Abstract
OBJECTIVE
To establish if corneal nerve loss, detected using in vivo corneal confocal microscopy (IVCCM), is symmetrical between right and left eyes and relates to the severity of diabetic neuropathy.
RESEARCH DESIGN AND METHODS
Patients (n = 111) with type 1 and type 2 diabetes and 47 age-matched healthy control subjects underwent detailed assessment and stratification into no (n = 50), mild (n = 26), moderate (n = 17), and severe (n = 18) neuropathy. IVCCM was performed in both eyes and corneal nerve fiber density (CNFD), branch density (CNBD), and fiber length (CNFL) and the tortuosity coefficient were quantified.
RESULTS
All corneal nerve parameters differed significantly between diabetic patients and control subjects and progressively worsened with increasing severity of neuropathy. The reduction in CNFD, CNBD, and CNFL was symmetrical in all groups except in patients with severe neuropathy.
CONCLUSIONS
IVCCM noninvasively detects corneal nerve loss, which relates to the severity of neuropathy, and is symmetrical, except in those with severe diabetic neuropathy.
Diabetic sensorimotor polyneuropathy (DSPN) is a length-dependent, symmetrical neuropathy with initial involvement of sensory and autonomic nerve fibers (NFs), followed by motor nerve involvement (1). It is the most common long-term complication of diabetes and is the main initiating factor for foot ulceration and lower extremity amputation with substantial associated healthcare costs (2). Conventional techniques of electrophysiology and quantitative sensory testing along with an assessment of neurological disability offer a relatively robust means of defining neuropathic severity (3) but have limitations in detecting the earliest stages of nerve damage (4,5).
In vivo corneal confocal microscopy (IVCCM) is a rapidly expanding technique to quantify the severity of neuropathy in DSPN (6). It has been used to demonstrate early nerve damage in diabetes and a range of other peripheral neuropathies (7,8) with good sensitivity and specificity (9). Recently, corneal nerve damage detected with IVCCM has been related to the level of previous glycemic exposure and blood pressure (10) and HbA1c even in healthy subjects (11). In a study of subjects with idiopathic small fiber neuropathy, corneal nerve damage was associated with higher serum triglycerides (8). It has also shown significant nerve regeneration before improvement in a range of established measures of neuropathy, including quantitative sensory testing, neurophysiology, and intraepidermal NF density, after simultaneous pancreas and kidney transplantation (12) and after an improvement in glycemia and cardiovascular risk factors for DSPN (13).
Corneal NF loss correlates with intraepidermal NF loss (4), and corneal NF length (CNFL), particularly, has shown superior discriminative capacity to diagnose DSPN (14). Recent studies show that quantification of corneal nerve morphology is highly reproducible and does not differ significantly between observers (15) and occasions (16) in subjects with diabetes and healthy individuals. As a functional correlate, corneal sensation has been found to decrease with increasing neuropathic severity (17).
Perkins et al. (18) and Bromberg and Jaros (19) have previously reported high interside symmetry of nerve conduction studies (NCS) consistent with the symmetrical nature of diabetic neuropathy. Whilst Petropoulos et al. (16) have shown that central corneal innervation is highly symmetrical between right eyes (REs) and left eyes (LEs) of young healthy subjects, it is unknown whether corneal nerve loss in diabetic neuropathy maintains its symmetry in different stages of DSPN. This is relevant to further establish parallels in terms of pathophysiology between corneal and peripheral somatic nerve damage but also has practical relevance when examining patients to allow examination of only one eye. The purpose of the present, cross-sectional, observational study was to establish if corneal nerve loss, detected using IVCCM, is symmetrical between REs and LEs with increasing severity of diabetic neuropathy.
RESEARCH DESIGN AND METHODS
Study subjects
Patients (n = 111) with diabetes and 47 age-matched control subjects were evaluated for the presence of DSPN based on the updated Toronto consensus criteria (20). This research adhered to the tenets of the Declaration of Helsinki and was approved by the North Manchester Research Ethics Committee. Informed written consent was obtained from all subjects prior to participation in the study. Participants were excluded if they had a positive history of malignant, connective tissue, or infectious disease, deficiency of vitamin B12 or folate, chronic renal failure, liver failure, active diabetic foot ulcers, or family history of peripheral neuropathy. Participants were also excluded if they had active ocular disease, systemic disease known to affect the cornea other than diabetes, or chronic corneal pathologies.
