Abstract
OBJECTIVE
To evaluate the prevalence of diabetic nephropathy and microalbuminuria in pregnant women with type 2 diabetes in comparison with type 1 diabetes and to describe pregnancy outcomes in these women following the same antihypertensive protocol.
RESEARCH DESIGN AND METHODS
Among 220 women with type 2 diabetes and 445 women with type 1 diabetes giving birth from 2007–2012, 41 women had diabetic nephropathy (albumin-creatinine ratio ≥300 mg/g) or microalbuminuria (albumin-creatinine ratio 30–299 mg/g) in early pregnancy. Antihypertensive therapy was initiated if blood pressure ≥135/85 mmHg or albumin-creatinine ratio ≥300 mg/g.
RESULTS
The prevalence of diabetic nephropathy was 2.3% (5 of 220) in women with type 2 diabetes and 2.5% (11 of 445) in women with type 1 diabetes (P = 1.00). The figures for microalbuminuria were 4.5 (10 of 220) vs. 3.4% (15 of 445) (P = 0.39). Baseline glycemic control was comparable between women with type 2 diabetes (n = 15) and type 1 diabetes (n = 26). Blood pressure at baseline was median 128 (range 100–164)/81 (68–91) vs. 132 (100–176)/80 (63–100) mmHg (not significant) and antihypertensive therapy in type 2 versus type 1 diabetes was used in 0 and 62%, respectively, at baseline, increasing to 33 and 96%, respectively, in late pregnancy. Pregnancy outcome was comparable regardless type of diabetes; gestational age at delivery: 259 days (221–276) vs. 257 (184–271) (P = 0.19); birth weight 3,304 g (1,278–3,914) vs. 2,850 (370–4,180) (P = 0.67).
CONCLUSIONS
The prevalence of diabetic nephropathy and microalbuminuria in early pregnancy was similar in type 2 and type 1 diabetes. Antihypertensive therapy was used more frequently in type 1 diabetes. Pregnancy outcome was comparable regardless type of diabetes.
Youth onset of type 2 diabetes continues to increase worldwide (1), and pregnancy in women with type 2 diabetes is a substantial clinical problem (2). Cross-sectional studies show a higher prevalence of albuminuria in young adults with type 2 diabetes compared with type 1 diabetes (3–5). Subjects with youth-onset type 2 diabetes have a fourfold increased risk of end-stage kidney disease compared with youth with type 1 diabetes (6). Literature focusing on kidney involvement in pregnant women with diabetes is scarce (7–11). To our knowledge, no studies using strict diagnostic criteria have described the prevalence of diabetic nephropathy and microalbuminuria in early pregnancy in women with type 2 diabetes. Likewise, recommendations for an antihypertensive strategy during these pregnancies is missing.
In pregnant women with type 1 diabetes, nephropathy is associated with poor pregnancy outcome in terms of increased rates of preeclampsia and preterm delivery (11–13). In these women, intrauterine growth restriction (11) occurs almost twice as often as in the general population (13), and in the late 1990s, preterm delivery before 34 weeks occurred in ∼30% (13). In women with type 1 diabetes and microalbuminuria, preterm delivery and preeclampsia are also frequent and serious complications (11,13,14).
In nonpregnant subjects with diabetes, inhibition of the renin angiotensin system is a cornerstone in the treatment of microalbuminuria and diabetic nephropathy (15–19). However, during pregnancy, antihypertensive therapy is often not indicated until sustained blood pressure elevations >150 mmHg systolic or 100 mmHg diastolic are documented (20), owing to concerns for reduced placental circulation, which may cause fetal hypoxia and intrauterine growth restriction (21).
In Copenhagen, we recommend blood pressure levels <135/85 mmHg and urinary albumin-creatinine ratio <300 mg/g during pregnancy in women with diabetes and diabetic nephropathy or microalbuminuria (14,22), regardless of the type of diabetes. Using this strategy, observational studies have shown that early initiation of intensive antihypertensive therapy during pregnancy leads to a reduced prevalence of preterm delivery in women with type 1 diabetes and diabetic nephropathy or microalbuminuria (14,22). Brown and Garovic (23) also recommend a lower threshold for antihypertensive treatment in pregnant women with diabetes and kidney involvement compared with nondiabetic pregnant women.
