Abstract
OBJECTIVE
To investigate the efficacy and tolerability of empagliflozin as add-on to metformin and sulfonylurea in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patients inadequately controlled on metformin and sulfonylurea (HbA1c ≥7 to ≤10%) were randomized and treated with once-daily empagliflozin 10 mg (n = 225), empagliflozin 25 mg (n = 216), or placebo (n = 225) for 24 weeks. The primary end point was change from baseline in HbA1c at week 24. Key secondary end points were changes from baseline in weight and mean daily glucose (MDG) at week 24.
RESULTS
At week 24, adjusted mean (SE) changes from baseline in HbA1c were −0.17% (0.05) for placebo vs. −0.82% (0.05) and −0.77% (0.05) for empagliflozin 10 and 25 mg, respectively (both P < 0.001). Empagliflozin significantly reduced MDG, weight, and systolic (but not diastolic) blood pressure versus placebo. Adverse events were reported in 62.7, 67.9, and 64.1% of patients on placebo and empagliflozin 10 and 25 mg, respectively. Events consistent with urinary tract infection were reported in 8.0, 10.3, and 8.3% of patients on placebo and empagliflozin 10 and 25 mg, respectively (females: 13.3, 18.0, and 17.5%, respectively; males: 2.7, 2.7, and 0%, respectively). Events consistent with genital infection were reported in 0.9, 2.7, and 2.3% of patients on placebo and empagliflozin 10 and 25 mg, respectively (females: 0.9, 4.5, and 3.9%, respectively; males: 0.9% in each group).
CONCLUSIONS
Empagliflozin 10 and 25 mg for 24 weeks as add-on to metformin plus sulfonylurea improved glycemic control, weight, and systolic blood pressure and were well tolerated.
Metformin is the standard first-line pharmacotherapy to achieve glycemic control in patients with type 2 diabetes (1). However, metformin alone frequently fails to maintain glycemic control in the long term (2), and most patients with type 2 diabetes will require additional therapies (1). Although initially effective, sulfonylureas are associated with low durability (2), and common side effects are hypoglycemia and weight gain (3–5). Furthermore, as type 2 diabetes progresses, with deterioration of β-cell function and increased insulin resistance (6), the use of agents utilizing pathways dependent on insulin becomes increasingly difficult. In addition, steady increases in weight are observed in patients with type 2 diabetes (7), which may be associated with worsening markers of insulin resistance (1). Thus, there is still a great unmet need for effective and well-tolerated antidiabetes agents that can be used in combination with existing treatments to improve glycemic control in patients with type 2 diabetes, in particular without the risk of hypoglycemia and weight gain.
The sodium glucose cotransporter 2 (SGLT2), located in the proximal tubule of the kidney, represents a promising target for the treatment of type 2 diabetes. SGLT2 is responsible for tubular reabsorption of ∼90% of the glomerular filtrated glucose (8). In patients with type 2 diabetes, inhibition of SGLT2 leads to reduced renal glucose reabsorption and increased urinary glucose excretion, resulting in a reduction in hyperglycemia, irrespective of β-cell function or insulin resistance (9).
Empagliflozin is a potent and selective inhibitor of SGLT2 (10). In phase II trials in patients with type 2 diabetes, a 12-week treatment with empagliflozin as monotherapy or as add-on to metformin resulted in reductions in HbA1c, weight, and blood pressure and was well tolerated (11,12). These effects were shown to be sustained for up to 90 weeks (13).
The aim of this study (EMPA-REG METSU) was to evaluate the efficacy, safety, and tolerability of empagliflozin (10 and 25 mg once daily) versus placebo over 24 weeks as add-on therapy to metformin plus sulfonylurea in patients with type 2 diabetes with inadequate glycemic control. In addition, the efficacy and safety of empagliflozin 25 mg was investigated in poorly controlled patients with HbA1c >10% in an open-label treatment arm.
RESEARCH DESIGN AND METHODS
Study design
This was a randomized, placebo-controlled, double-blind phase III study conducted from July 2010 to February 2012 in 148 centers in 12 countries (Canada, China, France, Germany, India, Korea, Mexico, Slovakia, Slovenia, Taiwan, Turkey, and the U.S.). The clinical trial protocol was approved by the institutional review boards and independent ethics committees and competent authorities of the participating centers, and the trial complied with the Declaration of Helsinki, in accordance with the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice. The trial is registered with clinicaltrials.gov (NCT01159600). All patients provided written informed consent.
Inclusion and exclusion criteria
This study enrolled patients (aged ≥18 years; BMI ≤45 kg/m2) with inadequately controlled type 2 diabetes (HbA1c ≥7 to ≤10%) despite a diet and exercise program and a stable regimen (unchanged for ≥12 weeks prior to randomization) of metformin immediate release plus a sulfonylurea. Patients with HbA1c >10% were eligible to participate in an open-label treatment arm.
Exclusion criteria included uncontrolled hyperglycemia (glucose level >13.3 mmol/L) after an overnight fast, confirmed by a second measurement), acute coronary syndrome, stroke or transient ischemic attack within 3 months prior to consent, indication of liver disease, impaired kidney function (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) during screening or run-in, contraindications to metformin or sulfonylurea according to the local label, gastrointestinal surgeries that induce chronic malabsorption, history of cancer (except basal cell carcinoma) or treatment for cancer within 5 years, blood dyscrasias or any disorders causing hemolysis or unstable erythrocytes, treatment with antiobesity drugs 3 months prior to consent, use of any treatment at screening that leads to unstable body weight, treatment with systemic steroids at time of consent, change in dosage of thyroid hormones within 6 weeks of consent, alcohol or drug abuse within 3 months of consent, and investigational drug intake within 30 days of the trial.
