Abstract
OBJECTIVE
To examine racial differences in sleep duration and its relationship with diabetes.
RESEARCH DESIGN AND METHODS
We used data from a nationally representative sample of U.S. adults (n = 130,943) participating in the National Health Interview Survey from 2004 to 2011. Usual sleep duration was self-reported and categorized as <7 h (short), 7 h (optimal), and >7 h (long). Diabetes status was based on self-reported diagnosis from a health professional.
RESULTS
Participants’ mean age was 50.6 years, 49% were men, and 13% were black. Compared with whites, blacks were more likely to report short sleep (37 vs. 28%) and less likely to get 7 h of sleep (24 vs. 33%). Diabetes (9,643 cases [9%] in whites and 3,612 cases [15%] in blacks) had a U-shaped distribution with sleep in whites (10, 7, and 9%, for short, optimal, and long sleep, respectively) and blacks (16, 13, and 15%). Suboptimal sleep duration was more strongly associated with diabetes in whites than in blacks among short (prevalence ratio 1.49 [95% CI 1.40–1.58] vs. 1.21 [1.09–1.34]) and long (1.32 [1.25–1.40] vs. 1.11 [1.00–1.23]) sleepers on the relative scale. Adjustment for socioeconomic status (SES) attenuated the short sleep–diabetes association in blacks (1.15 [1.02–1.29]), and the racial/ethnic difference in the short sleep–diabetes association became nonsignificant after SES adjustments.
CONCLUSIONS
Suboptimal sleep duration was positively associated with diabetes in blacks and whites, although diabetes prevalence was higher at any level of sleep in blacks. Socioeconomic factors appear to partly explain the association for short sleep in blacks as well as disparity between racial groups.
Although optimal sleep is increasingly considered an essential component of an overall healthy lifestyle (1), studies suggest that mean sleep duration has decreased over time (2). With links between short as well as long sleep duration and increased risks of obesity, hypertension, coronary heart disease, diabetes, stroke, cancer, and mortality (3), racial/ethnic differences in sleep sufficiency may contribute to ethnic disparities in these health outcomes. It has been suggested that sleep restriction is an independent risk factor for diabetes, and investigating racial/ethnic variations in the relationship between sleep duration and diabetes is particularly important, as blacks (and other low-resourced groups) experience a disproportionately high prevalence of diabetes compared with whites (4).
Based on an understanding that social and environmental conditions may account for racial/ethnic differences in sleep duration, a recent study investigated hypothesized explanations for racial differences in the sleep–diabetes association that focused on socioeconomic position and individuals’ living environments (5). The authors concluded that the black excess of diabetes was independent of sociodemographic and medical characteristics (5). They, however, considered a limited list of characteristics, which included sociodemographic (i.e., age, sex, and income) and medical (i.e., hypertension, heart disease, and depression) variables among a relatively small sample size.
Short and long sleep durations have been shown to increase risk of diabetes (6), and this relationship may differ by race/ethnicity (7). For instance, diagnosed diabetes is at least 60% higher among blacks compared with whites (8), and a higher prevalence of obesity (particularly among women) that leads to greater glucose intolerance and insulin resistance is believed to contribute substantially to this disparity (4). Sleep deprivation may increase the risk of becoming overweight or obese, thereby increasing the risk of diabetes (9), through several pathways that include hyperactivity of the orexin system, which plays a central role in wakefulness and promoting increased food intake (10). Blacks have reported a higher prevalence of both short and long sleep durations than whites (11), and sleep duration and quality have been shown to be significant predictors of glycated hemoglobin among black participants (12).
To determine both the role of socioeconomic factors in the sleep–diabetes relationship and whether they explain potential racial/ethnic differences in the association between suboptimal sleep duration and diabetes among blacks and whites, we analyzed data from a nationally representative sample of the U.S. black and white populations. We hypothesized that the sleep–diabetes association would be partly explained by differences in socioeconomic factors, health behaviors, and medical conditions and that socioeconomic factors would explain any black-white differences in sleep duration and diabetes associations.
