Abstract
OBJECTIVE
To compare a modified fixed meal dosing strategy to flexible meal dosing in hospitalized patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patients (N = 126) with refractory hyperglycemia or requiring at least 20 units of insulin per day were randomly assigned to fixed meal dosing (including withholding the dose if less than half of the meal tray was consumed) or flexible meal dosing based upon carbohydrate intake. The inpatient diabetes management team made all treatment adjustments. Outcomes included day 3 mean glucose, 72-h glucose trend analysis, hypoglycemia (<3.9 mmol/L), and inpatient diabetes treatment satisfaction.
RESULTS
The mean glucose on day 3 was 9.5 and 8.8 mmol/L in the fixed and flexible meal groups, respectively (P = 0.26). The frequency of hypoglycemia was 23 and 39% overall in the fixed and flexible meal groups (P = 0.08), with half of events occurring in the morning. There was a wide range of carbohydrate intake (median 51 g/meal, 10–90% range 26–72 g on day 3). The fixed dose group required significantly more prandial insulin overall and more correction insulin over time. There was no difference in composite treatment satisfaction or dosing miscalculations between groups.
CONCLUSIONS
A fixed meal dosing strategy provided similar glucose control as flexible meal dosing, when managed by an inpatient diabetes treatment team. However, a larger sample size would be needed to definitively evaluate a treatment effect of flexible meal dosing in the hospital. Further study is needed to improve the delivery of bolus insulin in hospitalized patients.
Hyperglycemia is common in the hospital (1) and is associated with poor outcomes (2). Subcutaneous insulin is traditionally favored for most general surgical and medical patients outside of the intensive care unit, using a regimen that includes basal, prandial, and correction insulin components (3,4). However, there are limited data to support individualized treatment approaches, particularly outside of the intensive care unit, where most patients receive care. In general, glycemic control is related to the intake of carbohydrates (5) and is limited by the occurrence of hypoglycemia. Data suggest that the most common cause of hypoglycemia in hospitalized patients with diabetes is a reduction in caloric intake (6,7). Variable carbohydrate exposure may be a result of tests that require fasting, late meal trays, patient food preferences, or symptoms that interfere with nutritional intake, such as anorexia and nausea. Therefore, optimizing prandial insulin dosing may provide better glycemic control.
One approach to optimize prandial insulin coverage is to deliver meals with fixed carbohydrate content, thereby enabling fixed meal dosing (4). However, this does not guarantee that a patient will eat the entire meal or that the patient will not consume additional carbohydrates. An alternative approach is to administer prandial insulin based upon carbohydrate intake. In a retrospective study, the introduction of flexible meal dosing according to carbohydrate intake resulted in an improvement in glycemic control compared with sliding scale alone (8). Carbohydrate counting has shown probable glycemic benefit in outpatients with type 1 diabetes (9), but less clearly in outpatients with type 2 diabetes (10). Unlike hospitalized patients, outpatients are generally otherwise healthy, with good oral intake and recognition of impending hypoglycemia.
The Centers for Medicare and Medicaid Services is increasingly emphasizing value-based purchasing decisions (11), recognizing that patient satisfaction is closely linked to clinical quality measures (12). Flexible meal plans are becoming more popular as a means of improving overall patient satisfaction in hospitals (13). A 2008 survey of 270 health care food and nutrition practitioners, suppliers, and manufacturers affiliated with the National Society of Healthcare Foodservice Management reported that 37% of respondents offered restaurant-style room service, with many more planning to make the conversion (13). Such meal plans generally still fall within the prescribed diet order, but it is less clear how this is carried out in practice among patients with diabetes. In patients with diabetes, food choices have received the lowest scores on inpatient diabetes treatment satisfaction questionnaires (14). A flexible meal plan was associated with more hypoglycemia but may improve treatment satisfaction and opportunities for nutrition education (15). Thus, it has become increasingly important to establish glycemic control strategies that address this growing trend.
This is the first randomized study to examine the use of prandial insulin dosing according to carbohydrate intake in hospitalized patients.
RESEARCH DESIGN AND METHODS
Study subjects were general medical or surgical patients admitted to a single large academic medical center (976 beds) between August 2010 and February 2012. The nurse-to-patient ratio varied based upon location, although none of the patients were critically ill. Patients requiring insulin therapy were randomized using a computer-generated process to either fixed prandial insulin dosing or flexible dosing based upon carbohydrate intake. Inclusion criteria included type 2 diabetes between the ages of 18 and 80 years with hyperglycemia or requiring at least 20 units of insulin per day in the 24 h prior to enrollment. Hyperglycemia was defined as a blood glucose 8.3–22.2 mmol/L on at least two occasions separated at least 4 h apart for the purposes of study inclusion. Exclusion criteria included major surgery or any surgery lasting >2 h, glucocorticoids, enteral or parenteral nutrition, pregnancy, current or planned intravenous insulin, prolonged NPO status (>24 h), expected length of stay <48 h (determined by the attending physician of the primary team), insulin pump, diabetic ketoacidosis, end-stage renal or liver disease, major surgery, inability to give informed consent in English, and sensitive admissions (prisoners and suicidality). This study was approved by The Ohio State University institutional review board, and all patients signed informed consent.