Clinical assessment and evaluation of peripheral neuropathy
All study participants underwent assessment of their clinical characteristics (BMI, HbA1c, lipid fractions, albumin-to-creatinine ratio [ACR], and estimated glomerular filtration rate [eGFR]) and detailed evaluation of signs of DSPN based on the simplified neuropathy disability score (NDS), vibration perception threshold (VPT), and NCS. The NDS, a scale of 0–10, was used to stratify the neuropathic severity of the study participants into none (0–2), mild (3–5), moderate (6–8), and severe (9 and 10), as described elsewhere (21). It is composed of Achilles tendon reflex testing (present [0], reduced [1], or absent [2]), temperature sensation (present [0] or absent [1]), pin-prick sensation (present [0] or absent [1]), and vibration perception scores of the great toe using a tuning fork (present [0] or absent [1]).
VPT was tested using a neurothesiometer (Horwell; Scientific Laboratory Supplies, Wilfrod, Nottingham, U.K.). Electrodiagnostic studies were undertaken using a Dantec Keypoint system (Dantec Dynamics, Ltd., Bristol, U.K.) equipped with a DISA temperature regulator to keep limb temperature constantly between 32 and 35°C. Peroneal motor and sural sensory nerves were assessed in the left lower limb (calf to ankle) to estimate sural sensory nerve amplitude (SSNamp), sural sensory nerve conduction velocity (SSNCV), peroneal motor nerve amplitude (PMNamp), and peroneal motor nerve conduction velocity (PMNCV) by a consultant neurophysiologist. The peroneal motor nerve study was performed using silver-silver chloride surface electrodes at standardized sites defined by anatomical landmarks, and recordings for the sural sensory nerve were taken using antidromic stimulation over a distance of 100 mm.
IVCCM and corneal sensation
All study subjects were scanned with a laser IVCCM (Heidelberg Retinal Tomograph III Rostock Cornea Module [HRT III RCM]; Heidelberg Engineering GmbH, Heidelberg, Germany) by a purpose-trained optometrist. This IVCCM uses a 670-nm wavelength helium neon diode laser, which is a class I laser and therefore does not pose any ocular safety hazard. A 63× objective lens with a numerical aperture of 0.9 and a working distance relative to the applanating cap (TomoCap; Heidelberg Engineering GmbH) of 0.0–3.0 mm was used. The size of each two-dimensional image produced was 384 × 384 μm, which has a 15° × 15° field of view and 10 μm/pixel transverse optical resolution. HRT III RCM uses an entirely digital image capture system, and all images are stored in an external hard drive. A drop of 0.4% benoxinate hydrochloride (Chauvin Pharmaceuticals, Chefaro, U.K.) was used to anesthetize each eye, and Viscotears (Carbomer 980, 0.2%; Novartis Pharmaceuticals, Surrey, U.K.) were used as the coupling agent between the cornea and the applanating cap. All subjects were asked to fixate on an outer fixation light throughout the IVCCM scan, and a charge-coupled device camera was used to image the cornea and correctly position the applanating cap onto the corneal apex. The overall examination took ∼5 min for both eyes of each subject, and in this study, two experienced examiners performed all IVCCM scans. All images were captured using the “section” mode in the Heidelberg Explorer of the HRT III RCM. There is no consensus on optimal IVCCM image sampling, but it has been proposed that any number between five and eight images will provide an acceptable level of accuracy to quantify the corneal subbasal nerve morphology (22). We selected and analyzed six high-clarity images per subject from the central subbasal nerve plexus captured by 1-μm intervals at the z-axis using the “section” mode. Criteria for image selection were depth, focus position, and contrast.
Corneal sensation
Corneal sensation was evaluated using a purpose-built noncontact corneal aesthesiometer (NCCA) (Anterior Eye Laboratory, Queensland University of Technology, Brisbane, Australia) as described elsewhere (17).
