Abstract
OBJECTIVE
Evidence supporting an association between complement (C) and type 1 diabetes (T1D) includes the identification of C-fixing islet cell autoantibodies in T1D sera and genetic associations with the major histocompatibility complex III C4 region on chromosome 6. Therefore, we investigated whether C activation was present in pancreata from those with or at increased risk (positive for T1D associated autoantibodies) for T1D.
RESEARCH DESIGN AND METHODS
Immunohistochemical techniques were used to measure the C degradation product C4d in organ donor pancreata from patients with T1D and type 2 diabetes and autoantibody-positive and autoantibody-negative subjects.
RESULTS
Median C4d antigen density differed across the groups (P < 0.0001) and was highest in patients with T1D. C4d immunostaining localized to the blood vessel endothelium and extracellular matrix surrounding blood vessels and exocrine ducts. Receiver operating characteristic analysis resulted in 81.8% sensitivity and 94.4% specificity for C4d staining.
CONCLUSIONS
These data suggest that C activation is occurring within pancreata from patients with T1D and C4d may be a biomarker for T1D.
A potential role for antibody-mediated complement (C) activation in the pathogenesis of type 1 diabetes (T1D) has been suggested from genetic (association with major histocompatibility complex III C4 region on chromosome 6) and immunological (presence of C-fixing islet autoantibodies in T1D sera) studies (1–4). Additional efforts support a role for C in the pathogenesis of complications (5,6). These data, along with the rarity of observed insulitis in humans, suggest the possibility of an alternative explanation of β-cell destruction; namely, that islet autoantibodies, present before disease onset, may fix C on the surface of β-cells and promote their lysis. Therefore, we quantified C4d, a marker of antibody-mediated C activation, in pancreata from individuals with T1D (including long-standing disease) and type 2 diabetes (T2D), as well as autoantibody-positive and autoantibody-negative nondiabetic subjects (7–9).
RESEARCH DESIGN AND METHODS
Human pancreata from cadaveric organ donors obtained from the Network for Pancreatic Organ Donors with Diabetes program were analyzed, including those with T1D (n = 11), those diabetes free with T1D-associated islet autoantibodies (AA+) (n = 16), autoantibody-negative control subjects (AA−) (n = 11), or those with T2D (n = 7) (10). The mean ± SE ages of the T1D, T2D, AA+, and AA− groups were 22.1 ± 2.2 years (range 10.7–37.0), 54.0 ± 4.6 years (39.3–74.0), 32.2 ± 4.2 years (2.2–69.2), and 32.1 ± 5.5 years (9.0–68.0), respectively. BMI was highest in T2D patients (35.5 ± 2.2 kg/m2) compared with T1D (24.2 ± 1.3), AA+ (24.4 ± 1.5), and AA− (25.7 ± 1.7) subjects. Caucasians comprised the majority of patients who had T1D (73%), were AA+ (69%), and were AA− (90%). Of T2D patients, only 43% were Caucasian, with the remainder being black (29%), Hispanic (14%), and Asian (14%). There were no differences in age and BMI between T1D patients and either the AA− or the AA+ group. As expected, islet autoantibodies negatively correlated with duration of disease within the T1D group. (r = −0.75, P < 0.05).
One or more islet autoantibodies (GAD 65 [GADA], insulin [IAA], insulinoma associated protein 2 [IA2], zinc transporter 8 [ZnT8]) were present in 23 subjects (16 without and 7 of 9 with T1D). No sera were available in two T1D patients. Three T1D patients were positive for a single autoantibody (n = 2 mIAA; n = 1 GADA), three had two autoantibodies (n = 2 IA2 and mIAA; n = 1 IA2 and ZnT8), and one had three autoantibodies (GADA, IA2, and ZnT8). Among AA+ subjects without diabetes, 12 were positive for a single autoantibody (n = 11 GADA; n = 1 ZnT8) and 4 were positive for two autoantibodies (n = 1 GADA and IA2, n = 2 GADA and mIAA, and n = 1 IA2 and ZnT8). All research procedures were approved by the University of Florida Institutional Review Board.
C4d analysis
Frozen pancreatic sections were analyzed using an anti-human C4d monoclonal antibody (clone 10–11; AbD Serotec, Raleigh, NC) according to manufacturer recommendations, with visualization using a DAB detection kit (Ventana Medical Systems, Tucson, AZ). Whole-section digital images were analyzed using ImageScope software (Aperio Technologies, Vista, CA). A positive pixel count (total positive pixels plus total negative pixels/total pixels) from sections incubated with negative control antisera was subtracted from the positive pixel count from sections incubated with anti-C4d antisera to provide a fraction (converted to %).