Treatment and interventions
After a 2-week open-label placebo run-in period, eligible patients were randomized (1:1:1) to receive once-daily (in the morning with water) empagliflozin 10 mg, empagliflozin 25 mg, or placebo as add-on therapy to metformin (≥1,500 mg/day or maximum tolerated dose or maximum dose according to local label) plus a sulfonylurea (greater than or equal to half the maximum recommended dose, or the maximum tolerated dose, or the maximum dose according to local label) for 24 weeks. Randomization was performed using a third-party interactive voice and web response system and was stratified by HbA1c (<8.5 and ≥8.5%), eGFR (≥90, 60–89, and 30–59 mL/min/1.73 m2; Modification of Diet in Renal Disease equation), and region (Europe, Asia, North America, and Latin America). Patients allocated to the open-label arm received empagliflozin 25 mg for 24 weeks without run-in. Study visits were scheduled at screening; at the start of the placebo run-in period (randomized patients only); and at weeks 0, 6, 12, 18, and 24. Patients were followed up for 1 week after the treatment period.
Rescue medication was initiated during the treatment period if, between weeks 1 and 12, a patient had a glucose level >13.3 mmol/L after an overnight fast or, between weeks 12 and 24, a patient had a glucose level >11.1 mmol/L after an overnight fast or HbA1c >8.5%. The initiation, choice, and dosage of rescue medication were at the investigator’s discretion, according to local prescribing information. In cases of hypoglycemia, rescue medication was reduced or discontinued. Where hyper- or hypoglycemia could not be controlled, the patient was discontinued from the trial.
End points and assessments
The primary end point was the change from baseline in HbA1c at week 24. Key secondary end points were change from baseline to week 24 in body weight and mean daily glucose (MDG) using an 8-point blood glucose profile.
Exploratory end points included the following: percentage of patients with baseline HbA1c ≥7.0% who had HbA1c <7% at week 24; change from baseline in fasting plasma glucose (FPG), waist circumference, and systolic and diastolic blood pressure (SBP and DBP) at week 24; percentage of patients with >5% reduction in body weight at week 24; and use of rescue medication. Change from baseline in 2-h postprandial glucose (PPG) was assessed in a subset of patients based on a meal tolerance test (MTT) performed at baseline and week 24.
Safety end points included vital signs, clinical laboratory parameters, 12-lead electrocardiogram, and adverse events (AEs; preferred terms coded according to the Medical Dictionary for Drug Regulatory Activities version 14.1). AEs included all events with an onset after the first dose of trial medication up to a period of 7 days after the last dose. AEs of special interest included confirmed hypoglycemic AEs (plasma glucose ≤3.9 mmol/L and/or requiring assistance) and events consistent with urinary tract infection (UTI) and genital infection. Events consistent with UTI and genital infection were identified from AEs reported spontaneously by the investigator using prospectively defined search categories based on 67 and 87 preferred terms, respectively.
Statistical analysis
Efficacy analysis was performed on the full analysis set (FAS), which included all randomized patients treated with one or more doses of study drug who had a baseline HbA1c value. Two-hour PPG was analyzed in the MTT set (patients in the FAS with valid baseline and one or more on-treatment MTT measurements). Safety and lipid parameters were analyzed in the treated set (patients treated with one or more doses of study drug).
Values observed after a patient started rescue medication were set to missing. The last observation carried forward (LOCF) approach was used to impute missing continuous efficacy data. MDG was also analyzed based on observed cases (OCs). Categorical efficacy variables were analyzed using noncompleters considered failure imputation. LOCF-IR imputation (i.e., LOCF without setting values after rescue therapy to missing) was used for analysis of lipid parameters. Analyses of efficacy end points in the open-label set were based on OC.
The primary end point was assessed using an ANCOVA model, with treatment, region, and eGFR at baseline as fixed effects and baseline HbA1c as a linear covariate. Key secondary and continuous exploratory end points were analyzed using the statistical model described for the primary end point, with the baseline value for the end point in question as an additional linear covariate. Changes over time in HbA1c, FPG, and blood pressure were analyzed using restricted maximum likelihood–based mixed model repeated measures. Categorical change in HbA1c and the proportion of patients with >5% weight loss were analyzed using logistic regression.
Treatment differences versus placebo in primary and key secondary end points were tested using a hierarchical testing approach for each dose at a significance level of 2.5% (two sided) to maintain the overall type I error at 5%. All other exploratory tests were two sided at a 5% level (no multiplicity adjustment). Safety analyses and analyses of efficacy end points in the open-label group were descriptive.
Further details on statistical analysis including sample size calculation are given in Supplementary Section 1.