Image analysis
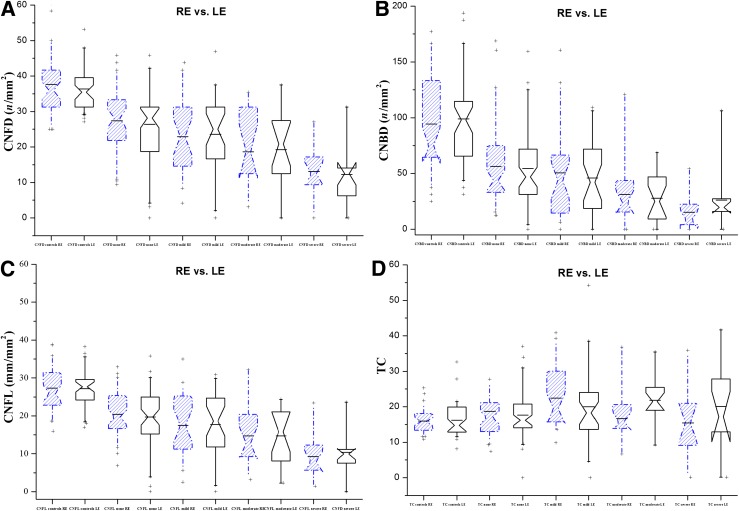
One examiner masked from the outcome of the medical and peripheral neuropathy assessment quantified the subbasal nerve morphology in 924 images of all study participants using semiautomated, purpose-written, proprietary software (CCMetrics; M.A. Dabbah, Imaging Science and Biomedical Engineering, University of Manchester, Manchester, UK). The specific parameters measured per frame were those we have previously established (9): corneal NF density (CNFD; n/mm2), corneal nerve branch density (CNBD; n/mm2), CNFL (mm/mm2), and tortuosity coefficient (TC) (23) (Fig. 1). CNFD is defined as the total number of main NFs per frame divided by the area of the frame in mm2 (area = 0.16033585 mm2) (Fig. 1). CNBD is defined as the total number of main nerve branches (nerve branches that stem from a NF) divided by the area of the frame. CNFL is the total length of NFs and nerve branches per frame. TC is a mathematical computation of the NF tortuosity as previously described by Kallinikos et al. (23), which is independent of the angle of the nerve in the image. A straight nerve equals a TC of zero, and the TC increases with increasing tortuosity of the NF.
Figure 1.
Box and whisker plots of the prevalence of symmetrical morphology in different stages of DSPN in the RE (dashed blue) and LE (solid black) for CNFD (A), CNBD (B), CNFL (C), and TC (D).
Statistical analysis
Statistical analysis was performed using StatsDirect for Windows (StatsDirect Ltd., Altrincham, Cheshire, U.K.), and OriginPro version 8.5 (OriginLab Corporation, Northampton, MA) was used to plot the results. Prior to statistical analysis, all the collected data were assessed for normality by relevant histograms and the Shapiro-Wilk test where appropriate. Under the assumptions of a 0.05 type 1 error and power of 0.95, we calculated that a minimum sample of 68 patients with diabetes/neuropathy was required to detect a statistically meaningful effect. The recruitment continued and reached 111 patients in total until each group contained at least 17 patients. Differences between REs and LEs and between groups (controls vs. none vs. mild vs. moderate vs. severe neuropathy) were tested by means of a paired Student t test and one-way ANOVA or nonparametric ANOVA (Kruskall-Wallis), respectively, and a P < 0.05 was considered significant. Post hoc analysis for multiple comparisons was performed using the Tukey (parametric) or the Conover-Inman test (nonparametric). The mean difference between the REs and LEs for each of the IVCCM parameters was calculated to define the magnitude of asymmetry, and the Spearman rank test was used to investigate the strength of the relationship between the variables. Box and whisker plots (Fig. 1A–D) were generated for CNFD, CNBD, CNFL, and TC to allow visual assessment of the data.
RESULTS
Clinical and peripheral neuropathy assessment
Among the 111 diabetic subjects, 61 (55%) were classified as having DSPN based on the case definition used in this study. There was no significant difference in age, BMI, and serum triglycerides, but HbA1c was significantly increased in the diabetes cohort (P < 0.0001) and was the highest in those with severe neuropathy (P < 0.001). Paradoxically, there was a trend for decreasing total cholesterol with increasing severity of neuropathy in diabetic patients compared with control subjects. There was an increase in ACR (P < 0.001) and a significant reduction in eGFR in diabetic patients with moderate (P < 0.001) and severe neuropathy (P < 0.001) (Table 1). When differences were adjusted for type of diabetes, duration, sex, and age, HbA1c tended to be higher in type 1 diabetes (P < 0.0001) whereas eGFR correlated with duration of diabetes (P < 0.0001) and age (P < 0.0001).
