Abstract
OBJECTIVE
Long-term diabetes leads to severe peripheral, autonomous, and central neuropathy in combination with clinical gastrointestinal symptoms. The brain-gut axis thus expresses a neurophysiological profile, and heart rate variability (HRV) can be correlated with clinical gastrointestinal symptoms.
RESEARCH DESIGN AND METHODS
Fifteen healthy volunteers and 15 diabetic patients (12 with type 1 diabetes) with severe gastrointestinal symptoms and clinical suspicion of autonomic neuropathy were included. Psychophysics and evoked brain potentials were assessed after painful rectosigmoid electrostimulations, and brain activity was modeled by brain electrical source analysis. Self-reported gastrointestinal symptoms (per the Patient Assessment of Upper Gastrointestinal Disorder Severity Symptom Index) and quality of life (SF-36 Short Form Survey) were collected.
RESULTS
Diabetic patients had autonomous neuropathy, evidenced by decreased electrocardiographic R-R interval (P = 0.03) and lower HRV (P = 0.008). Patients were less sensitive to painful stimulation (P = 0.007), had prolonged latencies of evoked potentials (P ≤ 0.001), and showed diminished amplitude of the N2–P2 component in evoked potentials (P = 0.01). There was a caudoanterior shift of the insular brain source (P = 0.01) and an anterior shift of the cingulate generator (P = 0.01). Insular source location was associated with HRV assessments (all P < 0.02), and the shift (expressed in mm) correlated negatively with physical health (P < 0.001) and positively with nausea (P = 0.03) and postprandial fullness (P = 0.03). Cingulate source shift was correlated negatively with physical health (P = 0.005) and positively with postprandial fullness (P ≤ 0.001).
CONCLUSIONS
This study provides evidence for interaction between autonomic neuropathy and peripheral nervous degeneration, as well as changes in dipole sources in diabetic patients with gastrointestinal symptoms. The findings may lead to improved treatment modalities targeting pharmacological neuroprotection or neuromodulation.
Diabetes is one of the leading causes of severe peripheral, autonomous, and central neuropathy. Diabetic peripheral neuropathy classically manifests as progressive symmetric thick-fiber (Aβ) and thin-fiber (Aδ) neuropathy affecting axons of the distal lower extremities. Patients may also suffer from diabetic autonomic neuropathy (DAN). According to the Toronto criteria, DAN is a disorder of the autonomic nervous system (ANS) in the setting of diabetes or metabolic derangements (1). DAN may affect cardiovascular, gastrointestinal, and urogenital systems and sudomotor function. Autonomic abnormalities can be classified as structural or functional disorders, and they may be subclinical, diagnosable only by tests, or clinical, with symptoms or signs (2).
As many as 50% of diabetic patients with long duration of the disease have severe gastrointestinal symptoms, including postprandial fullness, nausea, vomiting, bloating, early satiety, and abdominal pain. These symptoms likely represent clinical DAN, leading to significant reduction in quality of life and presenting a severe socioeconomic burden (3).
The underlying pathogenesis of DAN is multifactorial and comprises gastrointestinal motility dysfunction, metabolic insults to internal nerve fibers, neurovascular insufficiency, alterations in gastrointestinal hormone secretion, and abnormal interoception (combined perception of afferent neural trafficking from the internal organs). Clinical management of diabetic patients is therefore challenging, calling for a better understanding of the brain-gut axis. Introduction of such peptides as glucagon-like peptide 1 (GLP-1) agonists has contributed to understanding of this complex interaction. When released postprandially in the small intestine GLP-1 acts as a hormone or a signal to sensory vagal afferents. In the central nervous system, it primarily affects stimulation of glucose-dependent insulin secretion (4) and inhibition of glucagon secretion (5). When GLP-1 is released in brain hypothalamic nuclei from nerve endings originating from, for example, the solitary tract, however, it functions as a neuropeptide (6), affecting vagal activity and hence homeostatic regulation of the gut.
Psychophysical studies of the brain-gut axis in type 1 diabetes have focused on the upper gastrointestinal tract. Increased pain detection thresholds (hyposensitivity) to esophageal electrical stimulation were shown in patients with motor dysfunction and gastrointestinal discomfort (7), and interestingly hyposensitivity was accompanied by increased size of convergent somatic pain referrals in patients with evident DAN and gastrointestinal symptoms (8). Authors suggest that the latter finding involves central pain processing, indicating a central “neuropathy-like” component contributing to symptom generation. The mechanism in diabetes differs from classical neuropathy, however, in which hyperalgesia and allodynia (and not hyposensitivity) typically are present.
Neurophysiologically, laser evoked potentials (EPs) have been used to characterize patients with somatic neuropathic pain, with the reduced amplitudes reflecting Aδ-fiber reduction (9). Further, EPs have been used to explore the esophageal sensory afferents in type 1 diabetic patients, revealing similar findings: prolonged latencies and reduced amplitudes relative to healthy volunteers (10). Moreover, our group have shown central alterations in diabetic patients and reorganization of the cingulate-operculum network (11).
In light of previous findings, we wanted to study the brain-gut axis in diabetic patients. We hypothesized that the neurophysiological profile differed between diabetic patients and healthy volunteers, including 1) psychophysical response; 2) heart rate variability (HRV); 3) EP latency, amplitude, and topography; and 4) electrical brain activity according to dipolar source location. Furthermore, to characterize the consequence of altered interoception, we investigated the interactions of HRV, dipolar source location, gastrointestinal symptoms, and self-reported quality of life.