All oral and noninsulin injectable glucose-lowering therapies were discontinued. The total daily projected insulin dose (TDD) was based upon the pre-enrollment glucose and either weight (if insulin naïve) or total daily preadmission dose (if not insulin naïve), similar to studies of other inpatient populations (16,17). In subjects who were insulin naïve, the total dose of insulin was calculated as 0.4 or 0.5 units/kg if the enrollment glucose was >11.1 or <11.1 mmol/L, respectively. In subjects who were not insulin naïve, the total insulin dose was calculated as 120 or 100% of the total daily insulin dose at admission in subjects with an enrollment glucose of >11.1 or <11.1 mmol/L, respectively. One-half of the TDD was given as basal insulin analog (detemir). The dose was administered once daily at night unless the patient was already receiving twice-daily dosing of basal insulin. The inpatient diabetes management team wrote all insulin orders and made all insulin adjustments. The inpatient diabetes team was available by pager for questions or concerns after hours.
Prandial insulin (aspart) was administered immediately after the meal for all patients, since previous studies in other patient populations suggest that similar glycemic control could be achieved (18,19). In the fixed dose group, half of the TDD was divided into three equal fixed doses given immediately after each meal. The dose was held if the subject ate less than half of the meal. In the carbohydrate-based dose group, prandial insulin was based upon the following equation: carbohydrate-to-insulin ratio (CIR) = 400/TDD, as reviewed previously (20). Prandial insulin is routinely dosed according to carbohydrate intake at the study institution for the past 5 years. All meal trays come with a list of the carbohydrate content for each item, and nurses are trained to administer insulin based on carbohydrate intake. Nurses are instructed to contact the diabetes team for assistance with carbohydrate content of meals from outside the medical center. Supplemental (correction) insulin was provided at major meals and bedtime using correction factor = 1,700/TDD (20).
Daily adjustments were made according to a premeal glucose target of 7.8 mmol/L in increments of ±10–20% of the TDD, similar to the practice reported in other hospitalized patient populations (16,17). Prandial insulin was preferentially titrated first unless the subject was NPO or the total dose of prandial insulin administered in the previous 24-h period exceeded twice that of the basal insulin. In these cases, the dose of basal insulin was preferentially increased, provided that the fasting glucose was >6.7 mmol/L. Isolated fasting hyperglycemia was not specifically addressed during the 72-h study period. In the case of the fixed dose group, an absolute adjustment in the dose in units was made, whereas in the flexible dose group, an adjustment in the CIR was made (for example, a 20% increase for a CIR of 10 would be 8). An increase in dose of 10% was made if >50% of readings were above target in a given day, assuming there were no readings <4.4 mmol/L. Likewise, an increase in dose of 20% was made if all readings were >10 mmol/L.
Hypoglycemia was managed with a 10–20% reduction in prandial insulin, unless it occurred overnight, in which case, the basal insulin was reduced. Due to the occurrence of two subjects with a glucose <2.2 mmol/L, an amendment was implemented partway into the study to manage more advanced hypoglycemia (<2.8 mmol/L) with a 50% reduction in TDD. This amendment also allowed the provider to reduce the basal insulin up to 20% prior to a procedure for which the patient was NPO.
Capillary blood glucose checks were ordered before and 2 h after meals and at bedtime (7-point profiles) using the Accuchek Inform glucometer.
Patients at the study institution are typically able to customize meals with the assistance of a dietary technician by choosing from several options that fit within the diet ordered by the primary team. For this study, a “carbohydrate-controlled” diet was ordered for all patients. The actual content of this diet varies but also depends upon the calorie content ordered (for example, an 1,800-calorie diet consists of ∼45 g at breakfast and 65 g at lunch and dinner, but patients may choose to ask for supplemental items). Treatment satisfaction was measured at day 3 using the Diabetes Treatment Satisfaction Questionnaire for Inpatients (DTSQ-IP) (14). Each item is scored on a 7-point scale (0 to 6). Item 17 was excluded because it referred to a diabetes inpatient specialist nurse, which was not a feature of this study.
The intervention was limited to 3 days to allow subsequent customization of the insulin regimen outside of the study protocol, which was designed specifically to study prandial insulin, and also to allow patients in the fixed dose group to receive education on flexible meal dosing if desired. Furthermore, it was felt that 3 days would be sufficient for achieving a plateau in glucose levels (16,17). The primary outcome was day 3 mean 24-h glucose. Secondary outcomes included a trend analysis of glucose over the 72-h study period, frequency of hypoglycemia (defined as the proportion of subjects with a glucose value <3.9 and the proportion of subjects with a glucose value <2.2 mmol/L), frequency of protocol deviation (defined as any intentional or unintended deviation from any ordered dose of insulin, excluding rounding errors), proportion of patients with mean glucose on day 3 of 3.9–7.8 mmol/L, and diabetes treatment satisfaction. Additional measures, including nonfasting glucose, proportion of subjects with hypoglycemia <3.3 mmol/L, and proportion of patients achieving target glucose 7.8–10 mmol/L, were added in order to provide comparisons to recent publications and guidelines.
