) and exercise conditions (P < 0.001), respectively. As expected, 45 min of moderate-intensity exercise (125 ± 4 bpm) was more intense than the 45 min of ADL (100 ± 2 bpm; P < 0.001).
24-h glycemic control
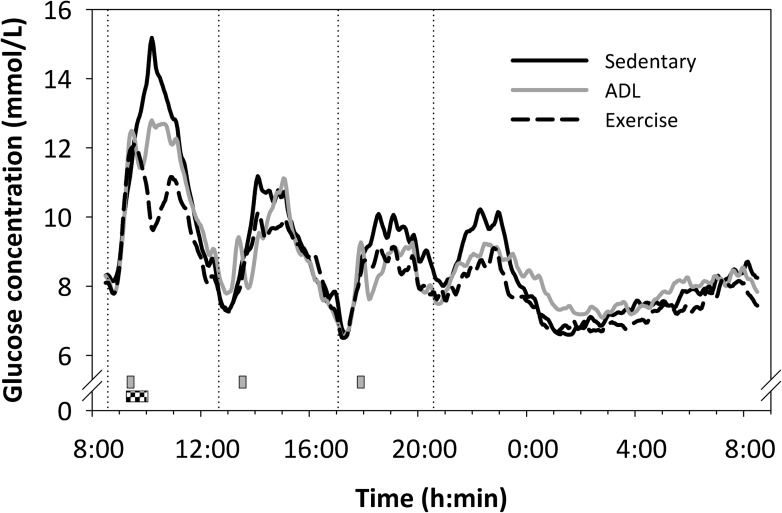
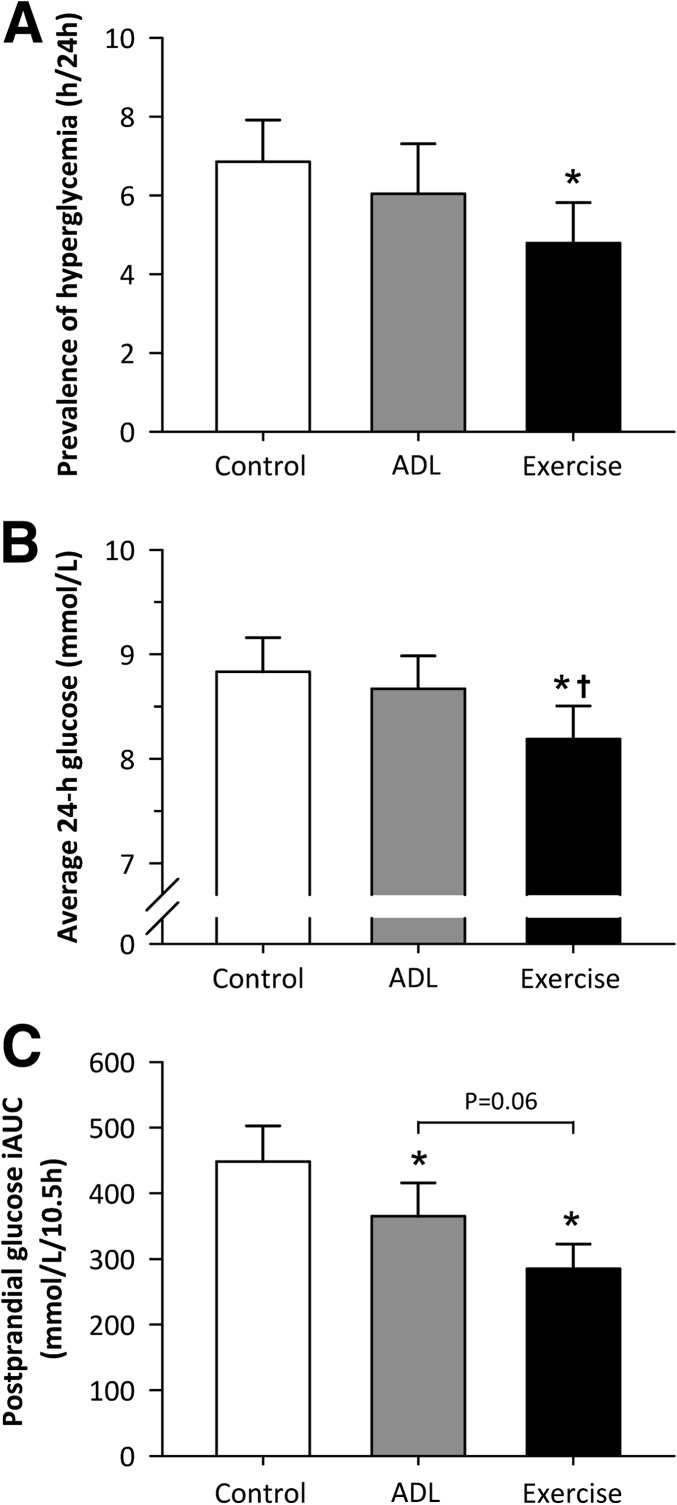
The 24-h glycemic profiles are presented in Fig. 1. During the sedentary control condition, hyperglycemia (blood glucose >10 mmol/L) was prevalent for 6 h 51 min (±1 h 4 min) throughout the day. A single bout of moderate-intensity exercise significantly reduced the daily prevalence of hyperglycemia to 4 h 47 min ± 1:02 (−34 ± 7%; P < 0.001; Fig. 2A). Although blood glucose concentrations were clearly attenuated after the onset of the 15-min bouts of ADL (Fig. 1), the prevalence of hyperglycemia over the course of 24 h was not significantly reduced (6 h 2 min ± 1 h 16 min; P = 0.67). Comparable findings were observed with respect to average 24-h blood glucose concentrations (Fig. 2B). A single session of moderate-intensity exercise significantly reduced average blood glucose concentrations by 0.6 ± 0.1 mmol/L (P < 0.001) relative to the sedentary control condition, whereas average 24-h blood glucose concentrations were basically unchanged during the ADL condition (−0.2 ± 0.1 mmol/L; P = 0.92).
Figure 1.
The 24-h glycemic profiles in type 2 diabetic patients under sedentary conditions and under conditions in which prolonged sedentary time was reduced by three 15-min bouts of activities of daily living (ADL; gray squares) or by a single 45-min bout of moderate-intensity endurance-type exercise (checkered square). The dotted lines indicate the ingestion of the main meals (0830, 1230, and 1700 h) or snack (2030 h). The error bars are not shown for clarity.