RESEARCH DESIGN AND METHODS
Subjects
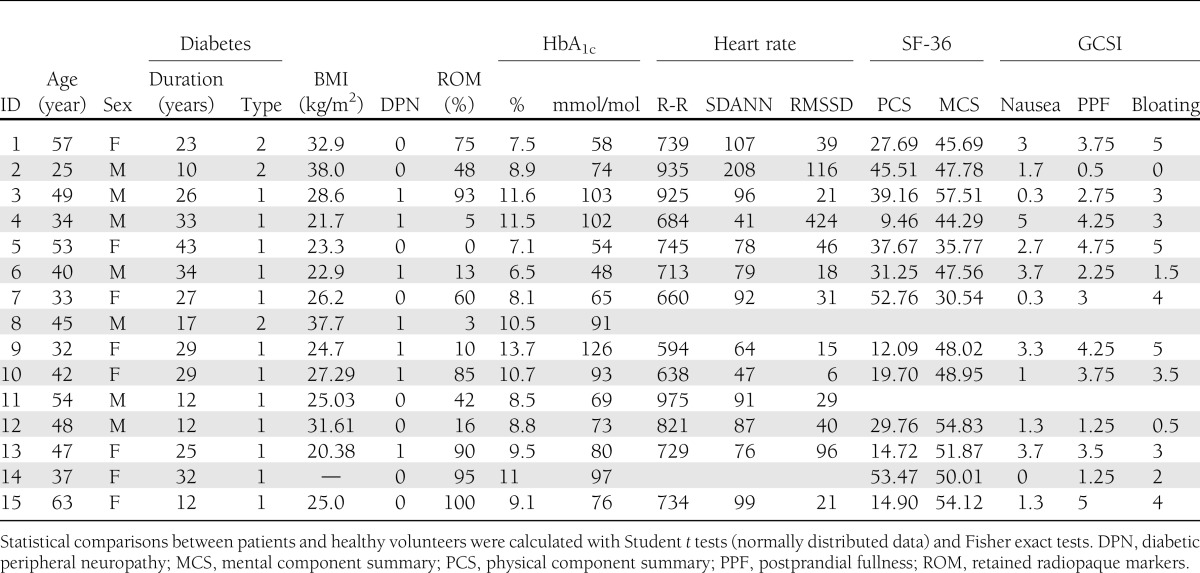
Data were collected from August 2010 until October 2011, and data regarding source connectivity of the acquired EPs have been reported previously by Lelic et al. (11). Fifteen diabetic patients with severe gastrointestinal symptoms were recruited at the Department of Endocrinology and Gastroenterology at Haukeland University Hospital in Bergen, Norway. All patients (10 females, mean 43.9 ± 9.5 years of age) had a verified diabetes diagnosis (12 with type 1 diabetes and 3 with type 2 diabetes), with an average disease duration of 24.3 ± 9.8 years. DAN was suspected on the basis of clinical gastrointestinal symptom scores, and after completion of gastric emptying tests, 9 of 15 patients were found to have gastroparesis (assessed as retained radiopaque markers in the stomach according to standard criteria) (12).
Patient characteristics are listed in Table 1. For those dependent on exogenous insulin, multiple insulin injection regimens or automatic insulin pumps were used. Fifteen age- and sex-matched healthy volunteers (10 females, mean 44.8 ± 9.3 years of age) served as controls. Seven of the healthy volunteers were recruited at the Department of Endocrinology and Gastroenterology at Haukeland University Hospital in Bergen, Norway, and the remaining eight healthy volunteers were recruited at the Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark. The local ethics committees approved the study protocols (Bergen 2010/2562–6 and Aalborg N-20090008).
Table 1.
Patient characteristics
Assessment of ANS activity
Diagnostic criteria and staging of cardiovascular autonomic neuropathy are still being debated, but cardiovascular reflex tests are the gold standard in clinical autonomic testing because they are valid, standardized, safe, noninvasive, and easily performed. Subjects refrained from drinking tea and coffee within 2 hours before the visit. ANS activity was assessed through HRV analysis of 24-h Holter electrocardiographic recordings (recording device 3-channel Lifecard CF; Del Mar Reynolds, Spacelabs Healthcare Inc., Snoqualmie, WA). The recording period commenced with 10 min of controlled respiration (15 breaths/min) in the supine position and the standing position before the subject was discharged for 24 h of continuous Holter monitoring. Heart rate and blood pressure were measured in supine and upright positions and continuously for every minute during the standing position. We assessed the HRV (Impresario Software version 3; Spacelabs Healthcare) throughout the full 24-h recording and characterized the recordings according to electrocardiographic R-R intervals, HRV within 5-min cycles (SDANN), and the root mean square of difference of successive normal R-R intervals (RMSSD). The R-R intervals represent the heart rate, SDANN represents the HRV, and RMSSD reflects parasympathetic tone (13).
Questionnaires
Symptom score.
To assess severity of patient symptoms, the Patient Assessment of Upper Gastrointestinal Disorder Severity Symptom Index was used. In its short form, the Gastroparesis Cardinal Symptom Index (GCSI), which is based on the first nine questions of Patient Assessment of Upper Gastrointestinal Disorder Severity Symptom Index, has proved to be a reliable and valid tool for measuring symptom severity in patients with gastroparesis and gastrointestinal dysfunction (14). The GCSI is based on three subscales: 1) nausea and vomiting (three items), 2) postprandial fullness and early satiety (four items), and 3) bloating (two items). Patients rate symptom severity during the preceding 2 weeks. The GCSI items range from 0 (no symptoms) to 5 (very severe symptoms).
Physical and mental health.
To assess physical and mental health of the patients, we used the SF-36 Short Form Survey, which is a multipurpose eight-scale profile of functional health and well-being scores, including two summary scores (physical component summary and mental component summary). The questionnaire covered 4 weeks before the experiment. The SF-36 has proved valid and useful in surveys of general and specific populations, in comparing the relative burdens of diseases, and in differentiating the health benefits produced by a wide range of varying treatments.