A sample size of 55 patients per group was estimated to provide at least 80% of the power to detect a 1.1 mmol/L difference in day 3 mean 24-h glucose level, assuming an SD of 1.9 mmol/L for both groups (α = 0.05; two-sided, two-sample Student t test) and a 10% dropout or crossover. The final enrollment number was increased to 126 due to higher than expected discharges prior to 72 h. For comparing the primary end point (day 3 24-h glucose level) between groups, a linear mixed model was applied, incorporating repeated measurements for each patient, with treatment group as the main fixed effect and patients as the random effect. Effects of other demographic factors (insulin naïvety, sex, age, BMI, duration of diabetes, daily carbohydrate intake, and NPO status) were explored in modeling procedures. For insulin, residuals from linear mixed models using the original scale were not normally distributed. Therefore, in order to test relevant within-group and between-group differences, natural log transformations were applied, and this largely resolved the violation of the normality assumption. Other secondary end points were analyzed using the Student t test (for normally distributed variables), Wilcoxon rank sum test (for nonnormally distributed variables), or Fisher exact test (for binomial variables) as appropriate. Data analyses were conducted in JMP 9.0, and models were performed in SAS version 9.3.
RESULTS
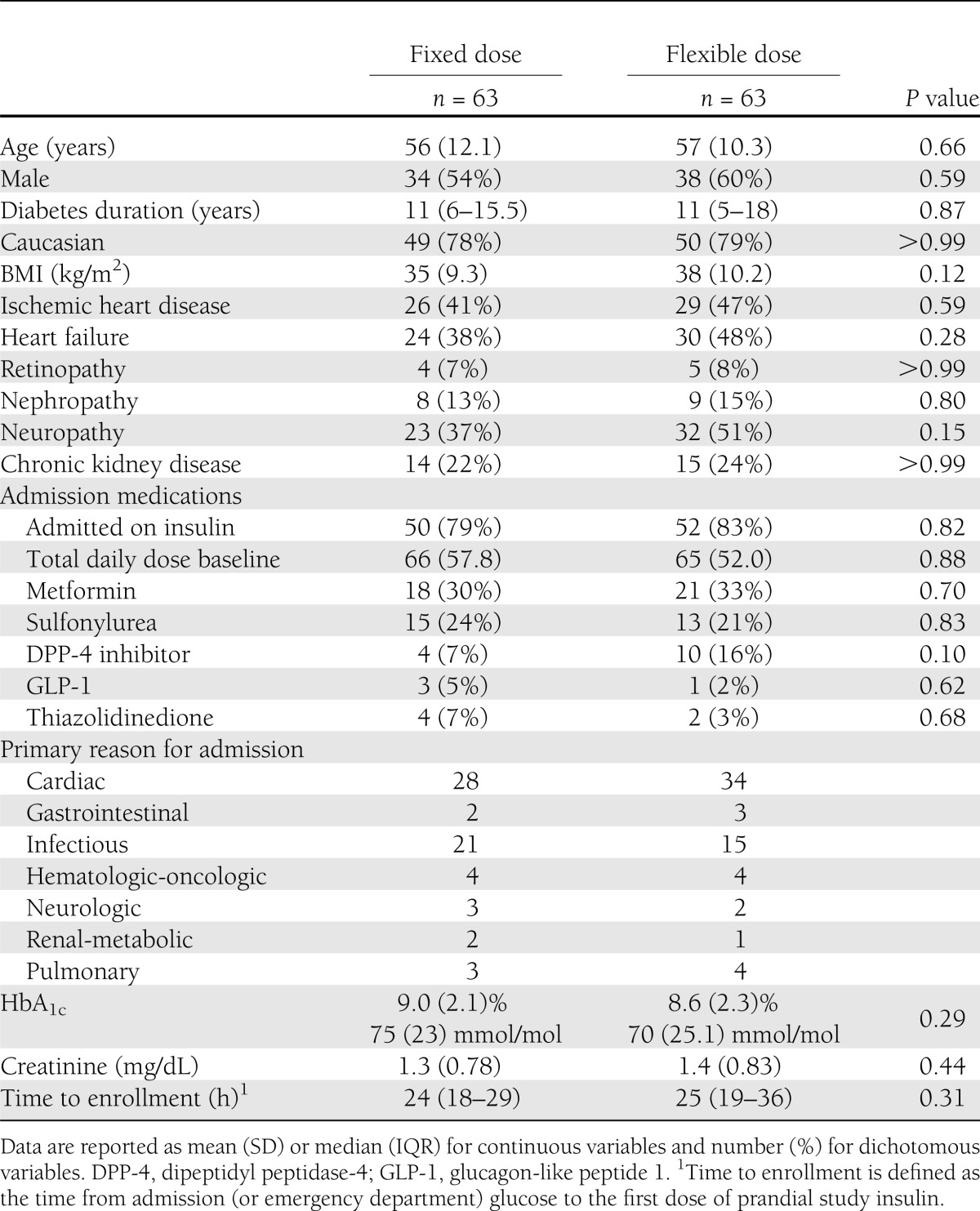
A total of 126 patients signed consent. Subjects had a mean age of 57 years, a median duration of diabetes of 11 years, and 85% were on insulin therapy prior to hospital admission. No data were available in five subjects due to screen failure (early discharge [one], steroids requiring intravenous insulin [one], leaving against medical advice [one], and consent withdrawal [two]). Data were available in 109 subjects on day 2 and 79 subjects on day 3 due to hospital discharge. Baseline characteristics are shown in Table 1. There were no differences between groups.