RESEARCH DESIGN AND METHODS
National Health Interview Survey
We analyzed data from a series of cross-sectional, nationally representative surveys from the National Health Interview Survey (NHIS), which uses a three-stage stratified cluster probability sampling design to conduct in-person interviews in samples of noninstitutionalized U.S. civilian households. A complete description of NHIS procedures is available elsewhere (13). In short, each week (on a continuous basis throughout the calendar year), a probability sample of households was interviewed by trained personnel from the U.S. Bureau of the Census to obtain information about health and other characteristics of each member of the sampled household. The interviews were conducted using computer-assisted personal interviewing. Information collected for all family members included household composition and sociodemographic characteristics, as well as indicators of health status, activity limitations, injuries, health insurance coverage, and access to and use of health care services. From each sampled family, one adult and one child (not included in this analysis) were randomly selected to provide more extensive health-related information. The response rate for the family sample was 84% (range 79–87), and the final response rate for sample adults, the basis for this analysis, was 67% (range 61–72). Our study was approved by the Harvard School of Public Health Institutional Review Board.
Study participants
Participants included self-reported non-Hispanic white or non-Hispanic black (henceforth, white and black) adults aged ≥25 years. Participants were excluded if they 1) were born outside the U.S.; 2) had an implausible BMI, i.e., either <15 or >70 kg/m2; or 3) had missing data on sleep or diabetes status. Our final sample comprised 130,943 adults (Supplementary Fig. 1). We excluded non–U.S. born participants for ease of interpretation because some evidence suggests that sleep patterns among immigrants differ from individuals born in the U.S. (14).
Measures
Sleep duration.
All sample adults who were 18 years old or older reported how many hours of sleep they, on average, get in a 24-h period. Interviewers were instructed to report the hours of sleep in whole numbers, rounding values of 30 min or more up to the nearest hour or otherwise rounding down. Usual sleep duration was self-reported and categorized as <7 h (short), 7 h (optimal), and >7 h (long).
Diabetes.
Participants were asked, “Have you EVER been told by a doctor or other health professional that you have diabetes or sugar diabetes?” Those reporting borderline diabetes (n = 1,793) were not considered to have diabetes, and participants 25 years old or younger who reported taking insulin for diabetes (n = 103) were excluded from the analysis to minimize the possibility of including type 1 diabetes cases.
Race/ethnicity.
Based on self-identification, participants were asked, “What race or races do you consider yourself to be?” They then selected one or more of the following categories: white, black/African American, American Indian/Alaskan native, Asian, and multiple race. Our analysis focuses on blacks/African Americans and whites for ease of interpretation.
Socioeconomic status.
Educational attainment was categorized as less than high school (<HS) (no high school diploma), high school (HS) (high school or general equivalency diploma), and greater than high school (>HS) (any education beyond high school). Household income was categorized into 0 to <35,000, ≥35,000 to <75,000, and ≥75,000 USD. Poverty status was based on the participants’ best estimate of total income of all family members from all sources. Occupation, based on the Standard Occupational Classification System, was analyzed with 22 robust categories. At the time of the survey, only adults working at a paying or nonpaying job during the week prior to the survey, who had a job or business but were not at work during the week prior to the survey, or who had ever worked were asked about their occupation. Class of work (based on current, longest held, or most recently held job or work situation) was classified as 1) an employee of a private company, a business, or an individual for wages, salary, or commission; 2) a federal, state, or local government employee; 3) self-employed in own business, professional practice, or farm; or 4) working without pay in a family-owned business or farm.
Health behaviors.
Smoking status was categorized as “ever” or “never.” Lifetime alcohol drinking status was assessed and classified as either “ever” or “never.” Leisure-time physical activity was analyzed as “none,” “low,” or “high.” Engagement in at least some level of activity based on reported number of moderate-to-vigorous activity bouts was dichotomized at their median and classified as “low” or “high.” Participants reporting “never” or “unable to do this type activity” were classified as “none.”
Medical conditions other than diabetes.
Sample adults were asked if they had ever been told by a doctor or other health professional that they had “hypertension, also called high blood pressure.” We combined reports of diagnosed heart conditions (e.g., coronary heart disease, myocardial infarction) to adjust for heart disease. Although depression was not consistently measured over the study period, a select number of participants who reported an activity limitation were asked whether they were chronically limited by depression, anxiety, or emotional problems.
Covariates.
Based on self-report, BMI was calculated by dividing weight in kilograms by the square of height in meters. Obesity was defined as BMI ≥30 kg/m2, overweight 25.0–29.9 kg/m2, normal weight 18.5–24.9 kg/m2, and underweight <18.5 kg/m2 (15). Self-reported health status was categorized as excellent/very good, good, or fair/poor. Marital status was categorized as married, divorced/separated/widowed, or never married, and regions of the country were categorized as South, Midwest, Northeast, and West.