Table 1.
Clinical and peripheral neuropathy status

Vibration perception, although within the normal range (<15 V), was elevated in diabetic patients without neuropathy (P = 0.02) and increased with increasing severity of neuropathy (P < 0.0001). SSNamp (P < 0.01) and SSNCV (P < 0.001) showed a progressive decline with increasing severity of neuropathy. Similarly, PMNamp and PMNCV also decreased, reaching significance (P < 0.0001) in mild, moderate, and severe neuropathy, respectively (Table 1). A longer duration of diabetes and age correlated significantly with VPT (P < 0.0001), PMNamp (P < 0.01), SSNamp (P < 0.0001), SSNCV (P < 0.0001), and PMNCV (P < 0.0001).
IVCCM and corneal sensation
CNFD (P < 0.001), CNBD (P < 0.001), and CNFL (P < 0.001) demonstrated a significant reduction between control subjects and diabetic patients with increasing severity of neuropathy (Table 2 and Fig. 1). Corneal sensation thresholds increased gradually and symmetrically in diabetic patients with increasing severity of neuropathy compared with control subjects (P < 0.001) (Table 2). There were no differences attributed to type of diabetes, sex, and age.
Table 2.
IVCCM and NCCA for REs and LEs in different stages of peripheral neuropathy

There were no significant differences between the RE and the LE in CNFD, CNBD, CNFL, TC, and NCCA for any stage of DSPN, confirming symmetrical corneal nerve damage across study subjects (Fig. 1A–D). Spearman correlation coefficients and the associated mean differences for each subject group and parameter are presented in Table 3. There was a strong relationship between the RE and LE for CNFD and CNFL for control subjects and diabetic patients with no, mild, and moderate neuropathy but not severe neuropathy. This association was maintained in control subjects and all groups of diabetic patients for CNBD. For TC, there was a less significant relationship between REs and LEs in control subjects and diabetic patients with “none” and “severe” neuropathy.
Table 3.
Mean difference and interside correlation of IVCCM parameters
CONCLUSIONS
DSPN is characterized by progressive distal and symmetrical sensory and autonomic nerve damage with eventual motor nerve involvement (1). It is hypothesized that the initial injury occurs in the thinly myelinated Aδ- or myelinated C-fibers where morphological alterations can be assessed with skin biopsy (24). Although NCS is the preferred end point for diagnosis and assessment of outcome in clinical intervention trials, it is limited to large nerves (18). IVCCM has emerged as a powerful technique to detect and stratify human DSPN as it allows direct, noninvasive visualization of the corneal subbasal nerves (6). Corneal innervation shares anatomical similarities with intraepidermal innervation, and corneal NF loss has been found to reflect intraepidermal NF loss (4).
Observational studies using IVCCM to evaluate peripheral neuropathy have reported on the concurrent validity (14), reproducibility (9,15), and optimization of image selection (22). In a previous study (16), we showed that corneal innervation patterns between REs and LEs in healthy subjects are symmetrical, with the exemption of branching, which showed wider limits of agreement. It is unknown, however, whether corneal nerve loss remains symmetrical in DSPN of varying severity. A robust test to diagnose DSPN should not only be able to detect changes but also have comparable properties to the clinical presentation and current end points of choice i.e., symmetrical involvement (18,24). This also has important practical relevance when undertaking IVCCM as symmetrical involvement will enable examination of one eye only, reducing the examination time.
