Abstract
OBJECTIVE
Emerging in vitro and animal evidence suggests that methylmercury could increase type 2 diabetes, but little evidence exists in humans. We aimed to prospectively determine associations of mercury exposure, as assessed by biomarker measurement, with incident diabetes.
RESEARCH DESIGN AND METHODS
We used neutron activation analysis to measure toenail mercury, an objective biomarker of methylmercury exposure, in 9,267 adults free of diabetes at baseline in two separate U.S. prospective cohorts. Incident diabetes was identified from biennial questionnaires and confirmed by validated supplementary questionnaire using symptoms, diagnostic tests, and medical therapy. Associations of mercury exposure with incident diabetes were assessed using Cox proportional hazards.
RESULTS
During mean ± SD follow-up of 19.7 ± 7.0 years, 1,010 new cases of diabetes were diagnosed. The 95th percentile of toenail mercury was 1.32 μg/g in men and 0.76 μg/g in women, corresponding to exposures ∼3.5-fold and 2-fold higher than the U.S. Environmental Protection Agency reference dose. In multivariable analyses, toenail mercury concentrations were not associated with higher incidence of diabetes in women, men, or both cohorts combined. Comparing the highest to lowest quintile of exposure, the hazard ratio (95% CI) for incident diabetes was 0.86 (0.66–1.11) in women, 0.69 (0.42–1.15) in men, and 0.77 (0.61–0.98) in the combined cohorts. Findings were similar when more extreme categories (deciles) of mercury were compared, and in analyses stratified by fish or omega-3 consumption, BMI, and age.
CONCLUSIONS
These findings from two separate large prospective cohorts do not support adverse effects of methylmercury on development of diabetes in men or women at usual levels of exposure seen in these populations.
Emerging evidence from in vitro models and animal experiments suggests that methylmercury could increase risk of type 2 diabetes. In these experiments, methylmercury causes dysfunction of pancreatic β-cells by increasing production of reactive oxygen species, increasing phosphatidylinositol 3-kinase activity and Akt phosphorylation, decreasing insulin secretion, and activating apoptosis (1,2). Given the large and growing health and economic burdens of diabetes (3), confirming and quantifying the potential effects of methylmercury on diabetes risk would be of tremendous public health consequence. For example, this would be crucial to inform potential extension of current recommendations on balancing benefits versus risks of fish consumption versus methylmercury exposure, which are focused on women of childbearing age and young children to optimize neurodevelopment in early life (4), to the general population to modify the risk of developing diabetes.
Very little evidence on methylmercury exposure and diabetes risk is available in humans, including mixed results from one ecological study (5), one small retrospective study (6), and one cross-sectional analysis (7). Findings on fish consumption, the major source of methylmercury, and diabetes risk have also been mixed, with some studies observing decreased risk, others increased risk, and others no significant association (8). Interestingly, the Coronary Artery Risk Development in Young Adults (CARDIA) cohort recently reported a positive association between chronic methylmercury exposure and diabetes (9). To our knowledge, no other studies have prospectively evaluated whether mercury exposure is associated with incident diabetes. To determine whether chronic methylmercury exposure might increase the risk of diabetes, we prospectively investigated the relationship between toenail mercury levels, an objective biomarker of methylmercury exposure, and incidence of diabetes among 9,267 men and women in two separate U.S. cohort studies.
RESEARCH DESIGN AND METHODS
Population and design
The designs of the Health Professionals Follow-up Study (HPFS) and Nurses’ Health Study (NHS) have been described (10,11). The HPFS enrolled 51,529 U.S. men, all health professionals, who were 40–75 years of age in 1986; and the NHS enrolled 121,700 U.S. women, all registered nurses, who were 30–55 years of age in 1976. In both cohorts, participants have been followed with biennial questionnaires on medical history, risk factors, lifestyle, and disease incidence. For the present analysis, we used prospectively collected data on toenail mercury concentrations from prior nested case-control studies of incident cardiovascular disease in both cohorts (12,13) (see Supplementary Data online for details). The study was designed by the authors and approved by the human subjects committees of all author institutions. All participants provided implied consent by return of completed questionnaires and toenail samples. Funding for the present investigation was provided by the National Institute of Environmental Health Sciences, National Institutes of Health.
From among NHS and HPFS participants who had stored toenail samples and were free of cardiovascular disease at the time of toenail sampling (1982–1983 in NHS; 1987 in HPFS), we measured toenail mercury concentrations in 9,308 men and women, including 4,654 participants who went on to develop coronary heart disease (CHD) or stroke during follow-up and 4,654 participants who did not develop CHD or stroke during equivalent follow-up (matched on month of toenail sample return, age, sex, race, and smoking status). We excluded dentists from these measurements, as their toenail mercury also reflects occupational exposure to inorganic (rather than methyl) mercury (14). For the present analysis, we excluded 41 participants with prevalent diabetes at the time of toenail sampling, leaving a total of 9,267 individuals with measured toenail mercury concentrations who were free of prevalent diabetes at baseline.