Statistical analysis
We pooled eight survey years (2004–2011) of NHIS data merged by the Integrated Health Interview Series (16), a federal effort to create consistent codes and documentation based on public use data files of the NHIS. Sampling weights that account for the unequal probabilities of selection resulting from the sample design, nonresponse, and oversampling of certain subgroups were used for all analyses. Taylor series linearization was used to calculate SEs (17). A two-sided P value <0.05 was considered statistically significant. STATA statistical software, version 12 (STATA, College Station, TX), was used for all analyses (18).
Continuous variables were expressed as means ± SE, whereas categorical variables were presented as absolute values with corresponding percentages. The Rao-Scott Second-order corrected Pearson statistic takes survey weights into account and was used to test for differences in prespecified sociodemographic, clinical, and behavioral characteristics between whites and blacks with regard to sleep duration and diabetes.
To estimate prevalence ratios (PRs) corresponding 95% CIs, we used Poisson regression models with a robust variance estimator, which is preferred over the traditional logistic regression model providing odd ratios that confer overestimates when the outcome is prevalent (≥10%) (19). Prespecified socioeconomic, health behavior, demographic, and clinical characteristics were entered into the model as a group in an inclusive stepwise manner, and white participants were used as the reference categories for the black-white comparisons because they had the largest sample size. For models stratified by race as well as a separate model with a multiplicative interaction term for race and sleep for which the value is to be added to the respective regression slope for black participants, we first adjusted for age (25–49, 50–64, and ≥65 years) and sex (male, female) and then for socioeconomic factors (i.e., household income, poverty status, education, occupation, and employment status). We subsequently adjusted for alcohol consumption, smoking status, leisure-time physical activity, and marital status before adjusting (in a separate model) for heart disease, hypertension, and standard BMI categories. During the adjustment for medical conditions, we excluded participants with chronic limiting depression, anxiety, or emotional problems, as during all survey years only a select number of participants (n = 3,110) who reported an activity limitation were asked about whether they were chronically limited by the aforementioned conditions. In sensitivity analyses, we examined separately whether sex and age modified the relationship between sleep and diabetes.
RESULTS
Among 130,943 participants, mean age was 50.6 years, 49% were men, 13% were black, and 30% had at least a college education. Usual sleep among all participants was 7.1 ± 0.01 h, and 9% reported having had a diabetes diagnosis.
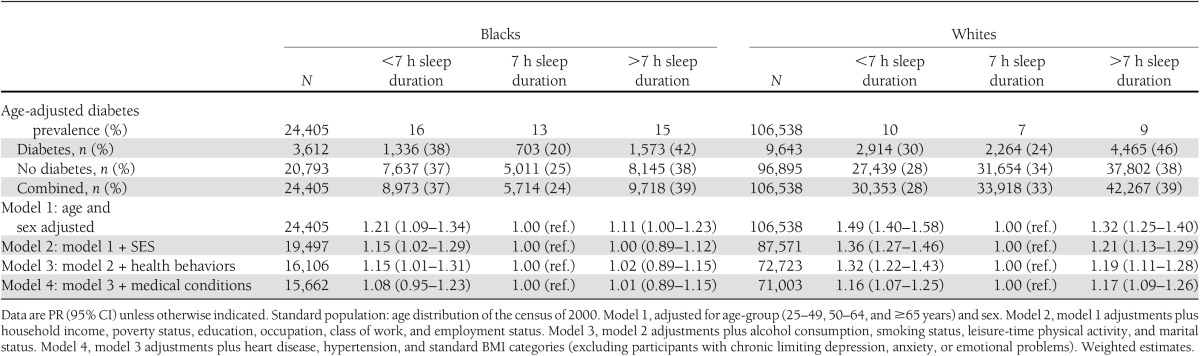
Sociodemographic, health behavior, and clinical characteristics of participants by sleep duration and race/ethnicity are provided in Table 1. Compared with blacks in all categories of sleep duration, whites were older, less likely to be women and to live in poverty, and more likely to have a college education, be married, and be self-employed. Across all sleep categories, blacks were more likely to live in the southern region of the country, be never smokers and alcohol consumers, and be more likely to report never engaging in leisure-time physical activity. Blacks were also more likely than whites to be overweight/obese and hypertensive as well as to report a fair/poor health status. Diabetes prevalence (9,643 [9%] in whites and 3,612 [15%] in blacks) varied by short, optimal, and long sleep duration for whites (10, 7, and 9%, respectively) and blacks (16, 13, and 15%, respectively) (Fig. 1A). The mean predicted probability of diabetes was also substantially higher in blacks compared to whites at every hour of sleep duration until the extreme at 11 hours of sleep or more (Fig. 1B).
