Abstract
OBJECTIVE
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease (ESRD). Obstructive sleep apnea (OSA) is common in type 2 diabetes and increases oxidative stress. Hence, OSA could promote the development and progression of DN.
RESEARCH DESIGN AND METHODS
This was a cohort study in adults with type 2 diabetes. Patients with known OSA or ESRD were excluded. DN was defined as the presence of albuminuria or an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. DN progression was based on eGFR measurements. OSA was defined as apnea hypopnea index (AHI) ≥5 events/h. Serum nitrotyrosine abundance (a marker of nitrosative stress) was measured by ELISA.
RESULTS
A total of 224 patients were included. OSA and DN prevalence was 64.3 and 40.2, respectively. DN prevalence was higher in patients with OSA (OSA+) compared with those without OSA (OSA−) (49.3% vs. 23.8%, P < 0.001). After adjustment, OSA (odds ratio 2.64 [95% CI 1.13–6.16], P = 0.02) remained independently associated with DN. After an average follow-up of 2.5 (0.7) years, eGFR decline was greater in OSA+ compared with OSA− patients (median −6.8% [interquartile range −16.1 to 2.2] vs. −1.6% [−7.7 to 5.3%], P = 0.002). After adjusting, both baseline OSA (B = −3.8, P = 0.044) and AHI (B = −4.6, P = 0.02) remained independent predictors of study-end eGFR. Baseline serum nitrotyrosine abundance (B = −0.24, P = 0.015) was an independent predictor of study-end eGFR after adjustment.
CONCLUSIONS
OSA is independently associated with DN in type 2 diabetes. eGFR declined faster in patients with OSA. Nitrosative stress may provide a pathogenetic link between OSA and DN. Interventional studies assessing the impact of OSA treatment on DN are needed.
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease (ESRD) in many countries (1) and has significant impact on patients and health care systems (2). DN progresses slowly, starting with microalbuminuria, which progresses into overt proteinuria in 20–40% of patients, and 20% of patients will have progressed to ESRD within 20 years after onset of overt proteinuria (1). The speed of DN progression is variable and largely dependent on blood pressure (BP), obesity, metabolic control, and other factors such as male sex and ethnicity (3,4).
The pathogenesis of DN is thought to be similar to other microvascular complications in which hyperglycemia and hypertension are thought to be fundamental to its development, as they promote increased oxidative and nitrosative stress (3). In addition, hemodynamic changes occur as a result of the activation of the renin-angiotensin-aldosterone (RAAS) and endothelin systems, resulting in increased systemic and intraglomerular pressure, causing hyperfiltration and albuminuria (3). Despite attempts to improve metabolic control and RAAS inhibition, DN remains very common, and many patients develop ESRD requiring renal replacement therapy. Hence, better understanding of the pathogenesis of DN is needed in order to develop more effective treatments.
Several reports, including our own, have shown a high prevalence of obstructive sleep apnea (OSA) in patients with type 2 diabetes (5,6). Patients with OSA and type 2 diabetes are at increased risk of diabetic peripheral neuropathy (6). Furthermore, OSA is associated with increased oxidative and nitrosative stress as well as impaired microvascular regulation in patients with type 2 diabetes (6). Hence, it is plausible that OSA complicating type 2 diabetes could facilitate the development and progression of microvascular complications including DN.
The primary aims of this study were to assess the relationship between OSA and DN and to assess the impact of OSA on the estimated glomerular filtration (eGFR) decline in patients with type 2 diabetes. Secondary aims were to assess the impact of OSA on the development of albuminuria and to explore the mechanisms by which OSA and DN could be linked.
RESEARCH DESIGN AND METHODS
We conducted a prospective observational cohort study in white European and South Asian adults with type 2 diabetes. Patients were recruited between 2009 and 2010 and were followed until the end of 2012. Patients with respiratory disease including previously diagnosed OSA or ESRD receiving renal replacement therapy were excluded. Patients were recruited consecutively from the diabetes clinics of two U.K. hospitals. Patients were approached consecutively in the waiting area by the investigator or a research nurse without any prior knowledge of their medical condition. Consent was obtained and ethnicity determined in accordance with the U.K. decennial census by the study participants. The project was approved by the Warwickshire Research Ethics Committee (REC no. 08/H1211/145).
OSA assessment
OSA was assessed by a single overnight home-based cardiorespiratory sleep study using a portable multichannel device (Alice PDX; Philips Respironics) and scored in accordance with the American Academy of Sleep Medicine guidelines (7). An apnea hypopnea index (AHI) ≥5 events/h was consistent with the diagnosis of OSA (8). Sleep studies with <4 h of adequate recordings were repeated and excluded if the quality remained poor. OSA severity was assessed based on the AHI categories (<5 events/h no OSA, 5 to <15 mild OSA, 15 to <30 moderate OSA, and ≥30 severe OSA), AHI (as a continuous variable), the time spent with oxygen saturations <80%, and the nadir oxygen saturations during sleep. Patients diagnosed with OSA were referred to the sleep clinic and treated as per routine clinical care. Patients with no or mild OSA were not offered continuous positive airway pressure (CPAP) treatment, while all patients with moderate to severe OSA were offered CPAP. Patients with moderate to severe OSA were hence divided into those compliant with CPAP (average usage >4 h/night on 70% of days) and those not compliant (including patients who declined treatment) (9). Data regarding CPAP compliance were downloaded directly from the CPAP equipment.