Table 1.
Baseline characteristics
There was no difference in mean glucose on day 3 between groups (Table 2). The mixed-model approach did not find a significant group-by-time interaction, indicating no difference in glucose change over time between the two groups (P = 0.47). There was no significant difference in nonfasting glucose or in the number of subjects at target glucose 3.9–7.8 mmol/L (27 vs. 33% in the fixed vs. flexible meal groups, respectively, P = 0.63). The percentage of subjects achieving mean glucose 3.9–10.0 mmol/L was also similar (70 vs. 71%, P > 0.99). There was no significant difference in the frequency of hypoglycemia between groups. Half of all hypoglycemia occurred at the prebreakfast time point.
Table 2.
Glucose data
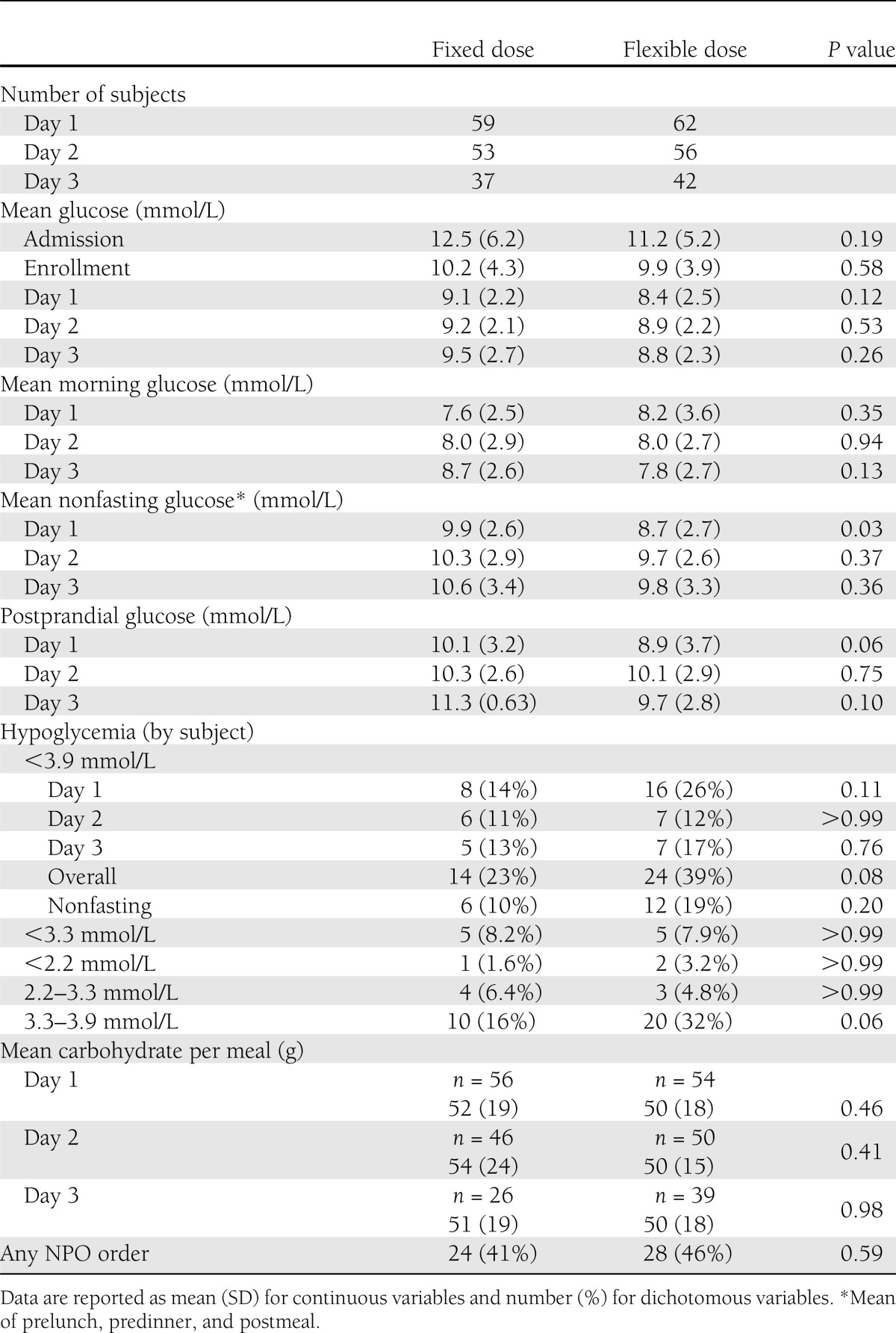
A large proportion of patients were NPO at some point during the study (41 and 46% of subjects in the fixed and flexible groups, respectively). However, when patients who were NPO were excluded (n = 12), mean glucose was similar on day 3 (9.4 vs. 8.8 mmol/L in the fixed and flexible groups, respectively, P = 0.37). In addition, carbohydrate intake was variable in patients who were eating. Carbohydrate intake was available in 56 subjects on day 3 and ranged from 5 to 122 g/meal (10–90% range 26–72 g). When stratified by carbohydrate intake, subjects eating >50 g/meal had a mean glucose of 10.9 ± 3.1 mmol/L in the fixed dosing group compared with 8.6 ± 2.0 mmol/L in the flexible group. However, in subjects eating <50 g of carbohydrates, the opposite trend was observed; the mean glucose was numerically smaller in the fixed meal group compared with the flexible meal group (8.2 ± 2.1 vs. 9.1 ± 2.5 mmol/L). There was no effect of BMI, insulin dose, duration of diabetes, age, sex, or race.