However, it has not been evaluated whether women with type 2 diabetes benefit from intensive antihypertensive therapy to the same degree as women with type 1 diabetes during pregnancy.
In this study, we evaluated the prevalence of diabetic nephropathy and microalbuminuria as well as pregnancy outcome in a recent cohort of pregnant women with type 2 or type 1 diabetes treated according to the same protocol for intensive antihypertensive treatment during pregnancy.
RESEARCH DESIGN AND METHODS
A retrospective cohort study among 665 singleton pregnancies in women with type 2 or type 1 diabetes with a living fetus at 22 weeks delivering at our center from January 2007 until October 2012 was conducted. The center is a referral center for all pregnant women with type 2 or type 1 diabetes from a geographically well-defined area of 2.5 million inhabitants. All women were asked to bring two urine samples for analysis of urinary albumin excretion at the first pregnancy visit. Urinary albumin-creatinine ratio was used in the majority of cases to evaluate kidney involvement since we previously reported a high concordance between albumin excretion in 24-h urine collection and urinary albumin-creatinine ratio (24). At least two values within the range of microalbuminuria or diabetic nephropathy were required to classify the patients. Forty-one women were identified with kidney involvement defined as presence of diabetic nephropathy (urinary albumin-creatinine ratio ≥300 mg/g) or microalbuminuria (urinary albumin-creatinine ratio 30–299 mg/g). At the first pregnancy visit, three women had nephrotic proteinuria (urinary albumin-creatinine ratio >2,000 mg/g).
One woman with type 1 diabetes and diabetic nephropathy had two pregnancies in the inclusion period; both pregnancies were included to ensure all available cases in the study period.
Women were recommended to perform self-monitored plasma glucose (SMPG) values seven times daily, before and 90 min after each main meal and before bedtime, and they were instructed to change insulin dose based on the SMPG values of the previous 3 days. Treatment targets were: preprandial SMPG of 4.0–6.0 mmol/L, 90-min postprandial SMPG of 4.0–8.0 mmol/L, and prebedtime SMPG of 6.0–8.0 mmol/L (25). HbA1c ≤5.6% in the second part of pregnancy was recommended (25).
The women visited our and/or their local diabetes clinic mainly at 2-week intervals during pregnancy. At each visit, weight, HbA1c, insulin dose, and blood pressure were recorded, and the urine was screened for proteinuria with a dipstick test (Urinstix; Bayer Diagnostics, Bridgend, U.K.) on sterile urine. Further analysis of urinary albumin-creatinine ratio was performed if proteinuria (>1+ on the dipstick) was present. The urinary albumin excretion was analyzed using an ELISA (26), and the blood creatinine concentration was measured by standard laboratory methods.
Blood pressure was measured with a digital blood pressure monitor (A&D Instruments, Abingdon, U.K.) in a sitting position after 5–10 min of rest.
If urinary albumin-creatinine ratio was ≥300 mg/g or blood pressure ≥135/85 mmHg, antihypertensive therapy was initiated or intensified. If ACE inhibitors were withdrawn during prepregnancy planning, another antihypertensive therapy was initiated unless the urinary albumin-creatinine ratio was close to normal (22).
Methyldopa (14,27) was the first-choice therapy in most cases, and, when indicated, labetalol and/or nifedipine (14) were added. If given before pregnancy, furosemide or thiazide was continued during pregnancy to reduce the risk of rebound fluid retention with increased blood pressure and urinary albumin excretion when discontinuing the drug (14,22).
Nonstress testing with cardiotocography was used routinely at least once weekly from 32–34 gestational weeks in addition to daily kick-counting. Fetal growth was evaluated routinely by obstetrical ultrasound at 28, 33, and 37 weeks of gestation. On clinical indications as judged by the obstetrician, flow measurements of the umbilical, cerebral, and uterine arteries were performed.