DN assessment
DN was assessed using eGFR, calculated using the four-variable Modification of Diet in Renal Disease equation (10) and the urinary albumin-to-creatinine ratio (ACR) of a single early-morning urine measurement. Microalbuminuria was defined as ACR >3.4 mg/mmol, and macroalbuminuria was defined as ≥30 mg/mmol (11–13). Urine samples with evidence of urinary tract infection were repeated when free from infection. DN was defined as the presence of albuminuria (micro or macro) or an eGFR <60 mL/min/1.73 m2 (14). ACR and eGFR were measured at baseline and study end. Study-end measurements were taken during patients visits to the follow-up appointments of the diabetes clinic. eGFR measurements during acute illness or after imaging that used contrast were excluded.
Nitrosative stress assessment
To explore possible mechanisms that might explain the relationship between OSA, DN, and progression of eGFR, we assessed serum nitrotyrosine levels. The rationale for the selection of serum nitrotyrosine was based upon prior reports of an association between nitrosative stress and DN in animal studies (15,16) as well as a single report that identified high levels of nitrotyrosine in the kidneys of patients with diabetes (17). All study participants were approached, and serum 3-nitrotyrosine was assessed in duplicate in all subjects who agreed using commercially available ELISA (Oxiselect; Cell Biolabs, San Diego, CA). The measurements had good precision (<10% difference between duplicate samples) and high reproducibility (correlation coefficient between repeated measurements = 0.9 and a mean [SEM] coefficient of variance of 10.9% [2.4%]) All assessments in the study (renal function, nitrosative stress, and biochemical profiles) were conducted blind to OSA status.
Outcome measures and analyses
Data analysis was performed using SPSS 21.0 software (SPSS, Chicago, IL). Data are presented as mean (SD) or median (interquartile range [IQR]). Independent continuous variables were compared using the Student t test or the Mann-Whitney test. Categorical variables were compared using the χ2 test. Correlations between continuous variables were performed using the Pearson or Spearman tests. Differences between independent groups were assessed by ANOVA.
DN
For assessment of whether OSA status, OSA severity, or hypoxemia measures are independently associated with DN, multiple logistic regression (forced entry) was used. Multiple linear regression (forced entry) was used to assess independent associations of continuous variables. Proportion of variation explained by models (R2) plus statistical significance and effect size of OSA, OSA severity, and hypoxia measures are reported. Variables included in both the logistic and linear regression models were based on known outcome-related risk factors or variables that differed between patients with and without OSA.
eGFR progression
For assessment of the impact of OSA on eGFR progression, only patients who had eGFR levels measured at baseline and study end were used. Baseline differences were accounted for by using linear regression with eGFR at study end and eGFR change from baseline as the outcome measures and OSA, baseline eGFR, and other confounders as the predictors. A repeat analysis assuming that baseline eGFR had not changed in those with missing eGFR at study end was performed. For assessment of progression of albuminuria, only patients with normal ACR at baseline were included and logistic regression was used.
Residuals and collinearity were considered in assessing fit of models to data. Sequentially removing variables involved in multicollinearity had limited impact on models estimates for the main exposure. Hence, final models presented include variables based on the known outcome-related risk factors, possible confounders, or variables that differed between patients with and without OSA, regardless of the presence of collinearity. Removing variables in order to minimize collinearity had little impact on the regression models.
Matched-groups analysis
For further exploration of the impact of baseline differences on the associations observed, a subgroup of 71 patients with and 59 without OSA were group matched for key DN risk factors. A P value < 0.05 was considered significant in all statistical testing.
RESULTS
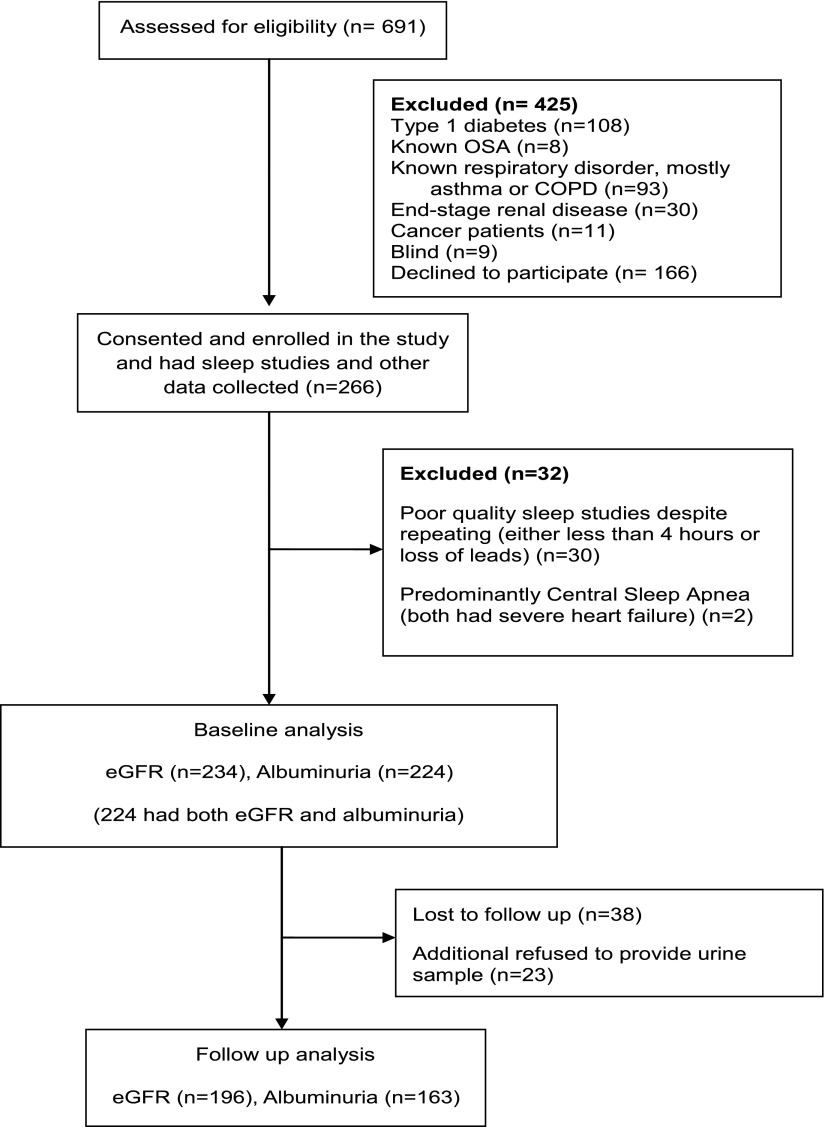
A total of 266 patients consented for the project; data regarding DN (both eGFR and albuminuria) at baseline were available in 224 patients. Baseline and study-end eGFR measurements were available in 196 patients. Please see Fig. 1 for details.