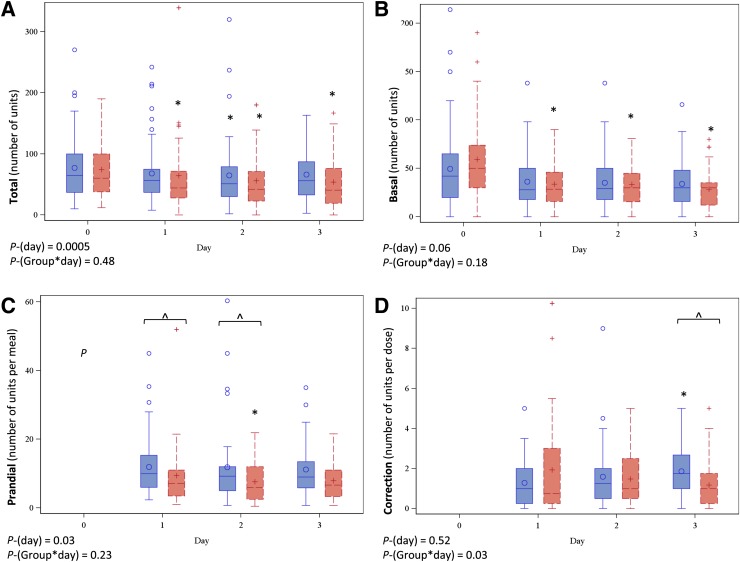
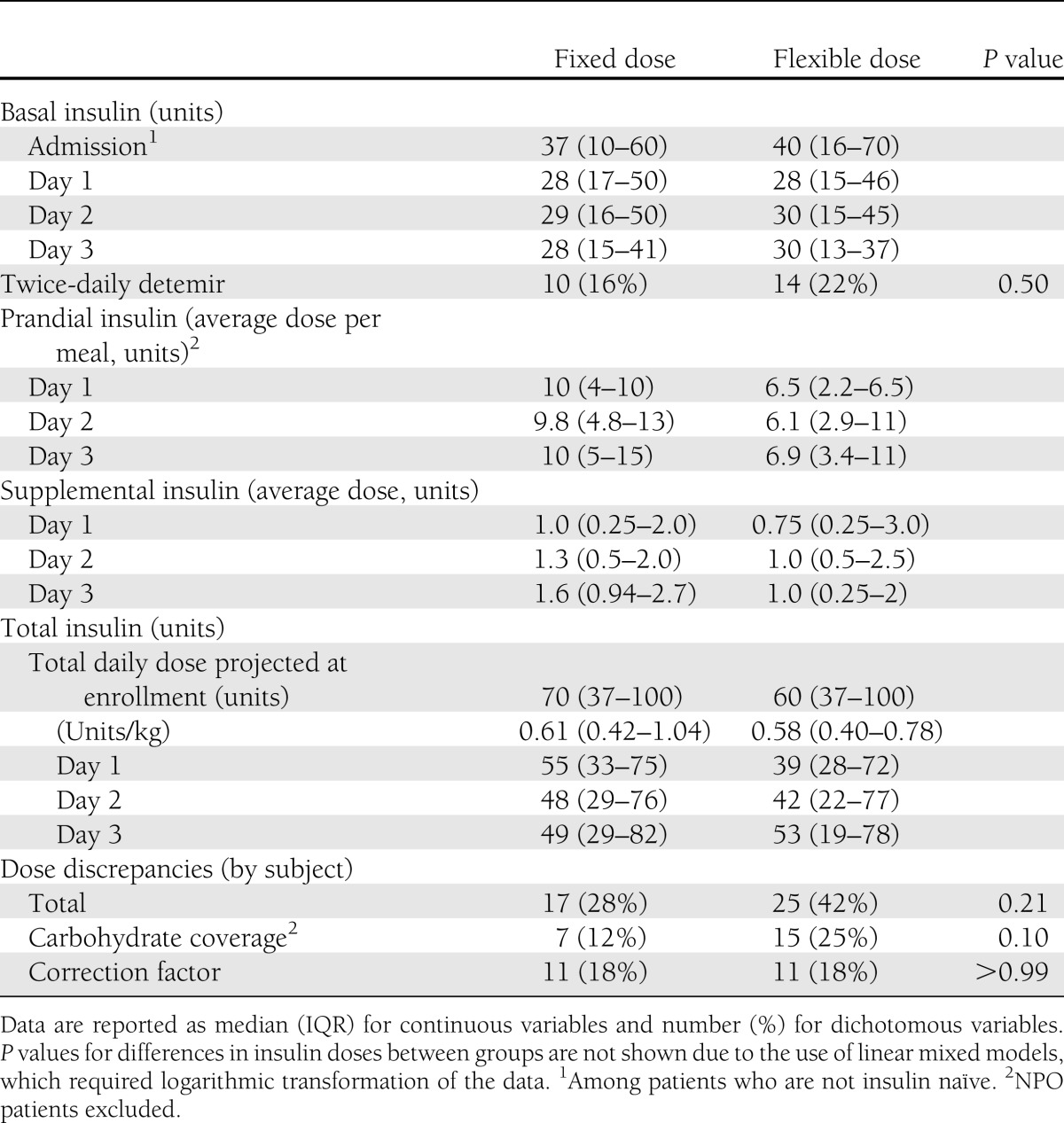
Total insulin decreased significantly from baseline in pooled data (P = 0.005) and at specific time points within both groups (Fig. 1). There was a significant reduction in basal insulin from baseline in the flexible but not the fixed dose group (Fig. 1). The mean prandial insulin dose decreased significantly over time in pooled data (P = 0.03), but particularly within the flexible dose group. The prandial insulin dose was lower in the flexible dose group than the fixed dose group overall (P = 0.01) (Fig. 1), primarily on days 1 and 2. Finally, the rate of change in correction insulin dose significantly differed between groups (P = 0.03), largely explained by an increase over time in the fixed dose group, particularly on day 3. There was no difference in the number of patients with any dose discrepancy between groups (Table 3). Overall, 18% of patient-days were accompanied by at least one deviation in insulin dose calculation or administration (excluding missing documentation or rounding errors); 9, 9, and 2% of patient-days had deviations in the carbohydrate, correction, and basal doses, respectively.
Figure 1.
Box plots summarizing total insulin dose (A), basal insulin dose (B), mean prandial dose per meal (excluding meals where patient was NPO) (C), and mean correction insulin per dose (D). Plots with solid lines and circles (○) indicate the fixed dose group, and plots with dashed lines and plus (+) signs indicate the flexible dose group. Day 0 indicates the TDD for total insulin and the admission dose for basal insulin. The horizontal line within the box represents the median value, and the top and bottom of the box represent the 75th and 25th percentiles, respectively. The whiskers represent the highest and lowest data point still within the following values: 75th percentile + 1.5 × (IQR) (top), and 25th percentile – 1.5 × (IQR) (bottom). If the data points do not reach the computed ranges, then the whiskers are represented as the highest and lowest data point. P-(day) indicates the P value for change in daily dose in the pooled sample, and P-(Group*day) indicates the P value for between-group difference in overall trend. *P < 0.05 vs. baseline within group; ^P < 0.05 between groups. P values determined from mixed linear models with natural log-transformed insulin values.
Table 3.
Insulin data