Statistical analysis
Potential group demographic differences were analyzed using one-way ANOVA with Bonferroni corrections. For nonparametric analyses, the Spearman rank correlation test was used, whereas Pearson testing was used for correlation assessment. Receiver operating curve (ROC) analysis was used to establish sensitivity and specificity, whereas mean values were analyzed using a Mann-Whitney test (nonparametric).
RESULTS
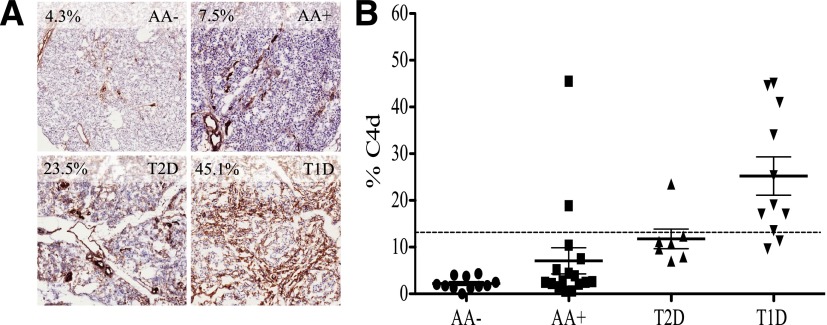
In all groups, C4d immunostaining was largely localized to the blood vessel endothelium and extracellular matrix surrounding blood vessels and exocrine ducts depending on vessel size (Fig. 1A and ref. 11 [see ref. 11 for login information]). C4d staining was detected on the endothelium of very small blood vessels/capillaries. On larger blood vessels, C4d staining localized to both the endothelium and extracellular matrix/adventitia, as well as extracellular matrix surrounding ductal structures.
Figure 1.
A: C4d immunostaining in representative tissues from T1D, T2D, and AA+ patients and AA− control subjects. Percentages in the upper left corner of each panel refer to the C4d antigen density. B: Comparison of C4d antigen density between groups. Clear differences between groups were present; P < 0.0001, ANOVA. T1D patients had higher C4d antigen density than both control subjects and nondiabetic AA+ subjects (P < 0.0001). Dotted line, ROC C4d cutoff of 12.95% (area under the curve 0.94, P < 0.0001). This cutoff yielded a sensitivity of 81.8% and specificity of 94.4% in distinguishing T1D patients.
Mean C4d antigen density differed between the three groups (P < 0.0001). C4d antigen expression was more prevalent in pancreata from patients with T1D (mean ± SEM 25.2 ± 4.1%) than from subjects without diabetes (2.2 ± 0.4%, P < 0.0001) or no diabetes yet AA+ (7.1 ± 2.8%, P < 0.0001) (Fig. 1B). C4d density did not differ between T2D, AA+, and AA− individuals. When comparing control subjects and T2D patients with T1D subjects, ROC analysis of C4d density yielded a sensitivity of 81.8% and specificity of 94.4% at a cutoff of 12.95% (Fig. 1B) (area under the curve 0.94, P < 0.0001), indicating that pancreatic C4d immunostaining can distinguish autoimmune T1D from nonautoimmune diabetes. These data were confirmed in blinded fashion by an expert pathologist. When converting percent C4d pixels into a rank order, C4d deposition was significantly correlated with computer-aided image analysis (ρ= 0.86, P < 0.001).
The mean C4d deposition among T1D patients with histological evidence of insulitis (17.8 ± 4.3%, n = 5), defined as six or more CD3+ cells adjacent to or within an islet, tended to be lower (P = 0.08) than in patients without insulitis (31.4 ± 5.7%, n = 6). In contrast to T1D patients with insulitis, among the AA+ subjects without diabetes the only subject with histological evidence of insulitis (Network for Pancreatic Organ Donors with Diabetes identification no. 6197) had higher C4d density (45.5%). This subject was positive for GADA and IA2. Islet autoantibody positivity did not correlate with C4d antigen density (P = NS). Finally, no correlations were found between C4d density and either cause of death or cardiac downtime prior to or during terminal hospital stay (data not shown).