Figure 1.
CONSORT diagram for study participants. COPD, chronic obstructive pulmonary disease.
Baseline data
The prevalence of OSA was 64.3% (144 of 224), with 38.4% (86 of 224) mild and 25.9% (58 of 224) moderate to severe. The prevalence of DN was 40.2% (90 of 224), with 33.0% (74 of 224) of patients exhibiting albuminuria, and 10.3% (23 of 224) was macroalbuminuria. The eGFR was ≥90, 60–89, 30–59, 15–29, and <15 mL/min/1.73 m2 in 45.5% (102 of 224), 37.9% (85 of 224), 15.2% (32 of 224), 1.3% (3 of 224), and 0% (0 of 224) of patients, respectively.
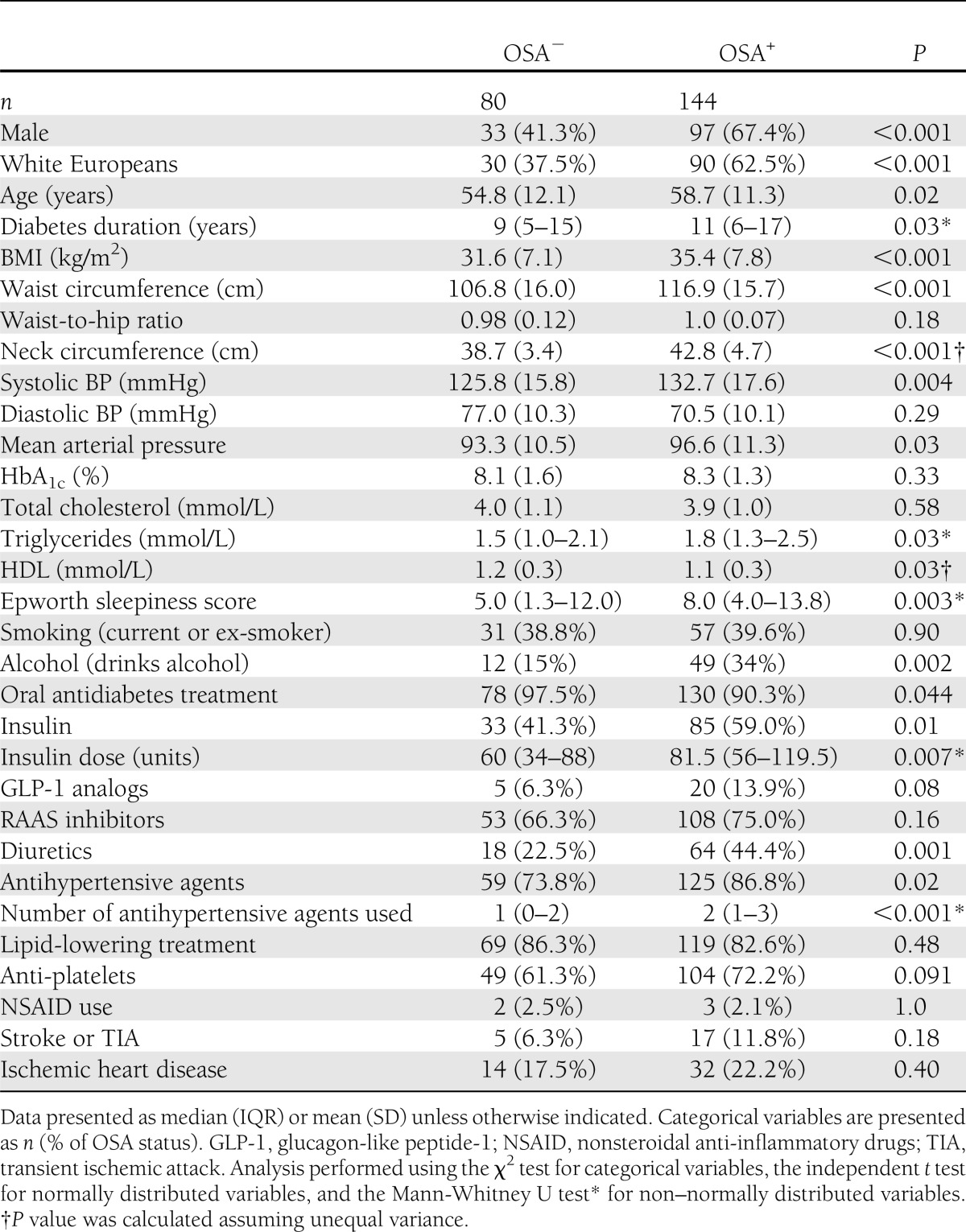
Baseline patients’ characteristics are summarized in Table 1. As expected, patients with OSA were older, heavier, and had a longer duration of diabetes and higher systolic BP, which required more antihypertensive medications. Patients with and without OSA had similar glycemic control and total cholesterol, as well as similar use of RAAS inhibitors.
Table 1.
Participant characteristics in relation to OSA status
DN is more common in patients with OSA.