Treatment satisfaction scores were similar between groups (69 ± 14 vs. 68 ± 13 out of 96 points in fixed and flexible groups, respectively, P = 0.69). In particular, there was no difference in the responses to glucose-specific items. The median score was 3 (P = 0.89) for item 2: “How often have you felt that your blood sugars have been unacceptably high recently?” The median score was 0 on item 3 (none of the time, P = 0.83): “How often have you felt that your blood sugars have been unacceptably low recently?” Hospital length of stay did not differ between groups (median 6 days [interquartile range (IQR) 3.75–10] in the fixed meal group vs. 6 days [IQR 5–9] in the flexible group, P = 0.91).
CONCLUSIONS
This study demonstrated that prandial insulin delivered using a flexible or fixed meal dosing strategy achieved similar mean glucose levels in hospitalized patients, with no difference in the frequency of hypoglycemia or overall patient satisfaction. This suggests that hospitals that allow more flexible meals can achieve similar glycemic control with a modified fixed meal dosing strategy compared with formal carbohydrate counting techniques, although a higher prandial or supplemental insulin dose may be required. However, the observed SD for mean glucose was higher than the estimates used for calculation of sample size, indicating that a larger study would be needed to definitively evaluate a treatment effect of flexible meal dosing. Additional calculations show that the treatment difference of 1.1 mmol/L postulated for this study would have necessitated 85 patients per group. Further study is also needed in patients who consume larger amounts of carbohydrates, where the flexible meal dosing strategy may provide better overall control.
It should be emphasized that all initial dose calculations and subsequent dose adjustments were performed by the inpatient diabetes treatment team. Therefore, it is unclear whether the results would extend to patients who are not followed by an inpatient diabetes treatment team (which is the majority of patients at the study institution), especially since custom carbohydrate and correction calculations were implemented. Gaps in knowledge regarding proper insulin dosing are reported to be major barriers to the achievement of glycemic control in the hospital among resident physicians (21,22). Currently, the institution’s prandial insulin order sets provide a choice of three default CIRs, which are designated as low (20), standard (10), or high (5). This simplifies insulin dosing (versus fixed meal dosing) at the study institution where the technique has become the most frequently ordered method of delivering short-acting insulin.