Table 1.
Sociodemographic, health behavior, and clinical characteristics by sleep duration and race/ethnicity among NHIS participants, 2004–2011 (N = 130,943)
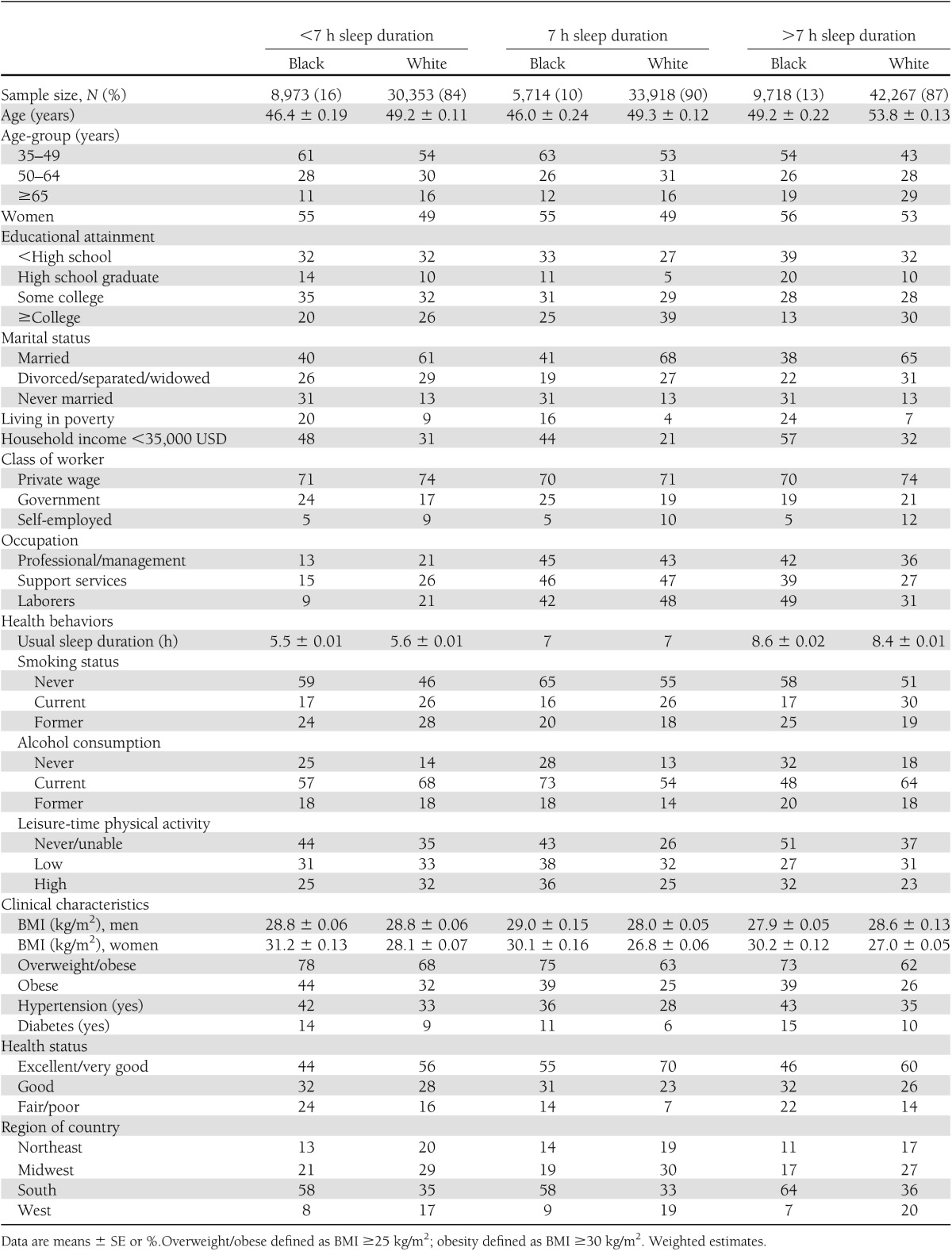
Figure 1.
Weighted estimates. Standard population based on age distribution of census of 2000, NHIS 2004–2011. A: Age-adjusted diabetes prevalence by sleep duration for blacks and whites. B: Mean predicted probability of diabetes by hours of sleep among blacks and whites. Postestimation means adjusted for age-group, sex, household income, poverty status, education, occupation, class of work, employment status, alcohol consumption, smoking status, leisure-time physical activity, marital status, standard BMI categories, heart disease, and hypertension (and excluding participants with chronic limiting depression, anxiety, or emotional problems).