Toenail mercury concentrations
Toenail concentrations of mercury and selenium, which in some animal models mitigate toxicity of mercury (15), were measured using neutron activation analysis by personnel unaware of the participants’ clinical information (12). This biomarker provides a measure of chronic methylmercury exposure over approximately the prior year. See Supplementary Data for details on analytic methods, validity, and reproducibility of these measures.
Ascertainment of incident diabetes
Potential cases of incident diabetes were identified from biennial questionnaires asking about any new physician-diagnosed diabetes. These potential cases were then reviewed and confirmed using a validated supplementary questionnaire that obtained data on symptoms, diagnostic tests, and medical therapy. Incident diabetes was diagnosed according to original National Diabetes Data Group criteria, based on the presence of one or more of the following: 1) classic symptoms plus a plasma glucose concentration ≥140 mg/dL (7.8 mmol/L) in the fasting state or a randomly measured plasma glucose concentration ≥200 mg/dL (11.1 mmol/L); 2) in the absence of symptoms, at least two elevated plasma glucose concentrations on different occasions (≥140 mg/dL fasting, ≥200 mg/dL randomly measured, or ≥200 mg/dL 2-h postoral glucose challenge); or 3) drug treatment with hypoglycemic medication (insulin or an oral hypoglycemic agent). These diagnostic criteria were modified after 1998 to account for the new lower diagnostic cutoff for fasting glucose concentration of 126 mg/dL. The validity of these supplementary questionnaire criteria for diagnosing diabetes in these cohorts has been demonstrated. In subsets of participants in whom diabetes was diagnosed based on these criteria and then compared with a full review of medical records, 98% in NHS and 97% in HPFS had a confirmed diagnosis of diabetes (16,17).
Covariates
Data on demographics, risk factors, and lifestyle habits were collected via validated self-administered questionnaires, using the closest report preceding toenail sample collection from each participant (18). Information on weight and height was obtained; self-reported weight was validated against technician-measured weight (r = 0.96) (19). Physical activity was assessed as metabolic equivalent tasks (METs) using validated questionnaires (20). Usual dietary habits were assessed using validated semiquantitative food frequency questionnaires that inquired about usual consumption of foods, beverages, and supplements over the prior year (21,22). Fish and seafood omega-3 consumption were quantified as previously described (23).
Statistical analysis
Associations of toenail mercury concentrations with incident diabetes were evaluated using Cox proportional hazards, with time at risk from the time of toenail sampling until the first event, death, or censoring at the date of return of the last questionnaire in 2008. The proportional hazards assumption was tested and not rejected based on Schoenfeld residuals. Mercury concentrations were evaluated as indicator categories in quintiles and also in deciles to evaluate a broader range of dose response. Because toenail mercury measures were measured as part of a prior nested case-control study of incident cardiovascular disease, participants were weighted according to their inverse probability of being sampled from the overall cohorts (SAS PROC PHREG, NORMALIZE option). Individuals selected as future cardiovascular case subjects were given a weight of 1, and control subjects received a weight of 17 in the NHS (women) and 24 in the HPFS (men). With such weighting, results can be interpreted as generalizable to the full cohorts.
Tests for trend were performed by assigning participants the median value in their quintile of exposure and evaluating this as a continuous variable. Statistical evaluation for interaction was performed by multiplying this variable by the effect modifier of interest and evaluating the Wald test for the multiplicative interaction term. Potential confounding was assessed using multivariable models adjusted for demographics, major diabetes risk factors, and lifestyle and dietary habits including fish and omega-3 fatty acid consumption. Multivariable modeling was guided by parsimony, clinical relevance of covariates, observed strength of association between covariates and exposure or outcome, and percent change in risk estimate when covariates were included. Missing covariates (<1%) were imputed using multiple imputation (24). We performed sensitivity analyses to minimize potential reverse causation due to the presence of potential unrecognized diabetes at baseline by excluding cases occurring during the first 2 years of follow-up, and to minimize potential misclassification due to exposure changes over time by restricting analyses to events within 10 years of toenail sampling. We also performed sensitivity analyses restricting to symptomatic cases who reported at least one classic symptom at the time of diagnosis. All analyses were performed using SAS version 9.1 (SAS Institute) with two-tailed α = 0.05.