Psychophysical assessment
To standardize influence of glucose and insulin levels on sensory assessments, all diabetic patients and healthy volunteers were asked to fast for 6 h (15). A hyperinsulinemic-euglycemic clamp technique (16) ensured continuous adjustment of the blood glucose level to 6 mmol/L throughout the whole study procedure.
Rectosigmoid sensation was assessed with a modified validated visual analog scale from 0–10 with anchor words. The scale has been used in numerous studies (17). The anchor words were 0, no sensation; 1, vague perception of mild sensation; 2, definite perception of mild sensation; 3, vague perception of moderate sensation; 4, definite perception of moderate sensation; 5, pain detection threshold; 6, slight pain; 7, moderate pain; 8, medium pain intensity; 9, intense pain; and 10, unbearable pain.
During electrical stimulation, the subjects were asked to notify when stimulation intensity reached 1, 3, 5, and 7 on the visual analog scale. Electroencephalography recording was done at pain detection threshold.
Electrical stimulation.
A rectal probe with a 6.2-mm outer diameter and two bipolar stainless steel electrodes mounted on the tip was constructed for rectosigmoid stimulation (Ditens A/S, Egaa, Denmark). Electrical stimulation was delivered by a computer-controlled constant current stimulator (DIGITIMER Ltd., Welwyn Garden City, U.K.). Stimuli were applied as 2-ms single square pulses. Current intensity was increased in steps of 1 mA. To blind the subject to stimulus intensity, intermittent sham stimuli were randomly delivered. To ensure mucosal contact was maintained, measurements of impedance were continuously assessed with a custom-made instrument (Aalborg University, Aalborg, Denmark), where impedance <2 kΩ indicated good contact.
EP analysis
The electrode cap contains 128 channels; however, 2 channels were used for ear references and 4 channels were used to detect eye and swallow movements. Calculations were therefore based on 122 channels.
Offline preprocessing of the averaged EPs was done using commercial software (Neuroscan version 4.3.1, Neuroscan, El Paso, TX). This procedure included the following preprocessing steps: 1) band-pass filtered between 0.5 and 30 Hz, 2) epoched from 100 ms before stimulus to 500 ms after stimulus, 3) epochs contaminated by eye movement were discarded, 4) epochs were averaged into EPs, and 5) EP signals were rereferenced to a common average reference such that inverse solutions were independent on location of the reference electrode.
Latencies and amplitudes of the most consistent components N2-P2 vertex complex were analyzed at the Fz (frontal) and Cz (central) electrodes, since brain activation following gut stimulation previously was reported in centrofrontal regions (17). Topographic mapping was made by spline interpolation, illustrating the scalp distribution of amplitudes derived from all 122 channels. The predominant activity (frontal, temporal, central, and occipital) was registered.
Dipolar source modeling
To analyze the origin of differences in EPs, underlying brain activity was estimated by use of the Brain Electrical Source Analysis software package (BESA Research 5.3; MEGIS GmbH, Gräfelfing, Germany).
On the basis of calculations of surface potential distributions from preset voltage dipoles within a standardized brain, an appraisal was made to fit between recorded and calculated field distributions. Residual variance (RV) describes the percentage of data that cannot be explained by the model. The method has been described in detail elsewhere (18). A symmetric constraint was applied to model bilateral sources (in insula and secondary somatosensory cortex) and one free-floating midline source in the cingulate cortex. These three brain areas were chosen to be regions of interest because they are key players of the so-called pain neuromatrix.
The individual dipolar sources were adjusted until the lowest RV was obtained. The model provides dipole coordinates (in mm) within the Talairach system (x, right [positive]/left [negative]; y, anterior [positive]/posterior [negative]; and z, up [positive]/down [negative]) and orientation and strength for each of the five dipoles. Euclidian distances were calculated as the three-dimensional vector, based on shifts in the x, y, and z Talairach coordinates, and thus represents the total shift (mm). To avoid multiple comparison problems, only dipoles showing differences between healthy volunteers and diabetic patients were used for further clinical correlations.
Statistical analysis
All descriptive data are presented as mean ± SD unless otherwise indicated. To test differences between diabetic patients and healthy volunteers, comparison of demographics, Holter monitoring, questionnaires, and EP amplitudes were done by Student t test and Fisher exact test.
To compare EP latencies and dipolar source location, a two-way ANOVA (within-subject factor EP component or source coordinate and between-subject factor patients vs. healthy volunteers) was used. If an overall significance was observed, Wald tests were used for post hoc analysis.
Comparisons of topographical distributions of surface potentials were done by χ2 tests.
For the dipolar sources that revealed significant differences in coordinates between healthy volunteers and patients, correlation analysis were done with the Pearson test (or Spearman ρ in case data were not normally distributed) between brain source locations and HRV and between GCSI score and SF-36. The software package Sigma Stat v.3.0 (IBM Corporation, Armonk, NY) was used in the analysis, and P ≤ 0.05 was considered significant.
RESULTS
All patients and healthy volunteers completed the study. No differences between patients and healthy volunteers were found in age or BMI (data not shown). A difference in mean HbA1c was shown, with a value in patients of 9.7% vs. 5.6% in healthy volunteers (P ≤ 0.001).
Cardiac autonomic parameters
Holter monitoring was done in 13 of 15 patients. In comparison with healthy volunteers, patients had decreased R-R interval (748 ± 112 ms vs. 828 ± 77 ms; P = 0.03), decreased SDANN (90 ± 38 vs. 125 ± 32; P = 0.008), and diminished—however insignificantly—vagal tone (RMSSD, 37.1 ± 30.3 vs. 41.0 ± 22.9; P = 0.72). Holter monitoring data are not shown.