Mild hypoglycemia (<3.9 mmol/L) was common in this study and tended to occur more frequently in the flexible dosing group (23 and 39% in the fixed dose and flexible dose groups, respectively), despite the algorithmic approach to insulin. However, most events were mild, and approximately half of hypoglycemia occurred in the morning, suggesting that basal insulin contributed as much as prandial insulin. The incidence of any glucose <3.3 mmol/L was 8% in both groups. By comparison, the frequency of hypoglycemia (<3.3 mmol/L) was 33% in a study of hospitalized subjects receiving detemir and aspart (17). In another study of surgical patients treated with glargine and apidra, the frequency of hypoglycemia (glucose <3.9 mmol/L) was 23% (16). However, subjects requiring >0.4 units/kg prior to admission were excluded in the latter study, and subjects had lower BMI and shorter duration of diabetes and were more likely to be insulin naïve than in the current study. Higher insulin dose was reported to be a risk factor for hypoglycemia in a pooled analysis of randomized trials (23). A national survey of hospitals reported a prevalence of hypoglycemia <3.3 mmol/L of 25–26% among hospitalized patients using basal insulin (24).
The dose of basal insulin tended to decrease over time in this study, in part due to morning hypoglycemia. This suggests that the starting dose of basal insulin (50–60% of the total home dose from all types of insulin) is too aggressive, given that most subjects were not insulin naïve. The need for basal dose reduction may have also masked a treatment effect of the intervention, since the need for reduction in the dose of basal insulin precluded any adjustment in prandial insulin. The total daily insulin dose also decreased over time in the study. In another randomized study, hypoglycemia was more frequent in patients who were admitted on insulin therapy and continued the home dose (17). Current guidelines suggest reducing the total home dose in patients with poor nutritional intake, impaired renal function, or admission glucose <5.6 mmol/L (4), but the current results suggest that this approach may not be sufficient. Although it has been noted that patients typically require more insulin during acute illness, this either resolves quickly or may be more relevant during critical illness (25). In general, the reduction in total insulin was more prominent in the flexible dosing group, which also required significantly less prandial insulin overall and a significant divergence from the fixed dose group in correction insulin requirements over time.
Meals with a set carbohydrate content may provide better glucose control with fixed meal dosing than that reported here. However, fixed carbohydrate meals may not represent usual practice in the typical inpatient setting and may affect patient satisfaction (15). In this study, although mean carbohydrate intake in a subset of patients was ∼50 g of carbohydrates, the range was high, which could significantly impact glycemic control, even with consistent carbohydrate plans. It was due to the concern for irregular intake that prandial insulin was dosed postmeal. Withholding the prandial insulin dose in cases where less than half the tray was consumed appeared to be an adequate strategy for addressing this concern at the lower end of the range of intake. In contrast, subjects with higher carbohydrate consumption may benefit from the flexible meal strategy, although it must be emphasized that this was a post hoc analysis, and it requires more evaluation. Unfortunately, carbohydrate intake was not documented in all patients consistently despite specific data collection efforts (the electronic medical record at the institution does not force the nurse to document the carbohydrate intake).
All nurses at the study institution are trained on dosing based upon carbohydrate intake, but additional interventions to ensure dosing accuracy appear to be necessary, regardless of the method of bolus insulin dosing. Problems related to irregular mealtimes, outside sources of food, timing of insulin administration relative to meals, calculation of insulin doses, and missed insulin doses could have masked potential treatment differences between groups, as dosing aberrations were common. The actual time of administration relative to oral intake was not recorded in this study, but this would be important for further study. Despite the extra calculations required for carbohydrate counting, dosing deviations were not significantly different between groups. Furthermore, 43% of bolus miscalculations were due to deviations in correction dosing and not the carbohydrate coverage. The use of custom CIR and correction factors is not standard in the study institution and might have contributed to these errors. Very little prospective data on subcutaneous insulin administration errors are available in the hospital. Frequent errors in insulin dosing were reported in a previous retrospective study, where there were 0.53 errors/patient day, with 64% of patients having at least one error (26). This error rate is higher than that reported here, possibly due to differences in the definition of error (26). Thus, continued opportunities exist for improving insulin dosing among hospitalized patients in general. The study institution recently implemented a fully computerized electronic medical record with capability for a built-in bolus insulin dose calculator. Bolus calculators are known to improve dosing accuracy in outpatients with type 1 diabetes using fixed or flexible dosing (27).
Finally, treatment satisfaction did not differ significantly between groups. However, in the original publication of DTSQ-IP, the best model could only account for 8.2% of the variability in inpatient treatment satisfaction (14). In particular, the DTSQ-IP may be insensitive for assessing this particular intervention, as not all elements apply and more subtle differences between groups cannot be excluded. Additional tools for assessing inpatient treatment satisfaction are needed.