Explanatory factors for the sleep–diabetes relationship among blacks and whites
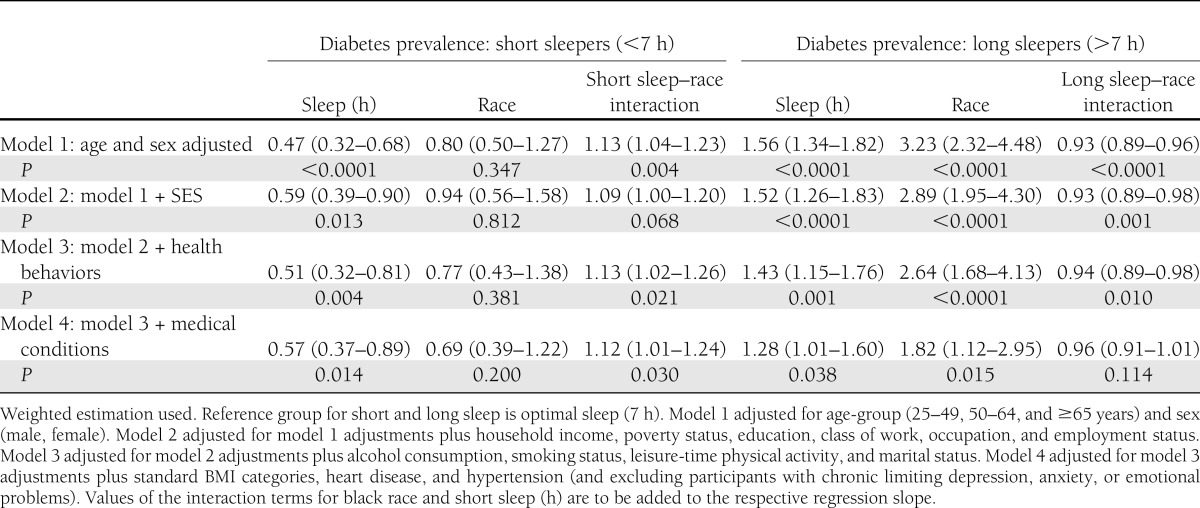
As displayed in Table 2, blacks were more likely to report short sleep duration (37 vs. 28%), less likely to get 7 h of sleep (24 vs. 33%), and had the same proportion of long sleepers (39 vs. 39%) compared with whites. There was a U-shaped association between diabetes and sleep duration in both blacks and whites, and nonsuboptimal sleep duration was more strongly associated with diabetes in whites than blacks among both short sleepers (PR 1.49 [95% CI 1.40–1.58] vs. PR 1.21 [95% CI 1.09–1.34]) and long sleepers (PR 1.32 [95% CI 1.25–1.40] vs. PR 1.11 [95% CI 1.00–1.23]) in age- and sex-adjusted models. After adjustment for household income, education, poverty, occupation, class of work, and employment, the sleep–diabetes association in blacks who were short sleepers was attenuated (PR 1.15 [95% CI 1.02–1.29]) but became nonsignificant only after adjustment for health behaviors and medical conditions (PR 1.08 [95% CI 0.95–1.23]). Long duration of sleep was no longer associated with diabetes prevalence in blacks after adjustment for socioeconomic status (SES) (PR 1.00 [95% CI 0.89–1.12]). The magnitude of attenuation after SES adjustment was similar for blacks and whites. Among whites, long and short sleep remained significantly associated with diabetes prevalence after further adjustment for health behaviors, marital status, BMI, heart disease, and hypertension among participants without chronic limiting depression, anxiety, or emotional problems.
Table 2.
Diabetes prevalence and PRs by sleeping duration for blacks and whites adjusted for individual socioeconomic, demographic, health behavior, and clinical characteristics: NHIS, 2004–2011
Explanatory factors for the black-white disparity in the sleep–diabetes relationship
Although the diabetes PRs were weaker in blacks compared with whites, blacks had a stronger association between diabetes and both short (P for interaction = 0.004) and long (P for interaction <0.0001) sleep in the age-adjusted model (Table 3). The association between diabetes and black race was stronger in long versus short sleepers, and short sleep remained significantly associated with diabetes (P for interaction = 0.030) in the fully adjusted model, while long sleep was significant until additional adjustment for medical conditions (P = 0.114). For short sleepers, the racial/ethnic difference in the sleep–diabetes association was nonsignificant after adjustment for socioeconomic factors (P = 0.068) but became statistically significant after additional adjustment for health behaviors and medical conditions (P = 0.030). For long sleepers, the racial/ethnic difference remained significant after adjustment for socioeconomic factors and health behaviors (P = 0.010) but became nonsignificant after controlling for medical conditions (P = 0.114).