Questionnaires
Questionnaires were completed by 15 patients. Differences between clinical gastrointestinal symptoms in patients and healthy volunteers were seen in the three GCSI subscales describing nausea (1.8 ± 1.4 vs. 0), postprandial fullness (2.9 ± 1.6 vs. 0.2 ± 0.3), and bloating (3.0 ± 1.7 vs. 0.3 ± 0.9; all P < 0.001). Data from questionnaires are not shown.
Differences between patients and healthy volunteers were also seen in the SF-36 physical component summary (31.0 ± 13.6 vs. 56.0 ± 3.6; P ≤ 0.001) and mental component summary (44.4 ± 10.2 vs. 50.9 ± 5.5; P = 0.04).
Electrical stimulation and EPs
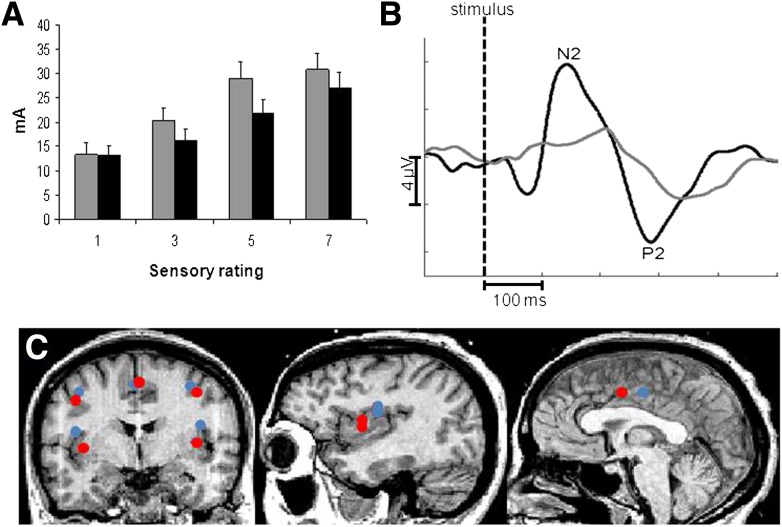
Electrical stimulation and EPs were done in 14 of 15 patients. Overall hyposensitivity to electrical rectosigmoid stimulation was seen in patients in comparison with healthy volunteers (F = 7.67, P = 0.007) (Fig. 1).
Figure 1.
A: Sensory profile at visual analog scale ratings of 1 (sensory detection threshold), 3 (moderate sensation), 5 (pain detection threshold), and 7 (moderate pain) in response to electrical stimulation of the rectosigmoid junction. Patients (gray) in general tolerate higher stimulation intensities than do healthy controls (black). B: EPs to rectosigmoid stimulation in representative subjects from each group (healthy volunteers in black and diabetic patients in gray) recorded at the central site on the scalp (Cz electrode). Prolonged latencies and reduced peak-to-peak amplitudes in the diabetic patient group indicate abnormal interoception. C: Localization of dipolar brain sources evoked by painful stimulations of the rectosigmoid in patients (red) and healthy volunteers (blue). The average of the individual Talairach coordinates deriving from the individual brain sources was projected to a standardized magnetic resonance image. The localization of the insular and cingulate dipole showed significant differences between the two groups.
EPs from the rectosigmoid stimulations showed prolonged latencies in the diabetic patients compared with healthy volunteers (F = 21.4; P ≤ 0.001). Post hoc analysis revealed significant differences between patients and healthy volunteers at both N2 (219.4 ± 49.2 ms vs. 165.1 ± 32.2 ms; P = 0.003) and P2 (337.8 ± 46.2 ms vs. 286.4 ± 31.1 ms; P = 0.003) (Fig. 1). EPs showed diminished N2–P2 peak-to-peak amplitudes in the patients (6.9 ± 3.3 µV vs. 3.3 ± 3.1 µV; P = 0.01). Patients and healthy volunteers showed no differences in the topographical distribution (P ≥ 0.7).
Dipolar source analysis
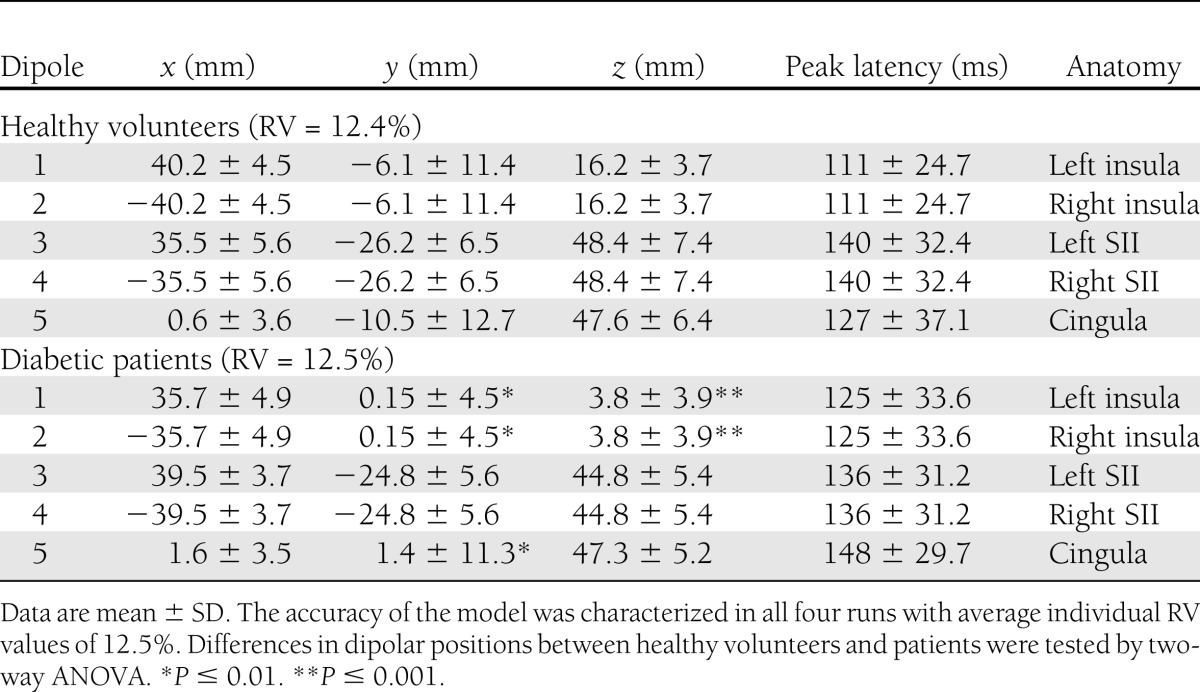
The dipolar source solutions in patients and healthy volunteers were applied to the recordings and then adjusted individually. We preferred the solution with the least RV, which was on average 12.4 ± 2.0% in healthy volunteers and 12.5 ± 1.8% in patients. In diabetic patients, the bilateral insular sources showed a shift in location (average Euclidian distance 16.0 ± 3.6 mm) compared with healthy volunteers (F = 6.6; P = 0.01). The most prominent finding in the post hoc test was on average