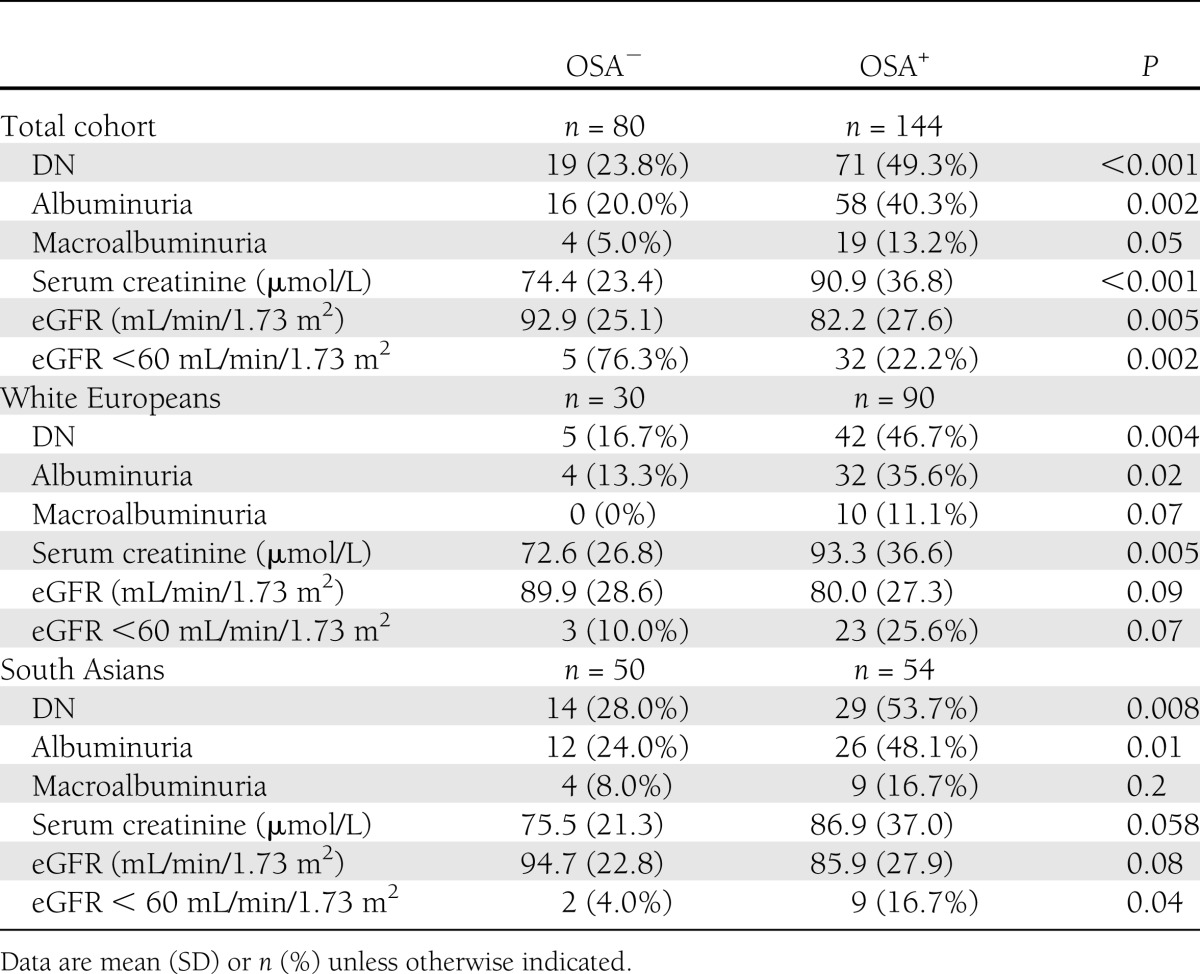
Patients with OSA had a higher prevalence of DN, serum creatinine, albuminuria, and macroalbuminuria and lower eGFR levels regardless of ethnicity (Table 2).
Table 2.
Relationship between OSA and DN in patients with type 2 diabetes in the total study population and in ethnicity subgroups
OSA is independently associated with DN: multivariable analysis.
In multiple logistic regression, OSA was independently associated with DN despite adjustments for potentially confounding variables (Table 3). Other independent associations with DN included age, diabetes duration, HbA1c, and triglycerides. The relationship between OSA severity and hypoxemia measures is summarized in the Supplementary Data.
Table 3.