Table 3.
Racial differences in diabetes PRs by sleep duration adjusted for individual-level socioeconomic, demographic, health behavior, and medical conditions: NHIS, 2004–2011
CONCLUSIONS
We found that blacks were more likely to report short sleep duration, less likely to get 7 h of sleep, and had the same proportion of long sleepers compared with whites. The prevalence of diabetes showed a U-shaped distribution with sleep duration in both whites and blacks, but blacks had significantly higher diabetes prevalence at a given sleep duration. Socioeconomic factors appeared to explain much (though not all) of the association between sleep and diabetes in blacks. The observed racial/ethnic disparity in the short sleep–diabetes relationship was partially explained by SES. Additional adjustment or behavioral factors and medical conditions, which are considered downstream products of SES, however, accentuated the racial disparity in the short sleep–diabetes relationship.
Based on both objective and subjective measures, previous studies have found that sleep duration differs for blacks and whites (20). Most studies, using objective measures like actigraphy or polysomnography, found that blacks have shorter sleep durations than whites (21). It has also been suggested that blacks have more extreme sleep durations than whites (22), which was consistent with our findings, as blacks were more likely to report short sleep and less likely to have sufficient sleep, and although the same proportion had long sleep, blacks were more likely to have extreme lengths of sleep (Supplementary Fig. 2).
Whites appear to get more and better-quality sleep compared with racial/ethnic minorities, and blacks are the most likely to get shorter, more restless sleep (23). Short sleep duration has been shown to have more deleterious health effects in blacks compared with whites, as blacks are at a particularly increased risk for metabolic disorders like insulin resistance and hypertension (24). Although not supported by results from this study, racial differences in sleep may occur because blacks are more likely than whites—due to lower SES—to be exposed to physical and social environments with greater noise and light pollution as well as stressful activity (e.g., single parenting, crime) that may interfere with the acquisition of optimal sleep (23). It is well known that blacks (especially women) experience more weight gain, and some physiologic studies suggest that insufficient sleep may lead to increased weight by influencing one’s appetite, levels of physical activity, and thermoregulation (25). For example, sleep deprivation has been linked to an increased appetite for sweet and salty foods, which could contribute to the poorer diet quality observed in blacks and disadvantaged populations (e.g., shift workers) (26). Sleep quality has also been shown to be mediated by perceived stress in blacks (27). Therefore, based on the interaction after SES adjustment alone, it is plausible that observed racial/ethnic disparities in sleep are partially explained by differences in SES because it is a strong determinant of the factors (e.g., health behaviors, medical conditions) that were subsequently adjusted. It has also been suggested that cultural values, beliefs, and practices may be important determinants of sleep sufficiency that should be investigated in addition to biopsychosocial determinants (28). Cultural factors (e.g., late-night socializing) may also impair optimal sleep (23).
Regarding the potential importance of socioeconomic factors on sleep, a recent study by Ertel et al. (29) investigated the interplay between socioeconomic factors, occupational exposures, and race/ethnicity on sleep duration, hypothesizing that low education and income as well as occupational exposures related to night work and job strain would account for the ethnic difference in sleep duration between African/Caribbean immigrants and whites. The authors found that socioeconomic and occupational characteristics explained some but not all of the ethnic difference in sleep duration among health care workers. It would be useful for a future study with comparable data to investigate these potential mechanisms in a nationally representative sample of U.S.-born blacks and whites. Another study investigating short sleep duration across income, education, and racial/ethnic groups over 34 years of follow-up found that socioeconomic position is a robust determinant of short sleep duration, even after adjustment for health-related characteristics linked to short sleep duration (22).
In terms of potential racial differences in the sleep–diabetes relationship, a previous cross-sectional study investigating socioeconomic, demographic, and medical determinants with just under 30,000 participants demonstrated a higher prevalence of diabetes among individuals with suboptimal sleep duration (30). The authors concluded that SES and comorbid conditions did not explain the excess diabetes that blacks experienced. They, however, adjusted for an incomplete list of socioeconomic characteristics by not incorporating occupational factors, which have been shown to be potentially important explanatory factors for black-white differences in sleep duration among immigrants (29). They also used a reference of 6–8 h of sleep and included participants starting at 18 years old whose individual socioeconomic factors may not accurately reflect their actual socioeconomic position and access to health-promoting resources, as individuals in this age-group are often in an unstable stage in life.