CONCLUSIONS
In this unique collection of pancreatic tissue from organ donors with long-standing T1D and AA+ and AA− control subjects, we directly investigated C activation. We showed that C activation was present in pancreatic tissue from patients with T1D and thus may be a novel immunohistochemical biomarker for T1D. Furthermore, increased pancreatic C4d deposition appears to distinguish T1D from T2D patients and nondiabetic control subjects. Whether the increased C deposition is directly or indirectly involved in the pathogenic mechanisms leading to T1D cannot be ascertained from this study. The elevated C4d density in one AA+ case that also presented with insulitis might suggest that C4d expression could be related to immune activation and beginning of β-cell destruction. Hyperglycemia and poor control may lead to enhanced C activation. Indeed, recent reports have suggested that serum peptides corresponding to C activation are increased in both humans with diabetes and animal models (12,13) and may be involved in both vascular (5,6) and renal complications (14) associated with diabetes. Complement activation products could not be measured in our study because serum was not collected in a manner that would avoid artifactual C activation. In addition, HbA1c measurements were not obtained in sufficient numbers of patients to test this hypothesis. Future studies will investigate C4d localization (i.e., vascular, exocrine, endocrine) and will determine whether C4d colocalizes with downstream, more final, effectors of the C cascade such as components of the membrane attack complex.
Acknowledgments
This work was supported by grants from the JDRF and the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
P.R. and C.W. designed and performed the experiments and wrote the manuscript. B.C. and M.C.-T. performed experiments and provided critical manuscript review. A.P., M.A., and D.S. provided experimental design and discussion input, as well as critical manuscript review. D.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
P.R. and C.W. contributed equally to this study.
References
- 1.Hägglöf B, Holmgren G, Holmlund G, Lindblom B, Olaisen B, Teisberg P. Studies of HLA, factor B (Bf), complement C2 and C4 haplotypes in type 1 diabetic and control families from northern Sweden. Hum Hered 1986;36:201–212 [DOI] [PubMed] [Google Scholar]
- 2.Bottazzo GF, Dean BM, Gorsuch AN, Cudworth AG, Doniach D. Complement-fixing islet-cell antibodies in type-I diabetes: possible monitors of active beta-cell damage. Lancet 1980;1:668–672 [PubMed] [Google Scholar]
- 3.Mustonen A, Knip M, Huttunen NP, Puukka R, Käär ML, Akerblom HK. Evidence of delayed beta-cell destruction in type 1 (insulin-dependent) diabetic patients with persisting complement-fixing cytoplasmic islet cell antibodies. Diabetologia 1984;27:421–426 [DOI] [PubMed] [Google Scholar]
- 4.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 [DOI] [PubMed] [Google Scholar]
- 5.Acosta J, Hettinga J, Flückiger R, et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci USA 2000;97:5450–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen TK, Tarnow L, Thiel S, et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 2004;53:1570–1576 [DOI] [PubMed] [Google Scholar]
- 7.Jen K-Y, Nguyen TB, Vincenti FG, Laszik ZG. C4d/CD34 double-immunofluorescence staining of renal allograft biopsies for assessing peritubular capillary C4d positivity. Mod Pathol 2012;25:434–438 [DOI] [PubMed] [Google Scholar]
- 8.Fedrigo M, Gambino A, Tona F, et al. Can C4d immunostaining on endomyocardial biopsies be considered a prognostic biomarker in heart transplant recipients? Transplantation 2010;90:791–798 [DOI] [PubMed] [Google Scholar]
- 9.Rangel EB, Malheiros DMAC, de Castro MCR, et al. Antibody-mediated rejection (AMR) after pancreas and pancreas-kidney transplantation. Transpl Int 2010;23:602–610 [DOI] [PubMed] [Google Scholar]
- 10.Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spectrum. Version 11.1.1.760. Aperio Technologies, Inc. [Internet]. Available from http://ahc-path-aperio11.ad.ufl.edu Accessed 29 May 2013 (To log in: Guest Login→Cases→C4D→brain icon; representative immunostained sections from each group)
- 12.Zhang Q, Fillmore TL, Schepmoes AA, et al. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. J Exp Med 2013;210:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Østergaard JA, Bjerre M, RamachandraRao SP, et al. Mannan-binding lectin in diabetic kidney disease: the impact of mouse genetics in a type 1 diabetes model. Exp Diabetes Res 2012;2012:678381 [DOI] [PMC free article] [PubMed]
- 14.Falk RJ, Sisson SP, Dalmasso AP, Kim Y, Michael AF, Vernier RL. Ultrastructural localization of the membrane attack complex of complement in human renal tissues. Am J Kidney Dis 1987;9:121–128 [DOI] [PubMed] [Google Scholar]