Assessing the impact of possible confounders on the association between OSA and DN using logistic regression models
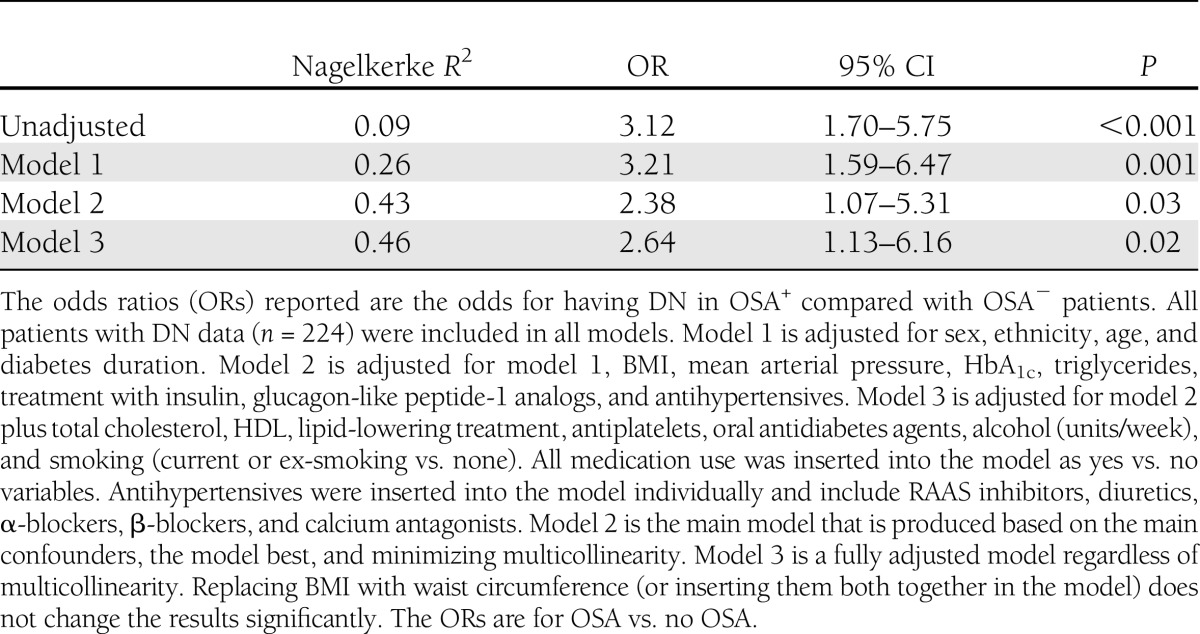
Longitudinal analysis
As indicated in Fig. 1, at study end eGFR data were available on 196 (124 [63.3%] with and 72 [36.7%] without OSA) patients and albuminuria data on 163 (105 [64.4%] with and 58 [35.6%] without OSA) patients. The follow-up analysis was based only on patients in whom both baseline and follow-up data were available, which comprised a total of 196 patients for eGFR progression, 163 for albuminuria progression, and 169 for DN progression. The average length of follow-up was 2.5 (0.7) years. There was no difference in the follow-up duration between patients with and without OSA (2.5 [0.6] vs. 2.4 [0.7], P = 0.51). Comparing patients who had study end point data with those who were lost to follow-up showed that the two groups were largely comparable.
OSA and the AHI are associated with a greater decline in eGFR.
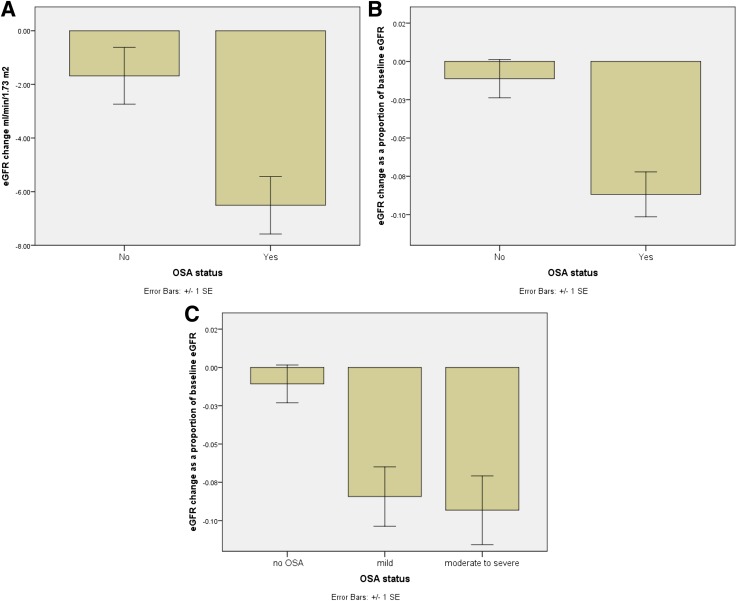
eGFR declined from baseline to the study end in patients with OSA (81.7 [27.8] vs. 75.2 [28.8] mL/min/1.73 m2 , P < 0.001) and without OSA (90.9 [23.2] vs. 89.2 [21.9] mL/min/1.73 m2, P = 0.1), but eGFR change was only significant in patients with OSA. The change in eGFR over the follow-up period was larger in the OSA group (−5.0 [−14 to 1.8] vs. −1.5 [−8.5 to 4.0] mL/min/1.73 m2, P = 0.006). Calculating the change in eGFR as a percentage of baseline eGFR showed a greater decline in the OSA group (−6.8% [−16.1 to 2.2%] vs. −1.6% [−7.7 to 5.3], P = 0.002) in a stepwise manner (−1.4% [−7.7 to 5.2] vs. −5.3% [−16.5 to 2.7] vs. −8.7% [−16.1 to 2.0], P = 0.003, for no OSA vs. mild vs. moderate to severe OSA) (Fig. 2). Rapid eGFR decline, defined as 4% decline of eGFR per year (18), was more common in patients with OSA compared with those without OSA (39.5% (n = 49) vs. 20.8% (n = 15), P = 0.007, respectively).
Figure 2.
The longitudinal impact of OSA on eGFR in patients with type 2 diabetes presented as eGFR change from baseline (A) and percentage of baseline eGFR (B and C). Data presented as mean and SEM.
In a linear regression model, baseline eGFR (R2 = 0.84, adjusted R2 = 0.84) was a predictor of study-end eGFR (B = 0.94, P < 0.001). After addition of OSA, age at diagnosis, diabetes duration, ethnicity, sex, BMI, mean arterial pressure, antihypertensive agent use, HbA1c, insulin use, oral antidiabetic agent use, total cholesterol, triglycerides, lipid-lowering therapy, antiplatelet use, and smoking to the model (R2 = 0.86, adjusted R2 = 0.85), OSA remained an independent predictor of study-end eGFR (B = −3.8, P = 0.04). The only other independent predictors were diabetes duration and baseline eGFR. After we replaced BMI with waist circumference (R2 = 0.86, adjusted R2 = 0.85), OSA remained independently associated with study-end eGFR (B = −4.2, P = 0.03).