Figure 2.
The impact of the three experimental conditions on the prevalence of hyperglycemia over the course of 24 h (A), average 24-h blood glucose concentrations (B), and the cumulative postprandial glucose iAUC (C). Data represent mean ± SEM. *Significantly lower compared with the sedentary condition (P < 0.05). †Significantly lower compared with ADL condition (P < 0.05).
Postprandial glycemic control
Moderate-intensity exercise strongly reduced the glycemic response to breakfast and, to a lesser extent, the glycemic response to lunch and dinner (P < 0.05 for all postprandial periods; Supplementary Table 1). The decrements in postprandial glucose concentrations observed in the ADL condition did not reach statistical significance for any of the postprandial periods (P > 0.05; Supplementary Table 1). More important, however, the cumulative glucose response to breakfast, lunch, and dinner (10.5-h iAUC; Fig. 2C) was significantly lower during both the ADL (365 ± 51 mmol/L/10.5 h; P < 0.05) and moderate-intensity exercise condition (285 ± 38 mmol/L/10.5 h; P < 0.001) compared with the sedentary control condition (448 ± 54 mmol/L/10.5 h). The 35 ± 5% reduction in the cumulative glucose iAUC during the moderate-intensity exercise condition was greater than the 17 ± 6% reduction observed in the ADL condition, although this observation did not reach statistical significance (P = 0.06).