a 12-mm caudal (downward) shift of the z coordinate (P ≤ 0.001) and on average an anterior (forward) shift of the y coordinate (P = 0.01) (Fig. 1C). Furthermore, the midline cingulate source showed a shift in location (Euclidian distance 15.9 ± 6.9 mm) between healthy volunteers and diabetic patients (F = 6.3; P = 0.01). The most prominent finding in the post hoc test was on average a 12-mm anterior (forward) shift (P ≤ 0.001). No differences between patients and healthy volunteers (Euclidian distance 8.9 ± 3.8 mm) were found in the secondary somatosensory cortex (F = 0.2; P = 0.6). Detailed results of dipolar positions are shown in Table 2, and source localization is depicted in Fig. 1.
Table 2.
Dipole coordinates and peak latencies for the five modeled dipoles in diabetic patients and healthy volunteers
Clinical correlations
Associations between insular activity and different HRV assessments were found. R-R interval (r = 0.61; P = 0.03), SDANN (r = −0.65; P = 0.02), and the ratio beats per minute/SDANN (r = 0.7; P = 0.008) were associated with abnormal insular activity. There was no association between insular activity and vagal tone (RMSSD, r = 0.38; P = 0.22). The results indicate a relative sympathetic dominance in diabetic patients with concomitant abnormal insular activation.
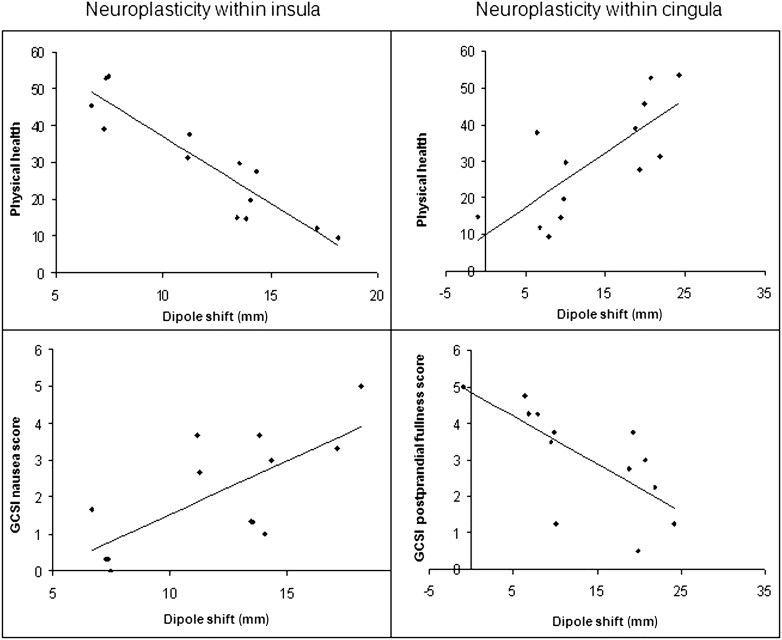
Furthermore, lowered insular activation was negatively correlated with physical health. Nausea (ρ = 0.59; P = 0.03), postprandial fullness (ρ = 0.60; P = 0.03), and physical health (ρ = −0.88; P ≤ 0.001) were associated with altered dipolar source localization. The results indicate increased symptoms relating to the gastrointestinal tract and decreased physical health with abnormal insular activation (Fig. 2), Finally, associations between altered cingulate activity and postprandial fullness (ρ = −0.83; P ≤ 0.001) and physical health (ρ = 0.71; P = 0.005) were found, indicating increased physical health and less postprandial fullness in diabetic patients with activation of the anterior cingulate cortex (Fig. 2).
Figure 2.
Altered brain activation is displayed in diabetic patients with autonomic neuropathy and gastrointestinal symptoms. Top left: Negative association between physical health score (SF-36) and altered insular activity after electrical stimulation of the rectosigmoid assessed as the Euclidian dipole shift. This means that increased caudoanterior insular reorganization was associated with inferior physical health experience. Bottom left: Positive association between reorganized insular activity (dipole shift) and nausea. Top right: Positive association between physical health score (SF-36) and the altered cingulate activity assessed as the Euclidian dipole shift. This means that increased anterior cingulate activity was associated with better physical health. Bottom right: Negative correlation between reorganized cingulate activity and postprandial fullness, meaning that the more anteriorly the cingulate activity is reorganized, the less complaints of postprandial fullness.