Replacing OSA with AHI as a marker of OSA severity in the above model demonstrated that baseline AHI was an independent predictor of study-end eGFR (B = −4.6, P = 0.02). Nocturnal hypoxemia measures were not independent predictors of study-end eGFR after adjustment.
Further analysis was performed by replacing the missing study-end eGFR measurements with the baseline eGFR measurements (giving a sample size of 234) and showed that baseline OSA (R2 = 0.88, adjusted R2 = 0.87) (B = −3.89, P = 0.02) and AHI (B = −3.45, P = 0.04) remained independent predictors of study-end eGFR.
The use of eGFR change instead of study-end eGFR as the outcome measure in the above-described regression models confirmed that OSA was an independent predictor of eGFR decline (Supplementary Data). For the impact of OSA on the development of albuminuria, see the Supplementary Data.
Matched group analysis.
We aimed to minimize the clinical differences between patients with and without OSA by re-examining our findings in a subgroup of patients who were group matched for key DN risk factors (Supplementary Table 1). Despite matching for important baseline differences, as predicted by the statistical adjustments, DN prevalence remained significantly higher in patients with compared with those without OSA (46.5 vs. 28.8%, P = 0.039). The change in eGFR in absolute terms (median −5.0 [IQR −14.0 to 1.0] vs. −2.0 [−7.0 to 5.0] mL/min/1.73 m2, P = 0.02) and as percent of baseline eGFR (−5% [−20 to 2%] vs. −2% [−8 to 6%], P = 0.016) was greater in the OSA group compared with those without OSA.
CPAP treatment and the longitudinal analysis.
Patients who were CPAP compliant had a slower eGFR decline than noncompliant patients (Supplementary Data).
Serum nitrotyrosine: an independent predictor of study-end eGFR.
Serum nitrotyrosine levels were available from 87 patients who had measurements of both baseline and study-end eGFR. There were no significant differences between patients who agreed and those who declined to provide serum samples (6). Serum nitrotyrosine levels were higher in patients with DN at baseline (n = 38) compared with those without (n = 45) (median 23.3 [IQR 17.7–34.8] vs. 20.8 [12.0–29.6] nmol/L, P = 0.04). There was also a modest correlation between serum nitrotyrosine abundance and baseline eGFR (r = −0.24, P = 0.03) as well as study-end eGFR (r = −0.26, P = 0.02). Serum nitrotyrosine correlated with the eGFR change when presented as percentage of baseline eGFR (r = −0.36, P = 0.001). Serum nitrotyrosine was nonsignificantly higher in patients with OSA compared with those without (22.8 [17.2–33.6] vs. 15.5 [11.8–26.5] nmol/L, P = 0.058).
For assessment of the relationship between serum nitrotyrosine and eGFR progression, multiple linear regression was used with study-end eGFR as the outcome measure and baseline eGFR and serum nitrotyrosine as the predictors (R2=0.84, adjusted R2=0.84). With use of this model, serum nitrotyrosine was an independent predictor of study-end eGFR (B = −0.25, P = 0.001). After addition of age at diabetes diagnosis, diabetes duration, sex, ethnicity, HbA1c, total cholesterol, triglycerides, mean arterial pressure, BMI, smoking, insulin use, antihypertensive use, and lipid-lowering therapy to the model (R2 = 0.87, adjusted R2 = 0.85), serum nitrotyrosine remained an independent predictor of study-end eGFR (B = −0.20, P = 0.02). Other independent predictors included baseline eGFR and diabetes duration. Interestingly, adding OSA to the same linear regression model (R2 = 0.88, adjusted R2 = 0.85) showed that serum nitrotyrosine remained an independent predictor of study-end eGFR (B = −0.20, P = 0.017), but OSA was not (B = −3.7, P = 0.23).
CONCLUSIONS
Our aim was to assess the relationship between OSA and DN and to study the impact of OSA on DN progression in patients with type 2 diabetes. We demonstrated a robust association between OSA and DN, which persisted after adjustment for a wide range of demographic and clinically relevant confounders. Importantly, baseline OSA status and AHI were independent predictors of future eGFR and eGFR change over an average of 2.5 years of follow-up. Serum nitrotyrosine abundance emerged as an independent predictor of study-end eGFR as well as the change of eGFR over the time course of the study despite adjustment for multiple confounders, including OSA. We have also identified a high prevalence of undiagnosed OSA in patients with type 2 diabetes.
The study sample was drawn from patients attending secondary care units for diabetes in the U.K. The participant characteristics were similar to those reported previously from a different region in the U.K. (19), suggesting that the current study sample was representative of the wider type 2 diabetic population in secondary care. However, whether our findings are applicable to patients typically managed in primary care and those with a shorter duration of diabetes remains to be examined. OSA prevalence in our sample is also consistent with other reports (5,20). Similarly, the prevalence of DN and albuminuria is consistent with that reported by others in patients with type 2 diabetes (12). It is important to note that patients with ESRD were excluded from our study. Patients with ESRD are well known to have a high prevalence of OSA (30–80%) (21); hence, our results are novel and important, as they apply to patients at an earlier stage of renal dysfunction.
As expected, demographic and metabolic factors differed between patients with and without OSA. Nevertheless, although these differences contributed to the observed relationship between OSA and DN, OSA remained independently associated with DN even after adjustment. Furthermore, OSA remained an independent predictor of study-end eGFR and eGFR change over the follow-up period after adjustment for a wide range of confounders. However, much of the variation of study-end eGFR is explained by baseline eGFR, which is consistent with previous studies (22). The matched group analysis also supported the findings of the multivariable analysis.