Preeclampsia was defined as two recordings of either systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and urinary albumin excretion >190 mg/24 h or proteinuria ≥1+ on a dipstick of sterile urine after 20 weeks of gestation. In women with diabetic nephropathy, the diagnosis was additionally based on a sudden increase of ≥15% in systolic or diastolic blood pressure (12).
Neonatal outcomes included early preterm delivery before 34 weeks, preterm delivery before 37 weeks, major congenital malformations (responsible for death, causing a significant future handicap, or requiring surgery), perinatal death (death occurring between 22 completed weeks and 1 completed week after delivery), low 5-min Apgar score (<7), transient tachypnea of the newborn (the need of continuous positive airway pressure for >1 h), neonatal hypoglycemia (plasma glucose <2.5 mmol/L) on the first plasma glucose value measured 2 h after delivery, or neonatal jaundice (need of photo therapy). Birth weight was evaluated by calculating SD z score to adjust for gestational age and sex and small and large for gestational age (weight for gestational age <10th percentile and >90th percentile, respectively) (28).
The study was approved by the Danish Data Protection Agency. According to Danish law, the regional committees for ethics and science did not have to be contacted.
Statistics
Data are given as median (range) or n (%). Differences between groups were analyzed using Fisher exact test and χ2 test, as appropriate, for categorical variables and Mann-Whitney test for continuous variables as data were nonnormally distributed.
The associations were considered to be statistical significant at a two-sided P value <0.05. All statistical analyses were performed using SPSS statistics 20.0 (SPPS, Chicago, IL).
RESULTS
The prevalence of diabetic nephropathy at baseline was 2.3% (5 of 220) in women with type 2 diabetes and 2.5% (11 of 445) in women with type 1 diabetes (P = 1.0). The prevalence of microalbuminuria was 4.5 (10 of 220) vs. 3.4% (15 of 445), respectively (P = 0.39), giving a total prevalence of kidney involvement in early pregnancy of 6.8% for type 2 diabetes vs. 5.8% for type 1 diabetes (P = 0.62).
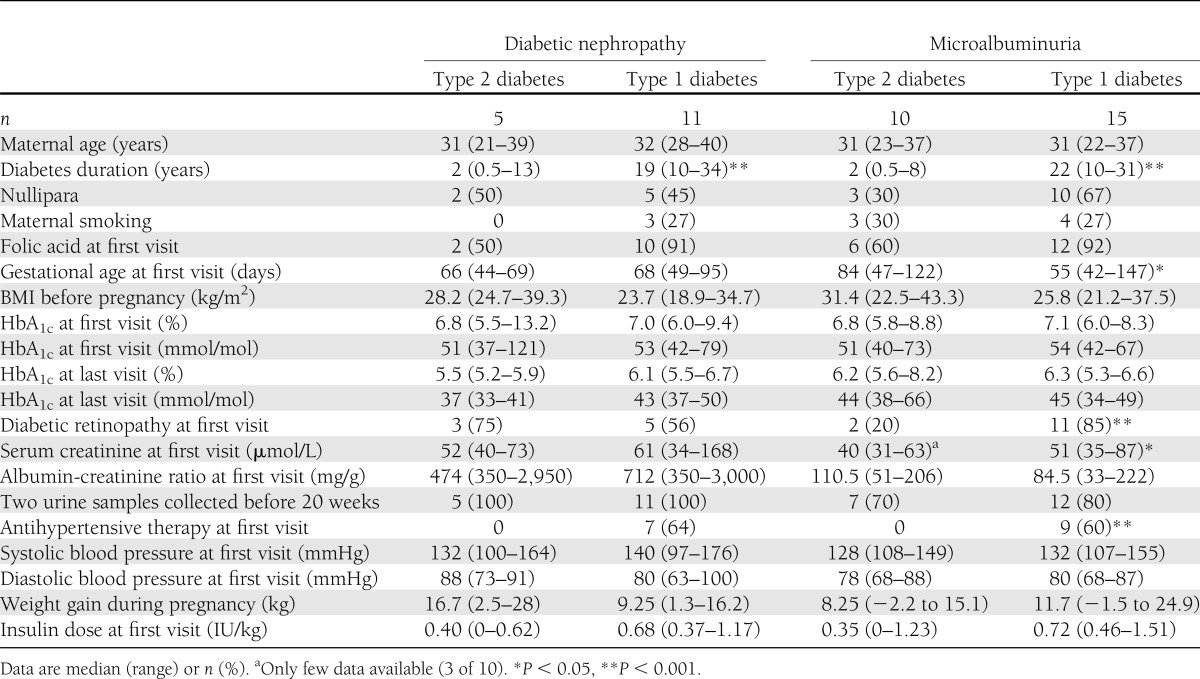
Baseline characteristics (Table 1) were comparable between women with kidney involvement with either type 2 diabetes (n = 15) or type 1 diabetes (n = 26), except for shorter duration of diabetes in women with type 2 diabetes. Elevated urinary albumin excretion rate was documented with two urine samples before 20 weeks in the majority of cases and with one urine sample in the remaining.