RESULTS
Patients
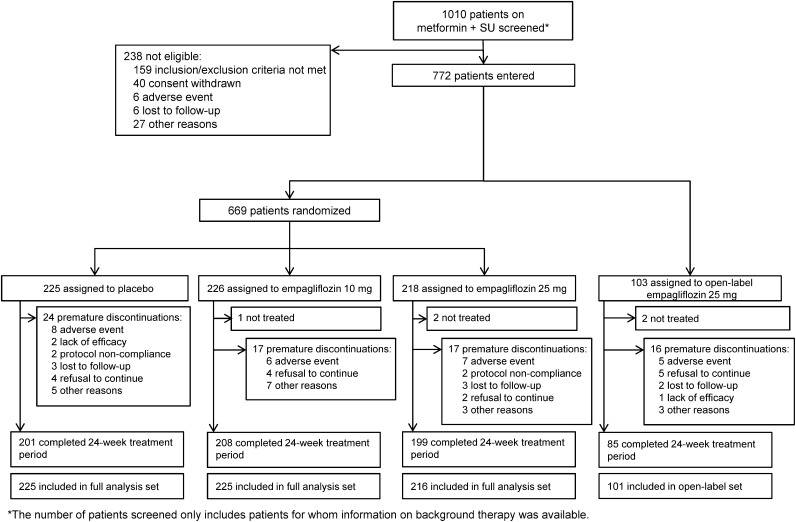
Patient disposition is shown in Fig. 1. A total of 669 patients were randomized, of whom 666 patients were treated double blind with study medication and comprised the FAS. Overall, 91.3% of randomized and treated patients completed the treatment period. A further 101 patients with HbA1c >10% were treated with open-label empagliflozin 25 mg, and 84.2% of these patients completed the treatment period. Two-hour PPG was evaluated in a subset of 124 patients (placebo, n = 35; empagliflozin 10 mg, n = 43; empagliflozin 25 mg, n = 46).
Figure 1.
Study flow.
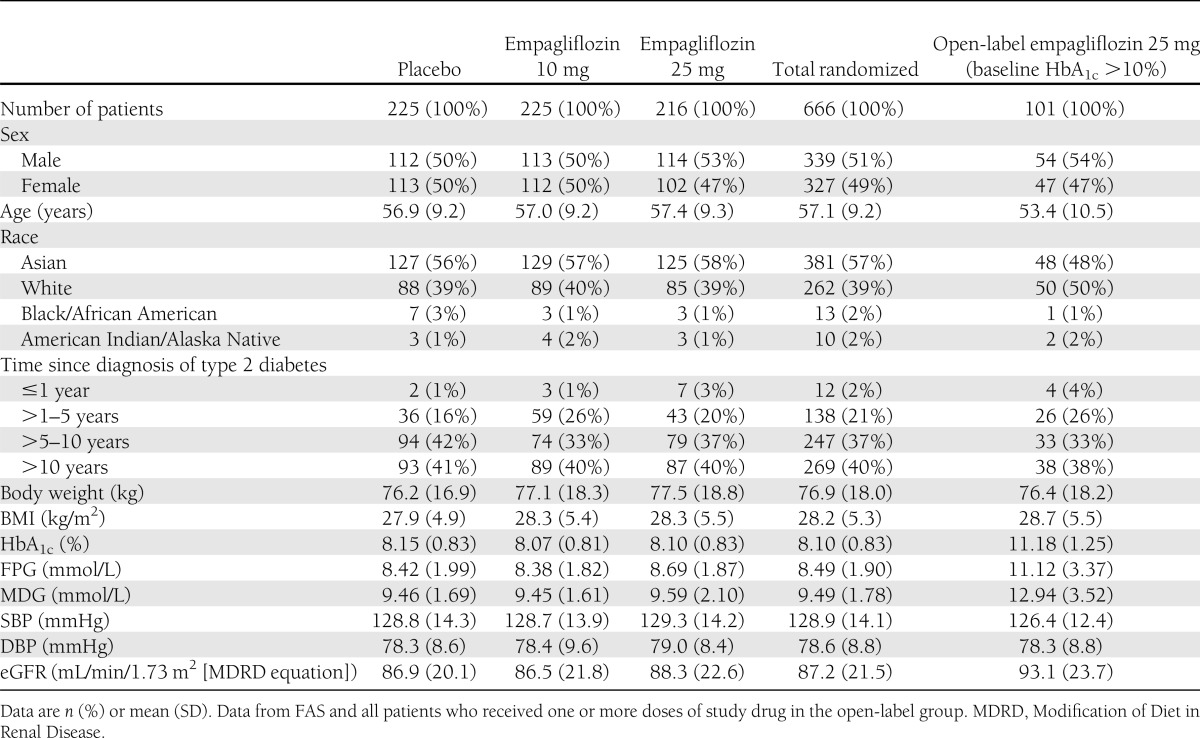
Baseline characteristics were balanced across treatment groups (Table 1). The mean (SD) age of randomized patients was 57.1 years (9.2); mean (SD) BMI was 28.2 kg/m2 (5.3). Mean (SD) baseline HbA1c in the randomized groups was 8.10% (0.83), 49.1% had a baseline HbA1c <8.0%, and 16.4% had a baseline HbA1c ≥9.0%.
Table 1.
Patient demographics and baseline characteristics
Efficacy
Randomized groups.