Plasma insulin concentrations
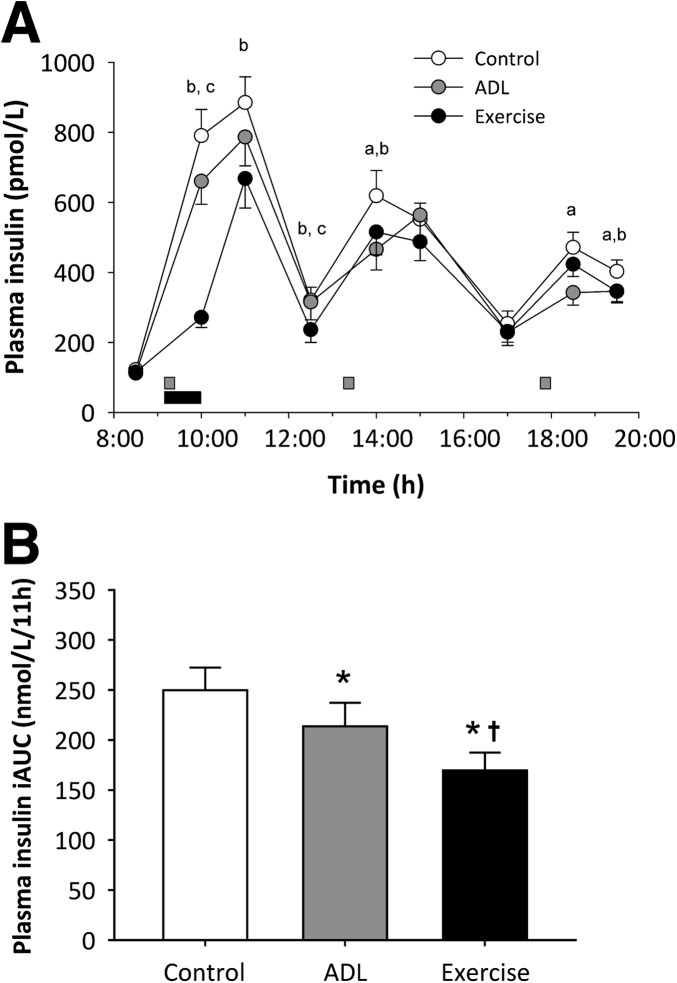
Plasma insulin profiles are shown in Fig. 3A. The postprandial increments in plasma insulin concentrations were significantly attenuated when ADL were performed in the early postprandial phase. A single bout of moderate-intensity exercise soon after breakfast blunted the subsequent increase in plasma insulin concentrations. The impact of moderate-intensity exercise on plasma insulin concentrations persisted for up to several hours after exercise. The resulting plasma insulin response to the standardized diet (11-h positive iAUC) was 17 ± 5% lower during the ADL condition (214 ± 24 nmol/L/11 h; P < 0.05) and 33 ± 4% lower during the exercise condition (170 ± 18 nmol/L/11 h; P < 0.001) relative to the sedentary control condition (250 ± 23 nmol/L/11 h). The insulin iAUC during the exercise condition also was lower compared with the ADL condition (P < 0.001).
Figure 3.
The impact of the three experimental conditions on insulin concentrations over time (A) and the postprandial insulin iAUC (B). Data represent mean ± SEM. aDifference between sedentary and ADL conditions (P < 0.05). bDifference between sedentary and exercise conditions (P < 0.05). cDifference between ADL and exercise conditions. *Significantly lower compared with sedentary condition (P < 0.05). †Significantly lower compared with ADL (P < 0.001).
CONCLUSIONS
The current study demonstrated that hyperglycemia is highly prevalent throughout the day in patients with type 2 diabetes. A single 45-min bout of endurance-type exercise strongly reduced the prevalence of hyperglycemia over the course of 24 h. An increase in ADL (three 15-min bouts of postmeal strolling) improved postprandial blood glucose homeostasis but did not significantly reduce the prevalence of hyperglycemia over 24 h.
Elevated blood glucose concentrations, which are most evident in the postprandial state, increase the risk for diabetes complications and mortality (1–6). In the current study, type 2 diabetic patients exceeded the recommended upper limit for postprandial glucose concentrations of 10 mmol/L during nearly 7 h per day (Fig. 2A). Strikingly, these hyperglycemic episodes occurred despite the fact that most patients were using oral blood glucose–lowering medication and were relatively well controlled according to the HbA1c level (6.9 ± 0.1% [52 ± 1 mmol/mol]; Table 1). These findings are in agreement with previous observations of the prevalence of hyperglycemia (8,28) and emphasize the need for additional treatment strategies in type 2 diabetes.