CONCLUSIONS
According to our hypothesis, patients with long-standing diabetes, severe gastrointestinal symptoms, and autonomic neuropathy had an altered neurophysiological profile relative to healthy volunteers. Patients showed hyposensitivity to rectosigmoid electrical stimulations, together with prolonged latencies and reduced amplitudes, indicating affection of visceral afferent neural trafficking. Furthermore, dipolar source analysis revealed a bilateral anterior-to-caudal shift of the insular source and an anterior shift of the cingulate source. The clinical implication was shown through correlations between altered brain activity and patient characteristics such as HRV assessments, gastrointestinal symptoms, and physical health. In conclusion, peripheral neuropathies, autonomous neuropathy, and altered dipolar localization seem to affect the interoception of the brain-gut axis in diabetic patients, leading to development and persistence of gastrointestinal symptoms.
Methodological considerations
The study included 15 diabetic patients with illness duration of approximately 25 years and severe gastrointestinal symptoms. Enrollment of a larger study population would have been preferable; however, recruitment was challenging. Even though results from small sample sizes should always be interpreted carefully, we found consistent differences between patients and healthy volunteers in this study.
The rectal probe used for electrical stimulations has previously shown to be robust and reproducible (19). The Brain Electrical Source Analysis software package has been used consistently in our hands to model brain activity in response to painful stimulation of the gut (17). Estimation of dipolar source locations with a spatial resolution comparable with functional magnetic resonance imaging and positron emission tomography, however, remains a challenge. In this study, 122-channel recordings improved the spatial outcome, and we characterized real-time brain activity on the basis of dipolar source locations (20). Nevertheless, even with optimal techniques, interpretation of source locations is limited for explaining the net effect of possibly multiple neural activities.
Cardiac autonomic neuropathy was used to assess generalized autonomic neuropathy. Because the heart is dependent on negative feedback controls, its activity contains hidden information of subtle variety of beat-to-beat periodicities (21). Autonomous assessment was therefore based on R-R interval, SDANN, and RMSSD. A limitation in this study is the lack of analyzing power spectrum bands. Bands at approximately 0.1 Hz represent sympathetic and parasympathetic influence, in contrast with the higher frequency band at 0.25 Hz that is mainly due to the respiratory-driven cardiac vagal efferent modulation. A more precise ratio between low- and high-frequency bands is thus more accurate in expressing the relative dominance of sympathetic modulation of sinoatrial activity (21). Nevertheless, even with the somewhat crude measures of R-R interval and SDANN, we showed autonomic nervous dysfunction, which was associated with the symptom generation, in this patient group.
Altered source localization in patients with long-standing diabetes
Previous studies have also indicated elevated perception thresholds to electrical stimulation of the gut in diabetic patients with concomitant DAN and different severities of gastrointestinal symptoms (7,8). Clinically, a larger subgroup of patients with longstanding diabetes suffers from severe gastrointestinal symptoms, which could indicate an overall hyperalgesia or allodynia of the internal afferents. It seems, however, as if this overall hyperalgesia is in opposition to the peripheral autonomic afferent neuropathy, which likely impairs the perception from the entire gastrointestinal tract. Source analysis solution suggested activation of bilateral insula, secondary somatosensory cortex, and cingulate cortex. These three brain areas are central actors of the so-called pain neuromatrix and are known to be involved in painful stimulations of the upper and lower gut; however, they cannot be considered pain-specific brain structures.
The insula is, through its connection with hypothalamus, thalamus, parabrachial nucleus, and solitary tract, crucial as a visceral sensorimotor area, representing the bodily states as well as coordinating activating networks devoted to sensory processing and homeostatic control (22,23). Furthermore, the insula is involved in emotional and affective aspects of pain-related learning and memory. This study revealed altered activation of the insular generator in patients with long-standing diabetes, evident as an anterior-to-caudal shift in dipolar source localization. Interestingly, this dipolar shift was positively correlated with diminished beat-to-beat interval and lower HRV, indicating a relative sympathetic dominance in diabetic patients with abnormal insular activation. Moreover, the insular dipole shift was negatively correlated with gastrointestinal symptoms and physical health. In other words, patients with altered insular activity reported more nausea and postprandial fullness and assessed poorer quality of life. These findings are supported by previous findings from our group, where lower insular source location after esophageal stimulation was associated with more nausea (24). Finally, it has been shown that direct electrostimulation of the insular region results in nausea and vomiting (25) and that insular lesions can cause ictal vomiting (26). Taken together, these data suggest a strong link between insular neuronal activity and gastrointestinal symptoms.
Cingulate activation has frequently been shown in experimental pain studies, and the cingulate cortex links perception and emotion (27,28). It is known that interoception is perceived in the posterior cingulate gyrus (Brodmann area 23), so the finding of an anterior shift is considered relevant for afferent activity from the internal organs. The experienced postprandial fullness was, however, less in patients with anterior reorganization. During food intake, the motivation to eat decreases and associated brain reward responses change, and it has been suggested that activation of especially the anterior cingulate cortex is involved in food-specific satiety (29). If so, the reported activation of the cingulate cortex and diminished postprandial fullness and improved physical health scores could give rise to speculation regarding whether those patients with enhanced anterior cingulate activity had better satiety control and therefore consumed less food.
Is autonomic nervous activity a biomarker of abnormal brain activity?