This study shows for the first time that DSPN, as detected by gold standard clinical and electrophysiological testing, is paralleled by significant corneal NF loss, which is highly symmetrical between REs and LEs except in those with severe neuropathy. Specifically, we demonstrate a dramatic stepwise reduction in CNFD, CNFL, and CNBD with an increase in TC using the latest third-generation IVCCM in diabetic patients with increasing severity of neuropathy compared with control subjects. This confirms and extends our findings using the less sensitive second-generation IVCCM (9). We have also found a significant increase in corneal sensation thresholds with increasing severity of neuropathy (17). The relationship between the right and left corneal innervation patterns was highly significant among control subjects and diabetic patients with increasing severity of neuropathy, except in patients with severe neuropathy. This may reflect variability and perhaps the patchy nature of central corneal nerve damage in advanced neuropathy, which has been shown recently in a small whole corneal nerve mapping study in a diabetic patient with severe neuropathy (25).
A study by Perkins et al. (18) and an earlier study by Bromberg and Jaros (19) found high interside symmetry of NCS in patients with varying degrees of DSPN. However, Perkins et al. (18) reported differences in each NCS parameter per nerve as a mean of the whole study cohort, regardless of the severity of neuropathy. To our knowledge, no previous studies have assessed whether small fiber involvement in DSPN is symmetrical. A potential limitation and a source of variation is the use of NDS, which is large fiber weighted to classify the severity of neuropathy. Thus, this may lead to variability when comparing to our findings using IVCCM, a small fiber measure, and may explain the large variation in corneal nerve measures among the different groups of neuropathic severity.
In conclusion, we confirm and extend our previous findings (9) in a large cohort of diabetic patients using the latest third generation IVCCM with optimal image clarity. We show that DSPN results in gradual and significant corneal NF loss with more advanced neuropathy, which is highly symmetrical except in patients with severe neuropathy.
Acknowledgments
This research was facilitated by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network. This research was funded by awards from the National Institutes of Health (R1-05991) and Juvenile Diabetes Research Foundation International (27-2008-362).
No potential conflicts of interest relevant to this article were reported.
I.N.P. designed the study, researched data, performed analysis, and wrote the manuscript. U.A., H.F., O.A., and A.M. researched data. P.G. collected data and performed analysis. G.P. researched data and contributed to discussion. A.J.M.B. reviewed the manuscript. M.T. researched data and reviewed the manuscript. R.A.M. designed the study, reviewed and revised the manuscript, and was the principal investigator of the study. R.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol 1996;55:1181–1193 [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486 [DOI] [PubMed] [Google Scholar]
- 4.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007;56:2148–2154 [DOI] [PubMed] [Google Scholar]
- 5.Løseth S, Stålberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol 2008;255:1197–1202 [DOI] [PubMed] [Google Scholar]
- 6.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003;46:683–688 [DOI] [PubMed] [Google Scholar]
- 7.Tavakoli M, Marshall A, Thompson L, et al. Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 2009;40:976–984 [DOI] [PubMed] [Google Scholar]
- 8.Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010;223:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010;33:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi F, Okino M, Ishibashi M, et al. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. Journal of Diabetes Investigation 2012;3:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu T, Ahmed A, Bril V, et al. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med 2012;29:e297–e303 [DOI] [PubMed] [Google Scholar]
- 12.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013;62:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 2011;28:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care 2012;35:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med 2011;28:1253–1260 [DOI] [PubMed] [Google Scholar]
- 16.Petropoulos IN, Manzoor T, Morgan P, et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea 2013;32:e83–e89 [DOI] [PubMed] [Google Scholar]
- 17.Tavakoli M, Kallinikos PA, Efron N, Boulton AJM, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care 2007;30:1895–1897 [DOI] [PubMed] [Google Scholar]
- 18.Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve 2002;25:212–217 [DOI] [PubMed] [Google Scholar]
- 19.Bromberg MB, Jaros L. Symmetry of normal motor and sensory nerve conduction measurements. Muscle Nerve 1998;21:498–503 [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye S, Boulton AJM, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 22.Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci 2012;89:812–817 [DOI] [PubMed] [Google Scholar]
- 23.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci 2004;45:418–422 [DOI] [PubMed] [Google Scholar]
- 24.Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology 2009;54:273–285 [DOI] [PubMed] [Google Scholar]
- 25.Edwards K, Pritchard N, Gosschalk K, et al. Wide-field assessment of the human corneal subbasal nerve plexus in diabetic neuropathy using a novel mapping technique. Cornea 2012;31:1078–1082 [DOI] [PubMed] [Google Scholar]