Although the acute impact of moderate-intensity to high-intensity exercise on 24-h blood glucose homeostasis has been well established (12–18), recent data also suggest an important role for light physical activity in maintaining or restoring proper glycemic control (21–23). Thus far, experimental studies investigating the impact of such light physical activity strategies on glycemic control in type 2 diabetic patients are lacking. The current study shows that the introduction of repeated bouts of ADL during sedentary behavior represents an effective interventional strategy to attenuate the increase in blood glucose and insulin concentrations after sequential meals in type 2 diabetic patients (Figs. 2C and 3B, respectively). As such, these findings confirm our hypothesis that simply performing ADL can improve postprandial blood glucose homeostasis. Considering the fact that postprandial hyperglycemia is a strong and independent risk factor for cardiovascular morbidity and mortality (4–6), our data indicate that even ADL can improve cardiovascular risk profiles in patients with type 2 diabetes. Hence, the beneficial impact of such light physical activity on postprandial glucose concentrations may, at least to some extent, explain the relationships observed between active and sedentary behavior and cardiovascular morbidity and mortality (29–32). The exact mechanisms by which light physical activity reduces postprandial blood glucose concentrations remain to be explored. Nonetheless, the concomitant reduction in postprandial insulin concentrations as observed in the current study (Fig. 3) suggests an important role for noninsulin-dependent (i.e., contraction-induced) glucose disposal. It also could be speculated that delayed gastrointestinal transit or splanchnic hypoperfusion attributable to the physical activity might have contributed to the lowered postprandial glycemic and insulinemic responses (33). However, this effect is likely to be small considering the low intensity of the ADL intervention.
Despite its positive effects on postprandial glucose and insulin concentrations, the ADL intervention did not significantly reduce the prevalence of hyperglycemic blood glucose excursions over the entire 24-h period (Fig. 2A and B). In contrast, a single 45-min bout of moderate-intensity endurance-type exercise was shown to reduce the daily prevalence of hyperglycemia by 34%, along with a reduction of 0.6 mmol/L in average 24-h blood glucose concentrations (Fig. 2A and B). Given the tight relationship between average 24-h glucose concentrations and HbA1c (34), the moderate-intensity endurance-type exercise intervention is more likely to affect long-term glycemic control (i.e., HbA1c) than the ADL intervention. Moreover, the reductions in postprandial glucose and insulin iAUC induced by moderate-intensity exercise (35 and 33%, respectively) were twofold greater than the decrements observed during the ADL condition (17% for both glucose and insulin iAUC). Thus, when matched for total activity duration, a single bout of moderate-intensity endurance-type exercise has a much greater impact on blood glucose homeostasis than repeated bouts of low-intense ADL.
We previously have speculated that the total volume of exercise (i.e., product of frequency, duration, and intensity) is of prime importance with respect to glycemic control (15). This theory also could explain the greater blood glucose–lowering effects observed in the endurance-type exercise condition as opposed to the ADL condition. The total energy expended during the 45 min of moderate-intensity endurance-type exercise (∼350 kcal) was approximately twofold higher compared with the 45 min (three 15-min bouts) of ADL (∼175 kcal). Interestingly, this difference in volume tends to be in agreement with the twofold greater decline in postprandial glucose and insulin iAUC observed during the exercise as opposed to the ADL condition. This observation suggests a dose–response relationship between the volume of aerobic physical activity and the subsequent improvements in blood glucose homeostasis. Such a graded dose–response relationship also has been observed recently between the volume of exercise training and improvements in insulin sensitivity (35). Moreover, for a fixed volume of exercise, high-intensity exercise was not found to be more effective than moderate-intensity in improving long-term glycemic control (i.e., HbA1c) (36,37). Taken together, it is tempting to speculate that equal volumes of ADL and moderate-intensity exercise lead to similar improvements in glycemic control. Future studies therefore should evaluate the dose–response relationship between the volume of ADL and subsequent improvements in blood glucose homeostasis. This also would provide an answer to the question of whether an increase in ADL provides any surplus benefit for those type 2 diabetic patients who already perform substantial levels of physical activity during daily life.
In conclusion, implementation of moderate-intensity endurance-type exercise or repeated bouts of ADL markedly improve postprandial blood glucose handling in type 2 diabetic patients. When matched for total duration, a single bout of moderate-intensity endurance-type exercise has a greater impact on daily blood glucose homeostasis than repeated bouts of ADL. Nonetheless, the introduction of repeated ADL bouts during prolonged sedentary behavior forms a valuable strategy in the management of blood glucose homeostasis in type 2 diabetes, especially in those patients who are unable or reluctant to perform structured exercise.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
J.-W.v.D. designed the study, collected the data, researched the data, and wrote the manuscript. M.V. collected the data, researched the data, and contributed to the discussion. W.v.M., C.D.A.S., and F.H. contributed to the discussion and critically reviewed and revised the manuscript. L.J.C.v.L. designed the study, researched the data, and wrote the manuscript. L.J.C.v.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented in abstract and poster form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank the excellent technical assistance of Jos Stegen, Antoine Zorenc, and Janneau van Kranenburg (Maastricht University Medical Centre+, Maastricht, the Netherlands), and are grateful for the enthusiastic support of the subjects who volunteered to participate in the study.