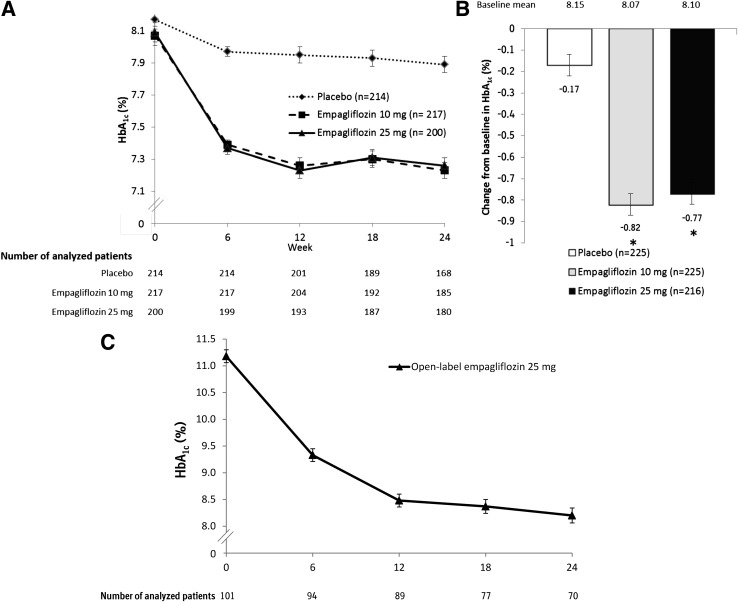
Adjusted mean HbA1c levels over the 24-week treatment period are shown in Fig. 2A. Reductions in HbA1c after 24 weeks were significantly greater in empagliflozin groups than in the placebo group, with adjusted mean (SE) changes of −0.17% (0.05) for placebo, compared with −0.82% (0.05) for empagliflozin 10 mg and −0.77% (0.05) for empagliflozin 25 mg (differences of adjusted means vs. placebo were −0.64% [95% CI −0.77 to −0.51] for empagliflozin 10 mg and −0.59% [−0.73 to −0.46] for empagliflozin 25 mg; P < 0.001 for both doses) (Fig. 2B and Supplementary Table 1). In patients with HbA1c ≥7.0% at baseline, a greater proportion of patients treated with empagliflozin 10 and 25 mg reached HbA1c <7.0% at week 24 (26.3 and 32.2%, respectively) compared with placebo (9.3%); odds ratios versus placebo were 3.85 (95% CI 2.17–6.85) for empagliflozin 10 mg and 5.22 (2.95–9.24) for empagliflozin 25 mg; P < 0.001 for both doses (Supplementary Fig. 1A, and Supplementary Table 1). In the subgroups of patients with normal renal function (eGFR ≥90 mL/min/1.73 m2), mild renal impairment (eGFR ≥60 to <90 mL/min/1.73 m2), and moderate renal impairment (eGFR ≥30 to <60 mL/min/1.73 m2), empagliflozin reduced HbA1c at week 24 versus placebo (P < 0.01 for each renal function subgroup) (Supplementary Table 2).
Figure 2.
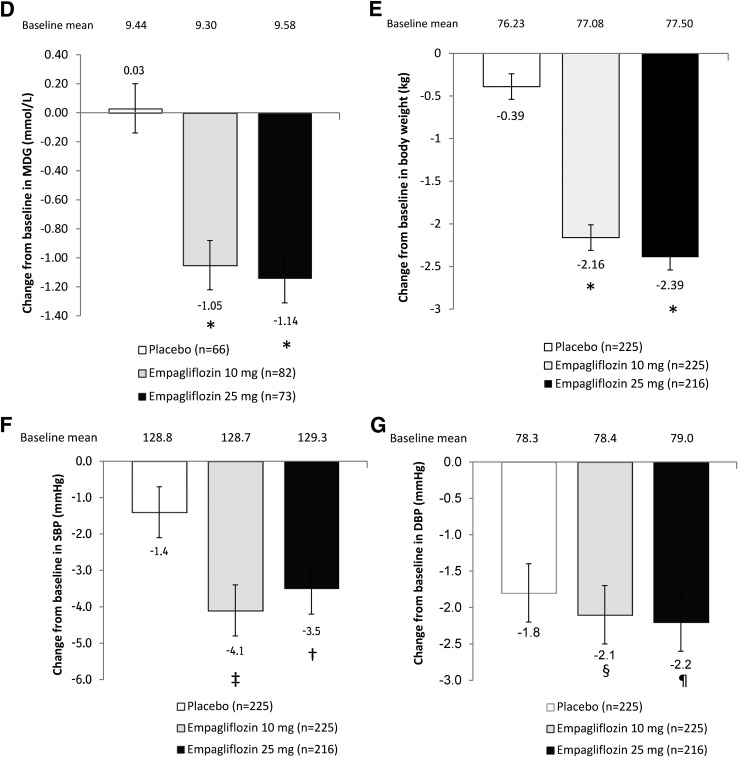
Effect of empagliflozin on efficacy parameters. A: HbA1c over time in randomized groups (mixed model repeated measures, FAS, and OC). B: Change from baseline in HbA1c at week 24 in randomized groups (ANCOVA, FAS, and LOCF imputation). C: HbA1c over time in the open-label treatment group (OC and descriptive statistics). D: Change from baseline in MDG at week 24 in randomized groups (ANCOVA, FAS, and OC). E: Change from baseline in weight at week 24 (ANCOVA, FAS, and LOCF). F: Change from baseline in SBP at week 24 (ANCOVA, FAS, and LOCF). G: Change from baseline in DBP at week 24 (ANCOVA, FAS, and LOCF). Data are adjusted mean (SE) for randomized groups and mean (SE) for open-label treatment group. *P < 0.001 vs. placebo; †P = 0.032 vs. placebo; ‡P = 0.005 vs. placebo; §P = 0.557 vs. placebo; ¶P = 0.534 vs. placebo.