Table 1.
Maternal characteristics among 41 women with type 2 diabetes or type 1 diabetes and kidney affection during pregnancy
Glycemic control was similar in women with type 2 and type 1 diabetes (Table 1). At first pregnancy visit, antihypertensive therapy was not used in any of the women with type 2 diabetes and in 16 (62%) women with type 1 diabetes (P < 0.001).
In women with type 2 diabetes, antihypertensive therapy was initiated during pregnancy in four (80%) women with diabetic nephropathy and in two (20%) women with microalbuminuria. Antihypertensive therapy was mainly initiated in early pregnancy. In two (40%) women with diabetic nephropathy, at least three classes of antihypertensive drugs were indicated.
Among women with type 1 diabetes, nine were treated with ACE inhibitors before pregnancy, and eight continued this treatment during the organogenesis. Antihypertensive therapy was initiated or continued during pregnancy in 25 of 26 women, and 14 (54%) women received at least two classes of drugs. In women with diabetic nephropathy, six (55%) received at least three classes of drugs. The maximum number of antihypertensive drugs given was four.
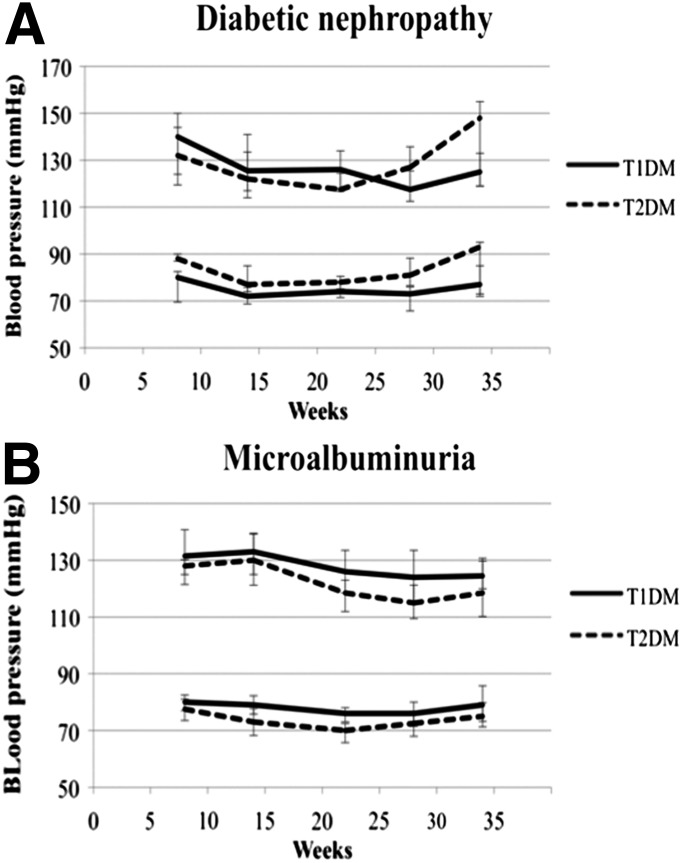
Blood pressure was stable during pregnancy in all groups without statistically significant differences (Fig. 1).