Footnotes
Clinical trial reg. no. NCT00945165, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2620/-/DC1.
References
- 1.Stratton IM. Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 1998;21:1167–1172 [DOI] [PubMed] [Google Scholar]
- 3.Zoungas S, Chalmers J, Ninomiya T, et al. ADVANCE Collaborative Group Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012;55:636–643 [DOI] [PubMed] [Google Scholar]
- 4.Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006;91:813–819 [DOI] [PubMed] [Google Scholar]
- 5.de Vegt F, Dekker JM, Ruhé HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926–931 [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Nathan DM, D’Agostino RB, Sr, Wilson PW, Framingham Offspring Study Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002;25:1845–1850 [DOI] [PubMed] [Google Scholar]
- 7.Praet SF, Manders RJ, Meex RC, et al. Glycaemic instability is an underestimated problem in Type II diabetes. Clin Sci (Lond) 2006;111:119–126 [DOI] [PubMed] [Google Scholar]
- 8.van Dijk JW, Manders RJ, Hartgens F, Stehouwer CD, Praet SF, van Loon LJ. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res Clin Pract 2011;93:31–37 [DOI] [PubMed] [Google Scholar]
- 9.Praet SF, van Loon LJ. Exercise: the brittle cornerstone of type 2 diabetes treatment. Diabetologia 2008;51:398–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998;49:235–261 [DOI] [PubMed] [Google Scholar]
- 12.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 2010;42:219–225 [DOI] [PubMed] [Google Scholar]
- 13.Praet SF, Manders RJ, Lieverse AG, et al. Influence of acute exercise on hyperglycemia in insulin-treated type 2 diabetes. Med Sci Sports Exerc 2006;38:2037–2044 [DOI] [PubMed] [Google Scholar]
- 14.van Dijk JW, Manders RJ, Tummers K, et al. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 2012;55:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dijk JW, Tummers K, Stehouwer CD, Hartgens F, van Loon LJ. Exercise therapy in type 2 diabetes: is daily exercise required to optimize glycemic control? Diabetes Care 2012;35:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab 2012;14:575–577 [DOI] [PubMed] [Google Scholar]
- 17.Mikus CR, Oberlin DJ, Libla J, Boyle LJ, Thyfault JP. Glycaemic control is improved by 7 days of aerobic exercise training in patients with type 2 diabetes. Diabetologia 2012;55:1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald AL, Philp A, Harrison M, Bone AJ, Watt PW. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med Sci Sports Exerc 2006;38:201–207 [DOI] [PubMed] [Google Scholar]
- 19.Healy GN, Dunstan DW, Salmon J, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007;30:1384–1389 [DOI] [PubMed] [Google Scholar]
- 20.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666 [DOI] [PubMed] [Google Scholar]
- 21.Lunde MS, Hjellset VT, Høstmark AT. Slow post meal walking reduces the blood glucose response: an exploratory study in female Pakistani immigrants. J Immigr Minor Health 2012;14:816–822 [DOI] [PubMed] [Google Scholar]
- 22.Nygaard H, Tomten SE, Høstmark AT. Slow postmeal walking reduces postprandial glycemia in middle-aged women. Appl Physiol Nutr Metab 2009;34:1087–1092 [DOI] [PubMed] [Google Scholar]
- 23.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 28.Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care 2005;28:2361–2366 [DOI] [PubMed] [Google Scholar]
- 29.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 2011;305:2448–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med 2012;172:494–500 [DOI] [PubMed] [Google Scholar]
- 31.Sluik D, Buijsse B, Muckelbauer R, et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: a prospective study and meta-analysis. Arch Intern Med 2012;172:1285–1295 [DOI] [PubMed] [Google Scholar]
- 32.Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: an epidemiological perspective. Sports Med 2001;31:101–114 [DOI] [PubMed] [Google Scholar]
- 33.van Wijck K, Lenaerts K, Grootjans J, et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol 2012;303:G155–G168 [DOI] [PubMed] [Google Scholar]
- 34.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubé JJ, Allison KF, Rousson V, Goodpaster BH, Amati F. Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc 2012;44:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balducci S, Zanuso S, Cardelli P, et al. Italian Diabetes Exercise Study (IDES) Investigators Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS ONE 2012;7:e49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen D, Dendale P, Jonkers RA, et al. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia 2009;52:1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.