Adjusted mean (SE) changes in MDG from baseline to week 24 using LOCF imputation were 0.00 mmol/L (0.10) for placebo, compared with −0.56 mmol/L (0.10) for empagliflozin 10 mg and −0.72 mmol/L (0.11) for empagliflozin 25 mg (differences of adjusted means vs. placebo were −0.56 mmol/L [95% CI −0.83 to −0.28] for empagliflozin 10 mg and −0.72 mmol/L [−1.02 to −0.43] for empagliflozin 25 mg; P < 0.001 for both doses) (Supplementary Table 1). As 46.9% of analyzed patients had missing or invalid MDG measurements at week 24 and therefore had their baseline values carried forward according to the LOCF imputation method, results based on the prespecified OC analysis may more accurately reflect the treatment effect of empagliflozin on MDG. Here, the adjusted mean (SE) changes from baseline to week 24 were 0.03 mmol/L (0.17) with placebo vs. −1.05 mmol/L (0.15) with empagliflozin 10 mg (P < 0.001) and −1.14 mmol/L (0.16) with empagliflozin 25 mg (differences of adjusted means vs. placebo were −1.08 mmol/L [95% CI −1.53 to −0.62] for empagliflozin 10 mg and −1.17 mmol/L [−1.64 to −0.70] for empagliflozin 25 mg; P < 0.001 for both doses) (Fig. 2D).
Reductions in FPG (Supplementary Fig. 1B and C) and 2-h PPG (Supplementary Fig. 1D) were significantly greater in the empagliflozin 10 and 25 mg groups than in the placebo group at week 24 (Supplementary Table 1). Further data on the effect of empagliflozin and placebo on these end points are given in Supplementary Section 2.
Treatment with empagliflozin resulted in a significantly greater reduction in body weight compared with placebo at week 24, with adjusted mean (SE) changes from baseline of −0.39 kg (0.15) with placebo compared with −2.16 kg (0.15) with empagliflozin 10 mg and −2.39 kg (0.16) with empagliflozin 25 mg (differences of adjusted means vs. placebo were −1.76 kg [95% CI −2.19 to −1.34] for empagliflozin 10 mg and −1.99 kg [−2.42 to −1.56] for empagliflozin 25 mg; P < 0.001 for both doses) (Fig. 2E and Supplementary Table 3). The proportion of patients with >5% reduction in body weight at week 24 was significantly greater with empagliflozin 10 mg (27.6%) and empagliflozin 25 mg (23.6%) than with placebo (5.8%; odds ratios vs. placebo were 6.36 [95% CI 3.36–12.02] for empagliflozin 10 mg and 5.19 [2.72–9.91] for empagliflozin 25 mg; P < 0.001 for both doses) (Supplementary Fig. 2A and Supplementary Table 3). The reduction in body weight in patients on empagliflozin 10 and 25 mg was accompanied by significant decreases in waist circumference at week 24 (adjusted mean [SE] changes of −1.46 cm [0.27] and −1.48 cm [0.28], respectively) versus placebo (−0.31 cm [0.28]; differences of adjusted means vs. placebo were −1.15 cm [95% CI −1.92 to −0.39] for empagliflozin 10 mg and −1.17 cm [−1.94 to −0.40] for empagliflozin 25 mg; P = 0.003 for both doses) (Supplementary Fig. 2B and Supplementary Table 3).
Changes from baseline in SBP over time are shown in Supplementary Fig. 3A. The adjusted mean (SE) change from baseline at week 24 was −1.4 mmHg (0.7) with placebo compared with −4.1 mmHg (0.7) with empagliflozin 10 mg (difference vs. placebo: −2.7 mmHg [95% CI −4.6 to −0.8]; P = 0.005) and −3.5 mmHg (0.7) with empagliflozin 25 mg (difference vs. placebo: −2.1 mmHg [−4.0 to −0.2]; P = 0.032) (Fig. 2F and Supplementary Table 4). Reductions in SBP with empagliflozin were not associated with increases in pulse rate. Changes from baseline in DBP over time are shown in Supplementary Fig. 3B. Changes from baseline in DBP with empagliflozin were not significant at week 24 compared with placebo (Fig. 2G and Supplementary Table 4).
In total, 33 patients (5.0%) in the randomized groups received rescue therapy. More patients received rescue therapy in the placebo group (26 patients [11.6%]) than in the randomized empagliflozin groups (5 patients [2.2%] on 10 mg, 2 patients [0.9%] on 25 mg). Of the seven patients who required rescue medication while receiving empagliflozin, five patients received a thiazolidinedione (four patients on empagliflozin 10 mg and one patient on empagliflozin 25 mg) and two patients received an α-glucosidase inhibitor (one patient on each dose).
Open-label group.