Figure 1.
Blood pressure during pregnancy among 41 women with type 2 (T2DM) or type 1 diabetes (T1DM). A: Women with nephropathy. B: Women with microalbuminuria. Data are expressed as median and interquartile range.
In early pregnancy, serum creatinine was within normal range in all women, except three (Table 1), and remained stable during pregnancy. No women developed end-stage kidney disease. Development of nephrotic proteinuria during pregnancy occurred in one (2.4%) woman with type 1 diabetes and diabetic nephropathy.
Among all 41 women, 11 (27%) received low-dose aspirin during pregnancy evenly distributed among the four groups. Five (20%) women with type 1 diabetes were treated with thyroid hormone replacement for hypothyroidism during pregnancy. None received antidepressant therapy.
Abnormal flow was measured by ultrasound in four women with type 1 diabetes (three with nephropathy and one with microalbuminuria) and was a contributing factor to induced preterm delivery based on obstetrical judgment. Three of these four women received antihypertensive treatment.
None of the routine nonstress tests alone led to induction of labor or cesarean section before planned.
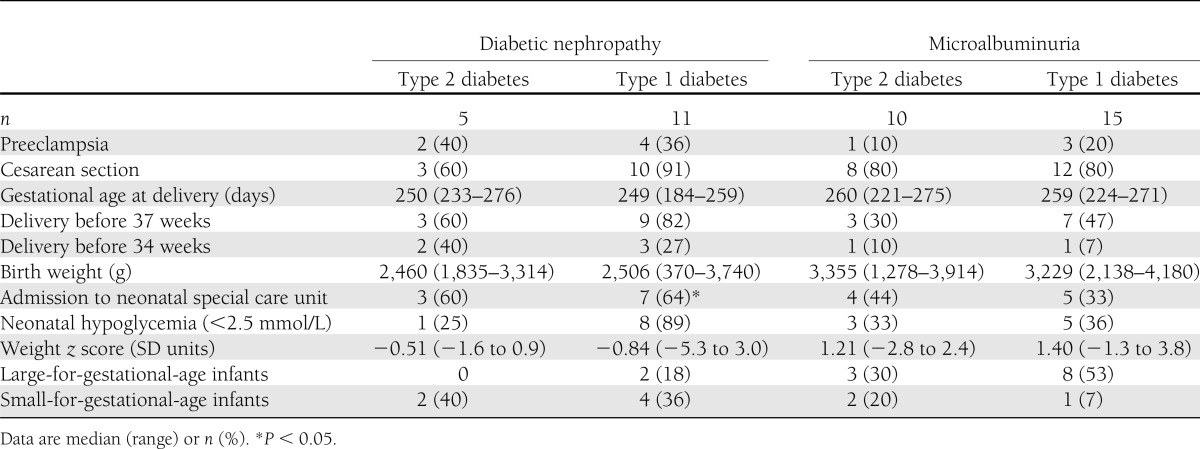
Pregnancy outcome was comparable in women with type 2 and type 1 diabetes (Table 2). The main causes for preterm delivery were preeclampsia, macrosomia, or suspected placental insufficiency. In women with type 2 and type 1 diabetes, birth weight was 3,304 g (1,278–3,914) and 2,850 (370–4,180), respectively (P = 0.67). Birth weight was <2,000 g in four (10%) infants of women with kidney involvement.
Table 2.
Pregnancy outcome among 41 pregnancies in women with type 2 or type 1 diabetes and kidney affection during pregnancy
Major congenital malformations were recorded in two infants (5%); one live-born with hypospadias (the mother received ACE inhibitor during the organogenesis), and one stillborn with multiple kidney and heart malformations (no inhibition of the renin angiotensin system during organogenesis). One infant with severe intrauterine growth restriction (z score −5.25) and abnormal umbilical flow was delivered at 30 weeks and died 3 days postpartum. The mother had type 1 diabetes and diabetic nephropathy and was treated with three antihypertensive agents during pregnancy. One year later, this woman completed an uneventful pregnancy while receiving four types of antihypertensive agents.