This is the first study to assess the impact of OSA on DN in patients with type 2 diabetes. Recently, some researchers postulated that sleep disorders (including OSA) might be a modifiable risk factor for the development and progression of chronic kidney disease, but to date there are little data to support such a link (23). The available evidence for a relationship between OSA and chronic kidney disease is generally conflicting and obtained from cross-sectional studies not specifically targeting patients with diabetes (24–31). In a study of 505 men, there was no association between eGFR and OSA after adjustment (24,25). In a cross-sectional study of 91 obese adults, there was no association between albuminuria and OSA, but AHI was independently associated with serum creatinine after adjustment (26). In another study of 496 adults, AHI was independently associated with albuminuria after adjustment (27), while other studies failed to identify such a relationship (28,29). For assessment of the issue of hypertension as a possible confounder for the effects of OSA on renal function, a cross-sectional study of 62 untreated hypertensive patients with OSA matched to 70 hypertensive patients without OSA found that albuminuria was 57% greater in patients with OSA versus those without and that albuminuria correlated with AHI and OSA severity (30).
Interestingly, our data demonstrated that even mild OSA (based on AHI) is associated with DN and worsening eGFR prospectively. We postulate that this may reflect the impact of intermittent hypoxemia, which is exaggerated in the presence of vulnerable tissue damaged because of chronic hyperglycemia. This finding is important for future interventional studies; as current guidelines do not recommend treating patients with mild OSA.
Although our data demonstrate an independent association between OSA and DN and that OSA is an independent predictor of worsening eGFR, OSA was not a predictor of the development of DN or albuminuria. This could be explained by the relatively short duration of follow-up or the small sample size of the subgroup that did not have DN (and/or albuminuria) at baseline. Another possible explanation is that OSA may not necessarily result in the development of DN, but once DN is present, OSA might contribute to a rapid progression and a decline in renal function. Furthermore, eGFR decline can be the only manifestation of DN progression in patients with diabetes without any evidence of albuminuria in up to 30% of cases (14,32).
There are several mechanisms that might explain the association between OSA and DN and the impact of OSA on eGFR. We have previously shown that OSA is associated with increased oxidative and nitrosative stress and impaired microvascular and endothelial regulation in patients with type 2 diabetes (6). Studies in patients with OSA but without diabetes have shown that OSA and/or intermittent hypoxia are associated with increased advanced glycation end products (33), altered protein kinase C signaling (34), decreased endothelial nitric oxide synthase and increased endothelin-1 levels (35), hypercoagulability (increased plasminogen activator inhibitor-1) (36), and increased inflammation (37). The repetitive episodes of reoxygenation after hypoxemia in OSA patients simulate ischemia-reperfusion injury, which results in the generation of reactive oxygen species (37). We have previously shown that OSA, AHI, and nocturnal hypoxemia are independently associated with serum nitrotyrosine abundance after adjustment for confounders in patients with type 2 diabetes (6). Our results give possible mechanistic insights into the pathophysiology of DN, since serum nitrotyrosine abundance emerged as an independent predictor of study-end eGFR despite adjustment for many possible confounders, suggesting that nitrosative stress is important in the progression of eGFR regardless of the cause of nitrosative stress (whether it was OSA, hyperglycemia, or hypertension). Our data are consistent with reports indicating an association of nitrotyrosine with DN in rodent models (38) and cell culture systems (39) and the demonstration of increased nitrotyrosine staining in the renal proximal tubules and thin limb of the loop of Henle in patients with DN (17). Furthermore, amelioration of nitrosative stress has been associated with improvement in DN in diabetic rodent studies (15,16). Interestingly, serum nitrotyrosine was a predictor of study-end eGFR even when OSA was included in the model. It is therefore tempting to speculate that nitrosative stress could mediate the relationship between OSA and eGFR, but our sample size is too small to confidentially draw such a conclusion and it seems likely that OSA might influence eGFR decline via multiple mechanisms such as those described above.
The impact of CPAP treatment was not in the scope of this study. Interestingly, in our cohort, in patients with OSA, the decline in eGFR was smaller in the CPAP-compliant group than those who were noncompliant with CPAP. Additionally, a larger proportion of patients in the noncompliant CPAP group developed albuminuria during follow-up. However, it is difficult to assess CPAP efficacy from observational data owing to the small numbers of patients who were compliant with CPAP treatment. Nonetheless, these data provide further justification for assessing the impact that CPAP can have on the development and progression of DN but also highlight the challenges of CPAP compliance in patient with type 2 diabetes.
Our study has several strengths and limitations. Use of home-based portable multichannel respiratory devices rather than inpatient overnight polysomnography may be considered a limitation, but this approach is well established and validated (40). We used a single random measurement of urine albumin to assess albuminuria rather than three measurements; again, this approach has been used previously by many other investigators (12,13), and recent data suggest that single urinary albumin measurements are accurate in predicting nephropathy and appropriate for epidemiological studies (13). The cross-sectional analysis of the relationship between OSA and DN makes it difficult to ascertain causality, but the longitudinal analysis of the impact of OSA on eGFR decline suggests a cause-effect relationship, although this needs to be tested in prospective interventional studies. The missing follow-up data, particularly for albuminuria, is a weakness and potential source for bias; however, no differences in characteristics of participants versus patients lost to follow-up were observed. A strength of our study was the well-characterized population, with measurement of a wide range of demographic and clinical variables, which allowed adjustment for a wide range of potential confounders. Furthermore, the study included patients of both sexes and South Asian and white European ethnicity and was prospective, which allowed the assessment of the impact of OSA on eGFR decline. In addition, we focused on patients relatively early in the course of DN; this population would be a prime target for treatments that might slow the progression toward ESRD.