The changes in HbA1c over time in the open-label group (baseline HbA1c >10%) are given in Fig. 2C. The mean (SE) change from baseline in HbA1c at week 24 was −2.89% (0.16). A total of 8.9% of patients achieved HbA1c <7.0% at week 24 (Supplementary Table 1). At week 24, the mean (SE) changes from baseline in MDG and FPG were −3.39 mmol/L (0.58) and −3.02 mmol/L (0.37), respectively; mean (SE) change in body weight was −1.76 kg (0.40), and mean (SE) change in waist circumference was −1.36 cm (0.54). A total of 18.8% of patients achieved a >5% reduction in body weight at week 24. Changes (SE) from baseline in SBP and DBP were −4.3 mmHg (1.2) and −3.4 mmHg (1.0), respectively. Eleven patients (10.9%) in the open-label group received rescue therapy.
Safety
AEs are summarized in Table 2. Most (96%) of patients with one or more AE reported only events of mild or moderate intensity. One patient in the empagliflozin 10 mg group died during the study due to an acute myocardial infarction, which was assessed as not being related to the study drug by the investigator. Confirmed hypoglycemic AEs were reported by more patients in the empagliflozin 10 mg (n = 36; 16.1%) and 25 mg (n = 25; 11.5%) groups than with placebo (n = 19; 8.4%). None of these events required assistance.
Table 2.
Summary of AEs
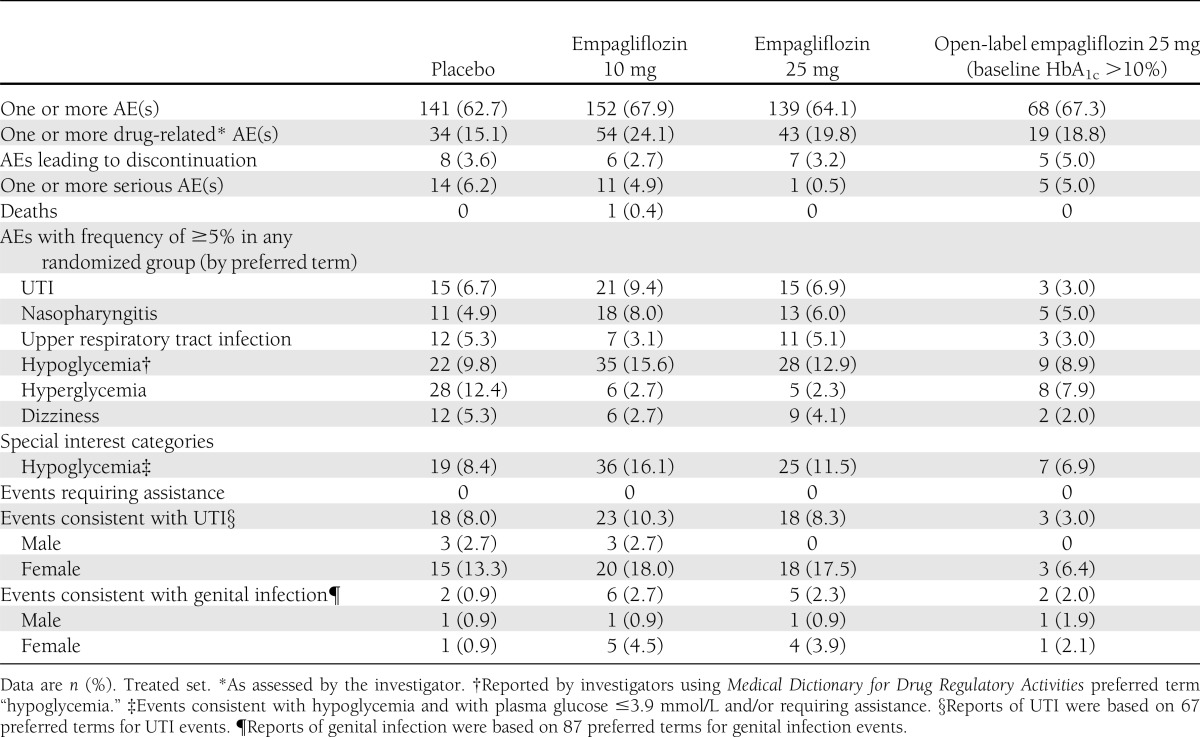
Events consistent with UTI were reported in 8.0% of patients on placebo, similar to the proportion on empagliflozin 25 mg (8.3%) and slightly lower than the proportion on empagliflozin 10 mg (10.3%). The majority of events consistent with UTI were mild in intensity, none was severe, and only one such event led to study discontinuation. No cases of pyelonephritis or urosepsis were reported. Most patients who reported any events consistent with UTI reported just one event; two patients in the empagliflozin 10 mg group and two patients in the empagliflozin 25 mg group reported two events. Events consistent with UTI were reported considerably more frequently in female patients (13.3–18.0%) than in male patients (0.0–2.7%). The proportion of patients who reported events consistent with genital infection was low, but higher with empagliflozin than with placebo (2.3–2.7 vs. 0.9%). Most were mild and none led to discontinuation. All but one patient (in the placebo group) who reported any events consistent with genital infection reported just one event. Events consistent with genital infection were reported more frequently in female patients (0.9–4.5%) than in male patients (0.9%).
In the open-label group, confirmed hypoglycemic events were reported for seven patients (6.9%). Events consistent with UTI were reported for three patients (3.0%), and events consistent with genital infection were reported for two patients (2.0%).