CONCLUSIONS
This study demonstrates that the prevalence of diabetic nephropathy and microalbuminuria in pregnant women with type 2 diabetes was comparable to the prevalence in pregnant women with type 1 diabetes. Using the same antihypertensive strategy, pregnancy outcome was comparable regardless type of diabetes in women with kidney involvement. Antihypertensive therapy was used more frequently in women with type 1 diabetes and did not seem to affect pregnancy outcome negatively.
The women with type 2 diabetes and kidney involvement had a relatively shorter diabetes duration compared with the women with type 1 diabetes. This is in accordance with the findings in the nonpregnant population of children and young adults with type 2 diabetes in whom microalbuminuria and diabetic nephropathy are also present in subjects with a relatively shorter duration of diabetes (3–5).
In Danish pregnant women with type 1 diabetes, the prevalence of diabetic nephropathy has declined from 5% in the late 1990s (12) to 2.5% in the current study, in which the same diagnostic criteria was used. The prevalence of diabetic nephropathy in type 1 diabetes in the current study is in accordance with a prevalence of 0.5–5.0% in other recent studies using slightly different diagnostic criteria for diabetic nephropathy (7–9,29). The figures for type 2 diabetes were 0.8 and 2.6% (8,9).
The prevalence of microalbuminuria in Danish pregnant women with type 1 diabetes has declined from 11% in the 1990s (12) to 4.5% in the current study, using the same diagnostic criteria. This is in accordance with international findings reporting prevalence of microalbuminuria in pregnant women with type 1 diabetes between 10 and 30% in the late 1990s (11,30) and recent findings of 4.5% (29).
For pregnant women with type 2 diabetes, the prevalence of microalbuminuria in the late 1990s was 13 (2) and 33% (31).
The decline in prevalence of diabetic nephropathy and microalbuminuria in women with type 1 diabetes is in line with a general trend toward lower prevalence of diabetic nephropathy in the Danish diabetic population (32). This may be explained by improved glycemic control (33,34) and more frequent use of inhibitors of the renin angiotensin system (16,17,35).
Preconceptionally, women with type 2 diabetes are mainly followed at the general practitioner and were rarely followed at a specialized diabetes center. This may explain the lack of antihypertensive treatment in women with type 2 diabetes and diabetic nephropathy or microalbuminuria at the first pregnancy visit.
The strength of this study is that the data are unselected from one large center covering the entire eastern part of Denmark. The same intensive antihypertensive strategy was used during the whole period, and the same few doctors treated all patients. Blood pressure was almost stable during pregnancy in all groups without statistically significant differences, but numbers were too small for firm conclusions. Glycemic control was generally good, contributing to affect pregnancy outcome positively (36).
The low number of cases, despite the long inclusion period, and the retrospective design of the study are weaknesses. We asked all women for two urine samples at the first pregnancy visit. The majority of women were compliant to this request; in women with diabetic nephropathy, it was 100%, but among women with microalbuminuria, two samples were obtained in 70–80% of the cases. Some of these women might have had normal urinary albumin excretion in the next sample, which could lead to a slightly lower prevalence of microalbuminuria. For clinicians caring for these high-risk pregnancies, it is clinically relevant to describe the prevalence and pregnancy outcome in pregnant women with type 2 diabetes and diabetic nephropathy or microalbuminuria, as this group of patients is expected to increase in the near future. Pregnancy outcome, especially preterm delivery and preeclampsia, were in accordance to our previous results (22) using the same antihypertensive protocol in pregnant women with type 1 diabetes and diabetic nephropathy or microalbuminuria. Thus, this study supports that intensified antihypertensive treatment reduces adverse pregnancy outcome as previously described in women with type 1 diabetes and nephropathy (11,37) or microalbuminuria (14).
Serum creatinine was used as an index of renal function. We found stable serum creatinine levels during pregnancy for both women with diabetic nephropathy and microalbuminuria, regardless type of diabetes in accordance with earlier findings (22,38).
The use of intensive antihypertensive therapy during pregnancy may raise concern about fetal intrauterine growth restriction by lowering the placental blood flow. In women with microalbuminuria, median birth weight z score was positive in both groups, indicating sufficient fetal growth despite antihypertensive therapy given in 68% of these women.