Our finding that diabetes remained significantly associated with long sleep in whites after all adjustments may have occurred because we were unable to account for factors like perceived stress that have been associated with long sleep in previous studies (31). Racial/ethnic differences in social and cultural factors (e.g., late-night socializing with friends and family) may start early in life and contribute to disparities in the sleep–diabetes association (32). The long sleep–diabetes association is also subject to reverse causation, as individuals at higher risk of developing diabetes—like those with depression or chronic disease—may sleep longer because of the underlying condition. Last, sleep quality may be lower in long sleepers, but these factors were not adjusted for in the analyses.
Potential mechanisms linking sleep to diabetes may differ between short and long sleep. For instance, suboptimal short sleep has been shown to increase insulin resistance, impair glucose metabolism (especially in the brain), and increase blood pressure, cortisol levels, and low-grade inflammation (33). Associated with hunger/appetite and caloric intake due to increases in ghrelin and decreases in leptin, sleep restriction may also lead to greater time to eat as well as fatigue leading to lower physical activity levels, thereby increasing risk of weight gain and subsequent health risks (33). Furthermore, dopamine levels and gastric reflux have also been considered important contributors to insomnia as well as to difficulties in maintaining sleep (34).
The potential mechanisms underlying the association between long sleep duration and increased diabetes risk are currently considered more speculative. Depression, unemployment, physical inactivity, poor health status, and chronic health conditions may contribute to suboptimal, long sleep and its association with diabetes (3,28). In terms of behavioral mechanisms, individual characteristics that could further impact sleep acquisition include smoking and alcohol consumption (28). Obstructive sleep apnea, as an example, is a known cause of daytime sleepiness or increased need for sleep and has been identified as a risk factor for insulin resistance and diabetes (35). An additional mechanism could be the sleep-inducing effects of proinflammatory cytokines that have been associated with elevated insulin resistance or obesity (36). Among those with diabetes, it is also possible that long sleep is a consequence of the diabetic or inflammatory state, as diabetes itself could impair the quantity of sleep owing to nocturia, neuropathic pain, or other pathologies that individuals with diabetes often experience (36).
Our study has several limitations that deserve to be mentioned. First, we had a cross-sectional study design, which is not ideal for assessing diabetes risks and elucidating causal factors, as the lack of temporality makes it difficult to determine whether the modifiable factors we adjusted for served as confounders or mediators of the sleep–diabetes relationship. Second, we also relied on self-reported sleep duration, whereas actigraphy and polysomnography (the gold standard) provide more objective measures (21). However, a moderate correlation (r = 0.45) between reported and measured sleep has been shown, and blacks and whites do not appear to appreciably differ in their reporting error regarding sleep duration (21). Third, sleep quality—affected by factors like sleep apnea—is an independent predictor of diabetes risk, but it was not assessed in our study (12). Fourth, we did not have access to disease verifications, but self-reports of diagnosed diabetes have been shown to be valid (37). Fifth, owing to data collection procedures, we were also unable to separate participants with type 1 versus type 2 diabetes in order to exclude participants with type 1 diabetes from the group reporting diabetes status. We were, however, able to exclude those who reported a diabetes diagnosis and insulin use when ≤25 years old. Furthermore, type 1 diabetes represents 5% of diabetes cases, which makes it reasonable to assume that the vast majority of cases were type 2 diabetes. Last, our adjustments for occupation categories may include a disproportionate number of shift workers, and most categories may be confounded by sleep duration. These categories also correlate with wealth or other parameters of interest. We, however, did not have data on shift work.
Our study had important strengths despite the limitations. For instance, our data were based on a large sample size with a large minority population where robust stratifications were possible. Modification testing for several a priori factors of interest was also possible because of the large sample size. Furthermore, our data are nationally representative, and we had access to recent data. We also directly estimated PRs rather than odds ratios for easier interpretation by public health professionals.
As suboptimal sleep duration was positively associated with diabetes in blacks and whites and modifiable socioeconomic characteristics appear to largely explain the association in blacks as well as the disparity between ethnicities for short sleepers, the main implication of our study is that there are modifiable, explanatory factors related to SES for the sleep-diabetes association as well as racial/ethnic disparities in the association. Our findings, along with those from previous studies, suggest that increased awareness and modification of physical, home, and working environments that may impair optimal sleep should be investigated (32,38). Future studies should use prospective designs to investigate racial differences in diabetes risk by sleep. Studies should also explore possible structural mechanisms/interventions related to socioeconomic factors to elucidate and address racial/ethnic disparities in the sleep–diabetes association.