Changes in laboratory values (electrolytes, hematocrit, and lipid parameters) are presented in Supplementary Table 5. There were small increases in hematocrit and small decreases in uric acid levels in the randomized empagliflozin groups. Electrolyte levels were unchanged across the groups. Changes in eGFR were small and comparable across the treatment groups. There was a small increase from baseline in HDL cholesterol with empagliflozin 10 and 25 mg (mean [SE], 0.05 mmol/L [0.01] for both doses) compared with placebo (−0.02 mmol/L [0.01]; P < 0.001). No major differences in mean (SE) changes from baseline in LDL cholesterol or triglycerides were noted between placebo and empagliflozin.
CONCLUSIONS
The current study was undertaken to establish the efficacy and safety of empagliflozin 10 and 25 mg once daily for 24 weeks in patients with type 2 diabetes who had inadequate glycemic control on metformin and sulfonylurea. Both doses led to clinically meaningful improvements in glycemic control, body weight, and SBP (but not DBP) with a good tolerability and safety profile.
Guidelines recommend that patients who fail to achieve adequate glycemic control with metformin monotherapy receive an additional agent such as sulfonylurea, a glucagon-like peptide-1 (GLP-1) receptor agonist, a dipeptidyl peptidase-4 (DPP4) inhibitor, a thiazolidinedione, or basal insulin. When combination therapy fails to achieve adequate glycemic control, insulin therapy is usually the most appropriate third-line treatment (1,14). Although efficacious, insulin is associated with side effects such weight gain and hypoglycemia (1,14). In this trial, the addition of empagliflozin to metformin and sulfonylurea led to significant reductions in HbA1c, with 26–32% of patients reaching HbA1c <7.0% at week 24 compared with only 9% in the placebo group. Improved glucose control was further demonstrated by significant reductions in MDG, FPG, and 2-h PPG with empagliflozin compared with placebo. Changes in MDG, assessed based on OC analysis, corresponded well with changes in HbA1c (15). A beneficial effect of empagliflozin on glucose control was also observed in patients with very poor glycemic control at baseline, in whom HbA1c was reduced from a mean of 11.2–8.2% at week 24. These results suggest that empagliflozin is efficacious over a broad HbA1c range. Further, empagliflozin was shown to be efficacious in a patient population that included a substantial number of patients with a long duration of type 2 diabetes (77% of randomized patients had been diagnosed >5 years before the trial). Significant reductions in body weight and waist circumference were observed with empagliflozin compared with placebo, and 24–28% of patients on empagliflozin had a >5% reduction in weight at week 24, compared with only 6% on placebo. Weight control is important for patients with type 2 diabetes to help improve glycemic control (16,17) and improve cardiovascular risk factors (1,18,19), and is particularly pertinent for patients taking a sulfonylurea. Treatment with sulfonylurea is associated with weight gain, a side effect that has been shown to reduce patient satisfaction and adherence to medication (20,21).
Hypertension is an important cardiovascular risk factor associated with type 2 diabetes (22,23). In this study, a significant reduction in SBP was observed with empagliflozin compared with placebo, without an increase in pulse rate. This may reflect osmotic diuresis associated with urinary glucose excretion (24), which may also explain the small increase in hematocrit. Unexpectedly, no reduction in DBP was observed at week 24. As a limitation of the study, changes in antihypertensive medication were allowed, possibly diluting the effect of empagliflozin versus placebo on blood pressure. A reduction in uric acid levels was observed in patients treated with empagliflozin, compared with an increase in patients on placebo. In many epidemiological studies, lower uric acid levels have been associated with lower cardiovascular morbidity and mortality (25,26).
Empagliflozin was well tolerated when used as add-on therapy to metformin plus sulfonylurea for 24 weeks. More patients reported serious AEs with placebo than empagliflozin, and the number of patients who discontinued due to AEs was similar between the randomized empagliflozin groups and the placebo group. More randomized patients reported confirmed hypoglycemic events with empagliflozin than with placebo. This suggests that empagliflozin in combination with sulfonylurea may increase the risk of hypoglycemia, whereas empagliflozin alone has a low risk of hypoglycemia (11). Importantly, empagliflozin as add-on to on metformin plus sulfonylurea did not cause any hypoglycemic events that required assistance.
Patients with type 2 diabetes, particularly female patients, are at increased risk of UTIs (27,28); however, the reported incidence varies between trials due to different reporting methods. In this trial, the proportion of patients with events consistent with UTI was slightly higher with empagliflozin 10 mg than with empagliflozin 25 mg or placebo, and the proportion of patients reporting genital infections was higher with empagliflozin than with placebo. This is consistent with data on other SGLT2 inhibitors, which show that a higher proportion of patients report genital infections with the SGLT2 inhibitor versus placebo, but the proportion of patients who report UTIs is similar or only slightly higher with SGLT2 inhibitor versus placebo (29,30).
Empagliflozin 10 and 25 mg showed no difference in efficacy or tolerability in this trial, even though a dose-dependent increase in urinary glucose excretion and a dose-dependent decrease in HbA1c have been reported in phase I/II studies (11,12,31,32). Dose dependency of empagliflozin will be evaluated based on the totality of data from phase III trials comprising more than 10,000 patients.