The prevalence of small-for-gestational age infants in women with diabetic nephropathy was 40 and 36% for type 2 and type 1 diabetes, respectively, which is in accordance with our earlier findings (12). The high rate of small-for-gestational-age infants is most likely explained by the diabetic microangiopathy per se (39).
To evaluate properly whether initiation of antihypertensive therapy affects uteroplacental and fetal hemodynamics in diabetic pregnancy, a prospective study with nonstress tests and careful ultrasound examinations before and after initiation of antihypertensive therapy is needed.
In women with increased risk of preeclampsia, including pregestational diabetes mellitus complicated with diabetic nephropathy or microalbuminuria, treatment with low-dose aspirin initiated before 12 weeks of gestation may slightly reduce this risk (20,40). In this study, only one-fourth (11 of 41) of patients received aspirin during pregnancy, a factor that should be improved in the future with the purpose to reduce the rate of preeclampsia.
In conclusion, the prevalence of diabetic nephropathy and microalbuminuria in early pregnancy among women with type 2 diabetes is comparable to that in pregnant women with type 1 diabetes. Pregnancy outcome was comparable in women with kidney involvement regardless type of diabetes. It seems reasonable to assume that pregnant women with diabetic nephropathy or microalbuminuria could be treated and controlled in a similar way regardless type of diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
J.A.D. researched data, wrote the manuscript, and contributed to discussions. B.Á. and N.F.C. researched data, contributed to discussions, and reviewed and edited the manuscript. J.M.M., L.R., and B.W.P. contributed to discussions and reviewed and edited the manuscript. E.R.M. contributed to the idea, researched data, contributed to discussions, and reviewed and edited the manuscript. E.R.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented as a poster at Diabetes, Hypertension, Metabolic Syndrome and Pregnancy, Florence, Italy, 14–16 March 2013; and in abstract form at the North Europe Young Diabetologists meeting, Korsør, Denmark, 28–30 August 2013.
References
- 1.Dart AB, Sellers EA, Dean HJ. Kidney disease and youth onset type 2 diabetes: considerations for the general practitioner. Int J Pediatr 2012;2012:237360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clausen TD, Mathiesen E, Ekbom P, Hellmuth E, Mandrup-Poulsen T, Damm P. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care 2005;28:323–328 [DOI] [PubMed] [Google Scholar]
- 3.Eppens MC, Craig ME, Jones TW, Silink M, Ong S, Ping YJ, International Diabetes Federation Western Pacific Region Steering Committee Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin 2006;22:1013–1020 [DOI] [PubMed] [Google Scholar]
- 4.Scott A, Toomath R, Bouchier D, et al. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J 2006;119:U2015. [PubMed] [Google Scholar]
- 5.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2007;30:2593–2598 [DOI] [PubMed] [Google Scholar]
- 6.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landon MB. Diabetic nephropathy and pregnancy. Clin Obstet Gynecol 2007;50:998–1006 [DOI] [PubMed] [Google Scholar]
- 8.Murphy HR, Steel SA, Roland JM, et al. Obstetric and perinatal outcomes in pregnancies complicated by type 1 and type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med 2011;28:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly with pre-existing diabetes: a population-based cohort study. Diabetologia 2012;55:936–947 [DOI] [PubMed] [Google Scholar]
- 10.Kitzmiller JL, Combs CA. Diabetic nephropathy and pregnancy. Obstet Gynecol Clin North Am 1996;23:173–203 [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic L, Inturissi M. Assessment of glycemic control. In Managing Preexisting Diabetes Mellitus for Pregnancy. Kitzmiller JL, Ed. Alexandria, VA, American Diabetes Association, 2008, p. 379–386 [Google Scholar]
- 12.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Mølvig J, Mathiesen ER. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care 2001;24:1739–1744 [DOI] [PubMed] [Google Scholar]
- 13.Dunne FP, Chowdhury TA, Hartland A, et al. Pregnancy outcome in women with insulin-dependent diabetes mellitus complicated by nephropathy. QJM 1999;92:451–454 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen LR, Müller C, Damm P, Mathiesen ER. Reduced prevalence of early preterm delivery in women with type 1 diabetes and microalbuminuria—possible effect of early antihypertensive treatment during pregnancy. Diabet Med 2006;23:426–431 [DOI] [PubMed] [Google Scholar]