Supplementary Material
Acknowledgments
C.L.J., S.R., and F.B.H. were supported by Transdisciplinary Research in Energetics and Cancer (TREC) (1U54CA155626-01).
No potential conflicts of interest relevant to this article were reported.
The funding sources were not involved in the data collection, data analysis, manuscript writing, or publication.
C.L.J. participated in the development of the study concept and design, acquired data (publically available), analyzed and interpreted data, drafted the manuscript, critically revised the manuscript for important intellectual content, performed statistical analysis, performed quality assurance and control, and gave final approval of the manuscript. S.R. interpreted data, critically revised the manuscript for important intellectual content, and gave final approval of the manuscript. I.K. and F.B.H. participated in the development of the study concept and design; interpreted data; critically revised the manuscript for important intellectual content; performed quality assurance and control; provided administrative, technical, and material support; obtained funding; supervised the study; and gave final approval of the manuscript. C.L.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0777/-/DC1.
References
- 1.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs 2004;19:56–59 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. QuickStats: percentage of adults aged >18 years who reported an average of <6 hours of sleep per 24-hour period? Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5708a8.htm. Accessed 15 January 2013
- 3.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 2010;71:1027–1036 [DOI] [PubMed] [Google Scholar]
- 4.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zizi F, Pandey A, Murrray-Bachmann R, et al. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. Am J Med 2012;125:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol 2009;19:351–357 [DOI] [PubMed] [Google Scholar]
- 7.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–2259 [DOI] [PubMed] [Google Scholar]
- 8.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 1998;21:518–524 [DOI] [PubMed] [Google Scholar]
- 9.Touma C, Pannain S. Does lack of sleep cause diabetes? Cleve Clin J Med 2011;78:549–558 [DOI] [PubMed] [Google Scholar]
- 10.Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001;21:1656–1662 [DOI] [PMC free article] [PubMed]
- 11.Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc 2008;100:317–322 [DOI] [PubMed] [Google Scholar]
- 12.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006;166:1768–1774 [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey. Hyattsville, MD, National Center for Health Statistics. Available from http://www.cdc.gov/nchs/nhis.htm Accessed 20 December 2012
- 14.Voss U, Tuin I. Integration of immigrants into a new culture is related to poor sleep quality. Health Qual Life Outcomes 2008;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Expert Committee on Physical Status. Use and Interpretation of Anthropometry. Physical Status: the Use and Interpretation of Anthropometry: Report of a WHO Expert Committee Geneva, World Health Org., 1995 (Tech. Rep. Ser., no. 854) [PubMed] [Google Scholar]
- 16.Minnesota Population Center and State Health Access Data Assistance Center Integrated Health Interview Series: Version 3.0. Minneapolis, University of Minnesota, 2010 [Google Scholar]
- 17.Wolters KM. Introduction to Variance Estimation. New York, NY, Springer-Verlag, 1990 [Google Scholar]
- 18.StataCorp. 2011. Stata Statistical Software: Released 12. College Station, TX: StataCorp LP [Google Scholar]
- 19.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med 2011;12:209–214 [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol 2007;17:948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med 2006;4:29–44 [DOI] [PubMed] [Google Scholar]
- 24.Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Ann Epidemiol 2010;20:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–850 [DOI] [PubMed] [Google Scholar]
- 27.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES). BMC Public Health 2010;10:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med 2013;79:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ertel KA, Berkman LF, Buxton OM. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep 2011;34:509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry 2000;47:921–927 [DOI] [PubMed] [Google Scholar]
- 31.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol 2009;169:1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin Crabtree V, Beal Korhonen J, Montgomery-Downs HE, Faye Jones V, O’Brien LM, Gozal D. Cultural influences on the bedtime behaviors of young children. Sleep Med 2005;6:319–324 [DOI] [PubMed] [Google Scholar]
- 33.Zimberg IZ, Dâmaso A, Del Re M, et al. Short sleep duration and obesity: mechanisms and future perspectives. Cell Biochem Funct 2012;30:524–529 [DOI] [PubMed] [Google Scholar]
- 34.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007;30:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–5771 [DOI] [PubMed] [Google Scholar]
- 37.Margolis KL, Lihong Qi, Brzyski R, et al. Women Health Initiative Investigators Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomfohr L, Pung MA, Edwards KM, Dimsdale JE. Racial differences in sleep architecture: the role of ethnic discrimination. Biol Psychol 2012;89:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.