To conclude, in this trial, empagliflozin at doses of 10 and 25 mg led to clinically meaningful improvements in glycemic control in patients with inadequate control on metformin plus sulfonylurea, led to significant reductions in body weight and SBP, and was well tolerated over 24 weeks. These results demonstrate the potential of empagliflozin as a third-line therapy in patients inadequately controlled with metformin plus a sulfonylurea.
Supplementary Material
Acknowledgments
This study was funded by Boehringer Ingelheim.
H.-U.H. is a member of the advisory boards for Daiichi Sankyo, Sanofi, Boehringer Ingelheim, and Roche. L.M. is an investigator and/or consultant and/or speaker without any direct financial benefit to him under contracts between his employer and the following companies: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Roche Pharma (neither he nor his family members hold stocks in pharmaceutical or device companies). E.S.-B., M.W., T.M., H.J.W., and U.C.B. are employees of Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
H.-U.H. and L.M. contributed to the acquisition and interpretation of data and drafted the manuscript. E.S.-B., M.W., T.M., and U.C.B. contributed to the study design and interpretation of data and reviewed and edited the manuscript. H.J.W. contributed to the interpretation of data and reviewed and edited the manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and approved the final version. M.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
An abstract of this study was presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21
–25 June 2013.
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Elizabeth Ng and Wendy Morris (Fleishman-Hillard Group, Ltd., London, U.K.) during the preparation of the manuscript.
Footnotes
Clinical trial reg. no. NCT01159600, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2673/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 3.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care 2008;31:2086–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng JM, Mellor DD, Masson EA, Allan BJ. Sulphonyurea as a cause of severe hypoglycaemia in the community. Prim Care Diabetes 2010;4:61–63 [DOI] [PubMed] [Google Scholar]
- 5.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410–1418 [DOI] [PubMed] [Google Scholar]
- 6.Campbell RK. Fate of the beta-cell in the pathophysiology of type 2 diabetes. J Am Pharm Assoc (2003) 2009;49(Suppl. 1):S10–S15 [DOI] [PubMed]
- 7.Morgan CL, Jenkins-Jones S, Evans M, Barnett AH, Poole CD, Currie CJ. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab 2012;14:424–432 [DOI] [PubMed] [Google Scholar]
- 8.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl 2011;S1–S6 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5–14 [DOI] [PubMed] [Google Scholar]
- 10.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012;14:83–90 [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 8 February 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Jelaska A, Seman L, Pinnetti S, Hantel S, Woerle HJ. Efficacy and safety of BI 10773, a new sodium glucose cotransporter (SGLT-2) inhibitor, in type 2 diabetes inadequately controlled on metformin (Abstract). Diabetes 2011;60:A271 [Google Scholar]
- 13.Woerle HJ, Ferrannini E, Berk A, Manun'Ebo M, Pinnetti S, Broedl UC. Safety and efficacy of empagliflozin as monotherapy or add-on to metformin in a 78-week open-label extension study in patients with type 2 diabetes. Diabetes 2012;61:LB13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handelsman Y, Mechanick JI, Blonde L, et al. AACE Task Force for Developing Diabetes Comprehensive Care Plan American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 2011;17(Suppl. 2):1–53 [DOI] [PubMed] [Google Scholar]
- 15.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
- 16.Feldstein AC, Nichols GA, Smith DH, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care 2008;31:1960–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shantha GPS, Kumar AA, Kahan S, Cheskin LJ. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ 2012;38:417–426 [DOI] [PubMed] [Google Scholar]
- 18.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med 2009;26:416–424 [DOI] [PubMed] [Google Scholar]
- 21.Pollack MF, Purayidathil FW, Bolge SC, Williams SA. Patient-reported tolerability issues with oral antidiabetic agents: associations with adherence; treatment satisfaction and health-related quality of life. Diabetes Res Clin Pract 2010;87:204–210 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cederholm J, Gudbjörnsdottir S, Eliasson B, Zethelius B, Eeg-Olofsson K, Nilsson PM, NDR Blood pressure and risk of cardiovascular diseases in type 2 diabetes: further findings from the Swedish National Diabetes Register (NDR-BP II). J Hypertens 2012;30:2020–2030 [DOI] [PubMed] [Google Scholar]
- 24.Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci 2011;32:63–71 [DOI] [PubMed] [Google Scholar]
- 25.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niskanen LK, Laaksonen DE, Nyyssönen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546–1551 [DOI] [PubMed] [Google Scholar]
- 27.Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J Diabetes Complications 2012;26:513–516 [DOI] [PubMed] [Google Scholar]
- 28.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol 2005;161:557–564 [DOI] [PubMed] [Google Scholar]
- 29.Janssen Pharmaceuticals,Inc. INVOKANA (canagliflozin) tablets prescribing information. Available from http://www.janssenpharmaceuticalsinc.com/assets/invokana_prescribing_info.pdf. Accessed 3 May 2013
- 30.Bristol-Myers Squibb/AstraZeneca EEIG. FORXIGA (dapagliflozin) summary of product characteristics. Available from http://www.forxiga.eu/sites/default/files/Forxiga%20Summary%20of%20Product%20Characteristics_SmPC_.pdf. Accessed 3 May 2013
- 31.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613–621 [DOI] [PubMed] [Google Scholar]
- 32.Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharm Drug Dev 2013;2:152–161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.