- 15.Parving HH, Smidt UM, Hommel E, et al. Effective antihypertensive treatment postpones renal insufficiency in diabetic nephropathy. Am J Kidney Dis 1993;22:188–195 [DOI] [PubMed] [Google Scholar]
- 16.Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ 1991;303:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathiesen ER, Hommel E, Hansen HP, Parving HH. Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ 1999;319:24–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes 2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathiesen ER, Rønn B, Storm B, Foght H, Deckert T. The natural course of microalbuminuria in insulin-dependent diabetes: a 10-year prospective study. Diabet Med 1995;12:482–487 [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence Guidance: Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London, National Institute for Health and Clinical Excellence, 2010 [Google Scholar]
- 21.Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S, CHIPS Study Group How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011;72:394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care 2009;32:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown CM, Garovic VD. Mechanisms and management of hypertension in pregnant women. Curr Hypertens Rep 2011;13:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justesen TI, Petersen JL, Ekbom P, Damm P, Mathiesen ER. Albumin-to-creatinine ratio in random urine samples might replace 24-h urine collections in screening for micro- and macroalbuminuria in pregnant woman with type 1 diabetes. Diabetes Care 2006;29:924–925 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care 2008;31:9–14 [DOI] [PubMed] [Google Scholar]
- 26.Feldt-Rasmussen B, Dinesen B, Deckert M. Enzyme immunoassay: an improved determination of urinary albumin in diabetics with incipient nephropathy. Scand J Clin Lab Invest 1985;45:539–544 [DOI] [PubMed] [Google Scholar]
- 27.Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol 1993;168:152–156 [DOI] [PubMed] [Google Scholar]
- 28.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848 [DOI] [PubMed] [Google Scholar]
- 29.McCance DR, Holmes VA, Maresh MJA, et al. Diabetes and Pre-eclampsia Intervention Trial (DAPIT) Study Group Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet 2010;376:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen DM, Damm P, Ovesen P, et al. Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes: results from a nationwide Danish study. Diabetes Care 2010;33:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cundy T, Slee F, Gamble G, Neale L. Hypertensive disorders of pregnancy in women with type 1 and type 2 diabetes. Diabet Med 2002;19:482–489 [DOI] [PubMed] [Google Scholar]
- 32.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003;26:1258–1264 [DOI] [PubMed] [Google Scholar]
- 33.Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet 1986;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 34.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 35.Bar J, Chen R, Schoenfeld A, et al. Pregnancy outcome in patients with insulin dependent diabetes mellitus and diabetic nephropathy treated with ACE inhibitors before pregnancy. J Pediatr Endocrinol Metab 1999;12:659–665 [DOI] [PubMed] [Google Scholar]
- 36.Holmes VA, Young IS, Patterson CC, et al. Diabetes and Pre-eclampsia Intervention Trial Study Group Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the Diabetes and Pre-eclampsia Intervention Trial. Diabetes Care 2011;34:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr DB, Koontz GL, Gardella C, et al. Diabetic nephropathy in pregnancy: suboptimal hypertensive control associated with preterm delivery. Am J Hypertens 2006;19:513–519 [DOI] [PubMed] [Google Scholar]
- 38.Rossing K, Jacobsen P, Hommel E, et al. Pregnancy and progression of diabetic nephropathy. Diabetologia 2002;45:36–41 [DOI] [PubMed] [Google Scholar]
- 39.Haeri S, Khoury J, Kovilam O, Miodovnik M. The association of intrauterine growth abnormalities in women with type 1 diabetes mellitus complicated by vasculopathy. Am J Obstet Gynecol 2008;199:278e1–5 [DOI] [PubMed] [Google Scholar]
- 40.Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet 1986;1:1–3 [DOI] [PubMed] [Google Scholar]