OSA is very common in patients with type 2 diabetes attending secondary care units in the U.K. Patients with OSA and type 2 diabetes are more likely to have DN compared with those with type 2 diabetes but without OSA despite adjustment for a wide range of confounders. Renal function (assessed by eGFR) declined faster in patients with OSA and type 2 diabetes compared with those with type 2 diabetes alone. The eGFR decline in patients with OSA was very clinically meaningful, as more patients in the OSA group had a rapid decline in eGFR (≥10% over 2.5 years), and indeed, 25% of patients with OSA exhibited an eGFR decline >16%. Nitrosative stress may provide an important pathogenetic link between OSA, type 2 diabetes, and DN. This study could form the basis for interventional studies to examine the impact of OSA treatment on the development and progression of DN in patients with type 2 diabetes.
Supplementary Material
Acknowledgments
This project was partially funded by the National Institute for Health Research (U.K.). A.A.T. was a research training fellow supported by the National Institute for Health Research.
This project was also funded by the U.K. Novo Nordisk Research Foundation and Sanofi-Aventis. No other potential conflicts of interest relevant to this article were reported.
A.A.T. conceived and designed the study, contributed to analysis and interpretation, wrote the first draft of the manuscript, and gave final approval of the manuscript. A.A. designed the study, reviewed the draft of the manuscript, and gave final approval of the manuscript. N.T.R. performed statistical analysis and interpretation, reviewed the draft of the manuscript, and gave final approval of the manuscript. S.B., K.D., Q.-A.A., M.K.P., and A.H.B. designed the study, reviewed the draft of the manuscript, and gave final approval of the manuscript. M.J.S. conceived and designed the study, contributed to interpretation, and gave final approval of the manuscript. A.A.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors acknowledge Dr. Fahmy Hanna, Helen Hodgson, and Rebecca Barakam from the University Hospital of North Staffordshire for their help with recruitment.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0450/-/DC1.
The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
References
- 1.Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract 2005;68(Suppl. 2):S3–S14 [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc 2011;86:444–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 2008;4:444–452 [DOI] [PubMed] [Google Scholar]
- 4.Afghahi H, Cederholm J, Eliasson B, et al. Risk factors for the development of albuminuria and renal impairment in type 2 diabetes—the Swedish National Diabetes Register (NDR). Nephrol Dial Transplant 2011;26:1236–1243 [DOI] [PubMed] [Google Scholar]
- 5.Foster GD, Sanders MH, Millman R, et al. Sleep AHEAD Research Group Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med 2012;186:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL, American Academy of Sleep Medicine, 2007 [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–276 [PMC free article] [PubMed] [Google Scholar]
- 9.Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest 2009;135:704–709 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 11.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006;69:2057–2063 [DOI] [PubMed] [Google Scholar]
- 13.Pugliese G, Solini A, Fondelli C, et al. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transplant 2011;26:3950–3954 [DOI] [PubMed] [Google Scholar]
- 14.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YJ, Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther 2008;324:658–663 [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues AM, Bergamaschi CT, Araújo RC, Mouro MG, Rosa TS, Higa EM. Effects of training and nitric oxide on diabetic nephropathy progression in type I diabetic rats. Exp Biol Med (Maywood) 2011;236:1180–1187 [DOI] [PubMed] [Google Scholar]
- 17.Thuraisingham RC, Nott CA, Dodd SM, Yaqoob MM. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int 2000;57:1968–1972 [DOI] [PubMed] [Google Scholar]
- 18.Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012;7:401–408 [DOI] [PubMed] [Google Scholar]
- 19.Abbott CA, Chaturvedi N, Malik RA, et al. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care 2010;33:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract 2007;13:355–362 [DOI] [PubMed] [Google Scholar]
- 21.Unruh ML, Sanders MH, Redline S, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol 2006;17:3503–3509 [DOI] [PubMed] [Google Scholar]
- 22.Skupien J, Warram JH, Smiles AM, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 2012;82:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 2012;60:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canales MT, Taylor BC, Ishani A, et al. Osteoporotic Fractures in Men (MrOS) Study Group Reduced renal function and sleep-disordered breathing in community-dwelling elderly men. Sleep Med 2008;9:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canales MT, Lui LY, Taylor BC, et al. Osteoporotic Fractures in Men (MrOS) Study Group Renal function and sleep-disordered breathing in older men. Nephrol Dial Transplant 2008;23:3908–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal V, Vanhecke TE, Rai B, Franklin BA, Sangal RB, McCullough PA. Albuminuria and renal function in obese adults evaluated for obstructive sleep apnea. Nephron Clin Pract 2009;113:c140–c147 [DOI] [PubMed] [Google Scholar]
- 27.Faulx MD, Storfer-Isser A, Kirchner HL, Jenny NS, Tracy RP, Redline S. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep 2007;30:923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casserly LF, Chow N, Ali S, Gottlieb DJ, Epstein LJ, Kaufman JS. Proteinuria in obstructive sleep apnea. Kidney Int 2001;60:1484–1489 [DOI] [PubMed] [Google Scholar]
- 29.Mello P, Franger M, Boujaoude Z, et al. Night and day proteinuria in patients with sleep apnea. Am J Kidney Dis 2004;44:636–641 [PubMed] [Google Scholar]
- 30.Tsioufis C, Thomopoulos C, Dimitriadis K, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis 2008;52:285–293 [DOI] [PubMed] [Google Scholar]
- 31.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung 2009;187:17–22 [DOI] [PubMed] [Google Scholar]
- 32.Kramer HJNQ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 33.Tan KC, Chow WS, Lam JC, et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 34.Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol 2008;294:H920–H927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation 2010;121:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rångemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep 1995;18:188–194 [DOI] [PubMed] [Google Scholar]
- 37.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 2009;32:447–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii H, Kono K, Nakai K, et al. Oxidative and nitrosative stress and progression of diabetic nephropathy in type 2 diabetes. Am J Nephrol 2010;31:342–352 [DOI] [PubMed] [Google Scholar]
- 39.Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med 2006;40:1454–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep 1997;20:1077–1085 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.