An association between DAN and altered source localization to interoception was found. The pathogenesis of gastrointestinal DAN is multifactorial and complex, including an array of metabolic, inflammatory, and cellular changes along with hyperglycemia and increased oxidative stress. Furthermore, autoimmune and genetic factors are involved. The nervous degeneration contributes directly and indirectly to Schwann cell dysfunction, affecting neurotransmission (30) and leading to impaired paranodal barrier function, damaged myelin, reduced antioxidative capacity, and decreased neurotrophic axonal support (31). Recently, much attention has been given to activation of the GLP-1 axis and its potential neuroprotective function (32). GLP-1 expression has been identified in neurons of the vagal nerve nodose ganglion, including sensory afferents critical to many autonomic reflexes regulating homeostatic control. Furthermore, diabetic patients with DAN have been shown to have altered incretin effect relative to patients without DAN (33). Increased levels of tumor necrosis factor-α have also been associated with cardiac autonomic neuropathy and HRV (34) and speculated to play a pathogenic role in development of diabetic neuropathy (35). Interestingly, recent findings support an anti-inflammatory effect of treatment with GLP-1 receptor activators (32).
Rectosigmoid hyposensitivity to electrical stimulation in diabetic patients strongly supports the presence of peripheral neuropathy. The phenomenon coexisted with corresponding EPs showing delayed latencies and diminished amplitudes. These findings suggest neuronal damage to the visceral afferent traffic, and traditionally this “bottom-up” model has been suggested; i.e., peripheral nerve damage causes central reorganization. Another consequence of altered visceral afferent input is a direct influence on an existing network between nucleus of the solitary tract and the nucleus of paragigantocellularis, which serves as a central integrator of autonomic descending regulation, particularly sympathetic outflow (36). A recent study from our group supports this approach. In diabetic patients, evidence of central nervous involvement were found, with reorganization of the cingulate-operculum source-connectivity associated with clinical symptoms. Other (perhaps synergistic) possibilities of the altered brain activation, however, may exist. In this study, diabetes adaptive reorganization in the insular generator was evident as an anterior-to-caudal shift in dipolar source localization, which was associated with 1) relative sympathetic dominance (vagal withdrawal) and 2) the total gastrointestinal symptom score. Even though diabetes often is associated with disturbances in the sympathovagal balance (21), no association between parasympathetic tone by itself and altered brain activity was found. The findings thus suggest alterations in the “net” interoception combining the afferent trafficking in sensory fibers. Because the ANS activity is processed in a central network, however, including the insula, prefrontal cortex, hypothalamus, amygdala, ventrolateral medulla, and nucleus of the solitary tract (37), any dysregulation of the ANS could alter brain activity. The caudal shift of insular activity found in this study thus may suggest dysregulation at the solitary tract nucleus level in the brain stem. In line with this, dysfunction at this level would induce changes to vagal efferent tone, affecting gut secretion and motility directly, which on the other hand could explain the association between insular dipole depth and patient symptom score. Another explanation addressing a “top-down” approach uncovers a strong link between insular activity and nausea (24), where similar findings between insular activity and symptom scores were found. Anterior reorganization of the cingulate generator of the EPs may reflect involvement of the anterior cingulate cortex (27,28). It could, however, also indicate enhanced frontal brain activity, such as emotional feedback modulation involving prefrontal cortices, being part of the ANS central network. Finally, to support the suggested central nervous involvement in diabetes, a recent study found microstructural neurodegenerative changes in brain areas involved in visceral sensory processing in patients with long-standing diabetes. Because the findings were associated with clinical gastrointestinal symptoms, mental well-being, and autonomic parameters, the authors advocate their functional significance (38).
Taken together, the findings give rise to future treatment possibilities including agents that directly support neurons and reparative processes after injury (39) or modalities with an effect on neuromodulation, such as peripheral pacing of visceral nerves (40).
Conclusion
This study provides evidence of peripheral nervous degeneration and altered brain activation in diabetic patients with autonomic neuropathy and gastrointestinal symptoms. Peripheral autonomous neuropathy likely contributed to both the gastrointestinal symptoms and the neurophysiological features of the patients. Furthermore, our findings also suggest that dysregulation of the central regulation of the ANS could play a key role. Abnormal brain activity may explain (at least in part) the development, appearance, and persistence of upper gastrointestinal symptoms in diabetic patients. We therefore suggest that future development of pharmacological compounds and electrical stimulation devices focusing on (central) neuroprotection should be considered in the treatment of severe diabetes.
Acknowledgments
The research leading to these results has received funding from the European Community's Seventh Framework Programme FP7/2007-2013 under grant agreement no. 223630.
No potential conflicts of interest relevant to this article were reported.