In conclusion, a prandial insulin strategy using a modified fixed meal dose provided similar glucose control compared with a flexible dosing regimen, when managed by an inpatient diabetes treatment team. The study illustrates the challenges and limitations of rigorous assessment of treatment regimens and the informed translation of such in a hospital setting. However, larger studies reflecting typical use would be necessary to definitively evaluate any treatment effect. Further study is needed to determine whether patients consuming more carbohydrates may benefit from flexible meal dosing. The results support the need for a reduction in the home dose of insulin at the time of admission and further research in methods of delivering timely, accurate meal dosing in the hospital.
Acknowledgments
This study was supported by The Ohio State University Clinical and Translational Research Center, National Center for Research Resources Award UL1-RR-025755, and National Institutes of Health (NIH) Grant K23-DK-080891.
This study was supported by an investigator-initiated research grant from Novo Nordisk. K.M.D. reports consulting fees from Eli Lilly and Company and Sanofi. No other potential conflicts of interest relevant to this article were reported.
K.M.D. performed study design, patient procedures, analysis of data, and preparation of the manuscript. C.S. performed patient enrollment and procedures and assisted with analysis of data. M.A.-R. assisted with analysis of data and preparation of manuscript. K.O. assisted with study design and reviewed the manuscript. K.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented orally at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT01101867, clinicaltrials.gov.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of Novo Nordisk, the National Center for Research Resources, or the NIH.
References
- 1.Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract 2011;17:853–861 [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 3.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists. American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009;15:353–369 [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 5.Rabasa-Lhoret R, Garon J, Langelier H, Poisson D, Chiasson JL. Effects of meal carbohydrate content on insulin requirements in type 1 diabetic patients treated intensively with the basal-bolus (ultralente-regular) insulin regimen. Diabetes Care 1999;22:667–673 [DOI] [PubMed] [Google Scholar]
- 6.Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med 1986;315:1245–1250 [DOI] [PubMed] [Google Scholar]
- 7.Vriesendorp TM, van Santen S, DeVries JH, et al. Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med 2006;34:96–101 [DOI] [PubMed] [Google Scholar]
- 8.Hirose M, Yamanaka H, Ishikawa E, Sai A, Kawamura T. Easy and flexible carbohydrate counting sliding scale reduces blood glucose of hospitalized diabetic patient in safety. Diabetes Res Clin Pract 2011;93:404–409 [DOI] [PubMed] [Google Scholar]
- 9.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman DP, Vaiana M, Romley JA. The emerging importance of patient amenities in hospital care. N Engl J Med 2010;363:2185–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med 2008;359:1921–1931 [DOI] [PubMed] [Google Scholar]
- 13.Aase S. Hospital foodservice and patient experience: what’s new? J Am Diet Assoc 2011;111:1118–1123 [DOI] [PubMed] [Google Scholar]
- 14.Sampson MJ, Singh H, Dhatariya KK, Jones C, Walden E, Bradley C. Psychometric validation and use of a novel diabetes in-patient treatment satisfaction questionnaire. Diabet Med 2009;26:729–735 [DOI] [PubMed] [Google Scholar]
- 15.Curll M, Dinardo M, Noschese M, Korytkowski MT. Menu selection, glycaemic control and satisfaction with standard and patient-controlled consistent carbohydrate meal plans in hospitalised patients with diabetes. Qual Saf Health Care 2010;19:355–359 [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovanovic L, Giammattei J, Acquistapace M, Bornstein K, Sommermann E, Pettitt DJ. Efficacy comparison between preprandial and postprandial insulin aspart administration with dose adjustment for unpredictable meal size. Clin Ther 2004;26:1492–1497 [DOI] [PubMed] [Google Scholar]
- 19.Ratner R, Wynne A, Nakhle S, Brusco O, Vlajnic A, Rendell M. Influence of preprandial vs. postprandial insulin glulisine on weight and glycaemic control in patients initiating basal-bolus regimen for type 2 diabetes: a multicenter, randomized, parallel, open-label study (NCT00135096). Diabetes Obes Metab 2011;13:1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract 2008;14:1095–1101 [DOI] [PubMed] [Google Scholar]
- 21.Cheekati V, Osburne RC, Jameson KA, Cook CB. Perceptions of resident physicians about management of inpatient hyperglycemia in an urban hospital. J Hosp Med 2009;4:E1–E8 [DOI] [PubMed] [Google Scholar]
- 22.Latta S, Alhosaini MN, Al-Solaiman Y, et al. Management of inpatient hyperglycemia: assessing knowledge and barriers to better care among residents. Am J Ther 2011;18:355–365 [DOI] [PubMed] [Google Scholar]
- 23.Farrokhi F, Klindukhova O, Chandra P, et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non-critically ill patients with type 2 diabetes. J Diabetes Sci Tech 2012;6:1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 2007;30:367–369 [DOI] [PubMed] [Google Scholar]
- 25.Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Association Diabetes in Hospitals Writing Committee Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 26.Deal EN, Liu A, Wise LL, Honick KA, Tobin GS. Inpatient insulin orders: are patients getting what is prescribed? J Hosp Med 2011;6:526–529 [DOI] [PubMed] [Google Scholar]
- 27.Sussman A, Taylor EJ, Patel M, et al. Performance of a glucose meter with a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabetes Sci Tech 2012;6:339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]