C.B., J.B.F., M.S., and A.M.D. designed the study. C.B., E.S., V.G., J.B.F., D.L., and G.D. collected the data. C.B., E.S., V.G., D.L., and B.B. analyzed the data. All coauthors cooperated in the interpretation of results. C.B. and A.M.D. prepared the manuscript. ANS expertise was provided by V.G. and M.S. Dipolar source expertise was provided by C.B. and D.L. Neurophysiological expertise was provided by A.M.D. Funding was provided by C.B., H.G., and A.M.D. All coauthors participated in critical revision of the manuscript. C.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Tesfaye S, Boulton AJ, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn R. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Autonomic nervous system testing. Diabetes Care 1992;15:1095–1103. [PubMed]
- 3.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001;161:1989–1996 [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–E206 [DOI] [PubMed] [Google Scholar]
- 5.Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 1988;123:2009–2013 [DOI] [PubMed] [Google Scholar]
- 6.Knauf C, Cani PD, Kim DH, et al. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes 2008;57:2603–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care 1991;14:1086–1089 [DOI] [PubMed] [Google Scholar]
- 8.Frøkjaer JB, Andersen SD, Ejskaer N, et al. Gut sensations in diabetic autonomic neuropathy. Pain 2007;131:320–329 [DOI] [PubMed] [Google Scholar]
- 9.Valeriani M, Pazzaglia C, Cruccu G, Truini A. Clinical usefulness of laser evoked potentials. Neurophysiol Clin 2012;42:345–353 [DOI] [PubMed] [Google Scholar]
- 10.Frøkjaer JB, Søfteland E, Graversen C, et al. Central processing of gut pain in diabetic patients with gastrointestinal symptoms. Diabetes Care 2009;32:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelic D, Brock C, Søfteland E, et al. Brain networks encoding rectal sensation in type 1 diabetes. Neuroscience 2013;237:96–105 [DOI] [PubMed] [Google Scholar]
- 12.Olausson EA, Brock C, Drewes AM, et al. Measurement of gastric emptying by radiopaque markers in patients with diabetes: correlation with scintigraphy and upper gastrointestinal symptoms. Neurogastroenterol Motil 2013;25:e224–e232 [DOI] [PubMed] [Google Scholar]
- 13.Novak V, Saul JP, Eckberg DL. Task Force report on heart rate variability. Circulation 1997;96:1056–1057 [PubMed] [Google Scholar]
- 14.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res 2004;13:833–844 [DOI] [PubMed] [Google Scholar]
- 15.Søfteland E, Dimcevski G, Graversen C, Nedrebø BG, Drewes AM, Frøkjær JB. Effects of isolated hyperinsulinaemia on sensory function in healthy adults. Exp Clin Endocrinol Diabetes 2011;119:604–609 [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 17.Brock C, Olesen SS, Valeriani M, Arendt-Nielsen L, Drewes AM. Brain activity in rectosigmoid pain: unravelling conditioning pain modulatory pathways. Clin Neurophysiol 2012;123:829–837. [DOI] [PubMed] [Google Scholar]
- 18.Valeriani M, Le Pera D, Tonali P. Characterizing somatosensory evoked potential sources with dipole models: advantages and limitations. Muscle Nerve 2001;24:325–339 [DOI] [PubMed] [Google Scholar]
- 19.Brock C, Nissen TD, Gravesen FH, et al. Multimodal sensory testing of the rectum and rectosigmoid: development and reproducibility of a new method. Neurogastroenterol Motil 2008;20:908–918 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs M, Wagner M, Kastner J. Confidence limits of dipole source reconstruction results. Clin Neurophysiol 2004;115:1442–1451 [DOI] [PubMed] [Google Scholar]
- 21.Kamath MV, Tougas G, Fitzpatrick D, et al. Assessment of the visceral afferent and autonomic pathways in response to esophageal stimulation in control subjects and in patients with diabetes. Clin Invest Med 1998;21:100–113 [PubMed] [Google Scholar]
- 22.Garcia-Larrea L, Perchet C, Creac’h C, et al. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain 2010;133:2528–2539 [DOI] [PubMed] [Google Scholar]
- 23.Isnard J, Magnin M, Jung J, Mauguière F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain 2011;152:946–951 [DOI] [PubMed] [Google Scholar]
- 24.Brock C, Graversen C, Frokjaer JB, et al. Peripheral and central nervous contribution to gastrointestinal symptoms in diabetic patients with autonomic neuropathy. Eur J Pain 2013;17:820–831 [DOI] [PubMed] [Google Scholar]
- 25.Afif A, Minotti L, Kahane P, Hoffmann D. Anatomofunctional organization of the insular cortex: a study using intracerebral electrical stimulation in epileptic patients. Epilepsia 2010;51:2305–2315 [DOI] [PubMed] [Google Scholar]
- 26.Catenoix H, Isnard J, Guénot M, Petit J, Remy C, Mauguière F. The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav 2008;13:560–563 [DOI] [PubMed] [Google Scholar]
- 27.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000;118:842–848 [DOI] [PubMed] [Google Scholar]
- 28.Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol 2004;115:2195–2222 [DOI] [PubMed] [Google Scholar]
- 29.Spetter MS, de Graaf C, Viergever MA, Smeets PA. Anterior cingulate taste activation predicts ad libitum intake of sweet and savory drinks in healthy, normal-weight men. J Nutr 2012;142:795–802 [DOI] [PubMed] [Google Scholar]
- 30.Askwith T, Zeng W, Eggo MC, Stevens MJ. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: implications for pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab 2009;297:E620–E628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol 2002;50:293–321 [DOI] [PubMed] [Google Scholar]
- 32.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin 2011;27:547–558 [DOI] [PubMed] [Google Scholar]
- 33.Kazakos KA, Sarafidis PA, Yovos JG. The impact of diabetic autonomic neuropathy on the incretin effect. Med Sci Monit 2008;14:CR213–CR220 [PubMed] [Google Scholar]
- 34.Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO. Association of serum adipocytokine levels with cardiac autonomic neuropathy in type 2 diabetic patients. Cardiovasc Diabetol 2012;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Clemente JM, Mauricio D, Richart C, et al. Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2005;63:525–529 [DOI] [PubMed] [Google Scholar]
- 36.Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci 2003;18:2103–2109 [DOI] [PubMed] [Google Scholar]
- 37.Sandroni P. Testing the autonomic nervous system. IASP Newsl 1998. Available from http://www.iasp-pain.org/AM/Template.cfm?Section=Technical_Corner&Template=/CM/ContentDisplay.cfm&ContentID=2188 Accessed 10 February 2013 [Google Scholar]
- 38.Frokjaer J, Andersen L, Brock C, et al. Altered brain microstructure assessed by diffusion tensor imaging in patients with diabetes mellitus and gastrointestinal symptoms. Diabetes Care 2013;36:662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmood D, Singh BK, Akhtar M. Diabetic neuropathy: therapies on the horizon. J Pharm Pharmacol 2009;61:1137–1145 [DOI] [PubMed] [Google Scholar]
- 40.Andersson S, Ringström G, Elfvin A, Simrén M, Lönroth H, Abrahamsson H. Temporary percutaneous gastric electrical stimulation: a novel technique tested in patients with non-established indications for gastric electrical stimulation. Digestion 2011;83:3–12 [DOI] [PubMed] [Google Scholar]