RESULTS
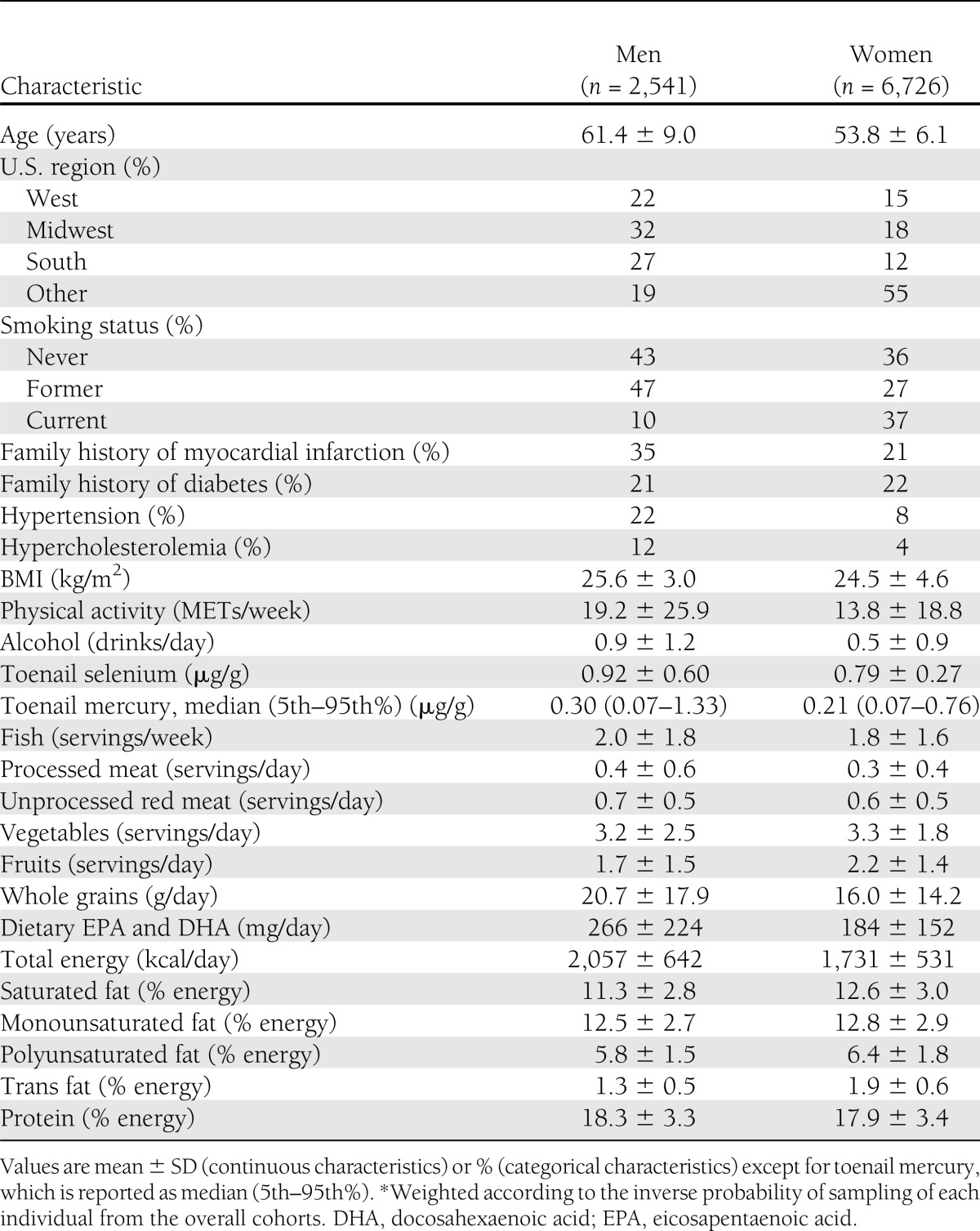
At baseline, mean ± SD age was 61.2 ± 8.9 years among men and 53.7 ± 6.1 years among women (Table 1). The 95th percentile of toenail mercury concentration was 1.32 μg/g in men and 0.76 μg/g in women, corresponding to concentrations in hair of ∼3.67 and 2.11 μg/g (12), respectively, or ∼3.5- and 2-fold higher than the U.S. Environmental Protection Agency (EPA) reference dose exposure corresponding to 1.0 μg/g in hair (25). Unadjusted (bivariate) associations of baseline characteristics of participants in these cohorts according to toenail mercury concentrations have been previously reported (12,13). In brief, higher mercury concentrations were associated with more frequent hypercholesterolemia, slightly lower BMI, modestly higher physical activity, and greater alcohol use. Mercury concentrations were also positively associated with fish consumption (Spearman r = 0.39) and dietary factors related to fish consumption, including slightly lower intakes of saturated fat, monounsaturated fat, trans fat, and dietary cholesterol and slightly higher intakes of protein and polyunsaturated fat. Mercury concentrations were not significantly associated with age, smoking, family history of diabetes, or presence of hypertension.
Table 1.
Baseline characteristics of 9,267 U.S. men and women in two separate prospective cohorts*
During 19.7 ± 7.0 years of follow-up, 1,010 new cases of diabetes were diagnosed. The median duration of follow-up from time of toenail sampling to diagnosis of diabetes was 12.7 years (interquartile range 7.5–18.1). In both age- and sex-adjusted as well as multivariable-adjusted analyses, toenail mercury concentrations were not associated with higher incidence of diabetes in women, men, or overall (Table 2). Comparing the highest to the lowest quintile of mercury exposure, the fully adjusted hazard ratio (HR) (95% CI) for incident diabetes was 0.86 (0.66–1.11) (P trend = 0.21) in women, 0.69 (0.42–1.15) (P trend = 0.12) in men, and 0.77 (0.61–0.98) (P trend = 0.04) in both cohorts combined.
Table 2.
Multivariable-adjusted risk of incident diabetes according to mercury exposure among 9,267 U.S. men and women in two separate prospective cohorts*
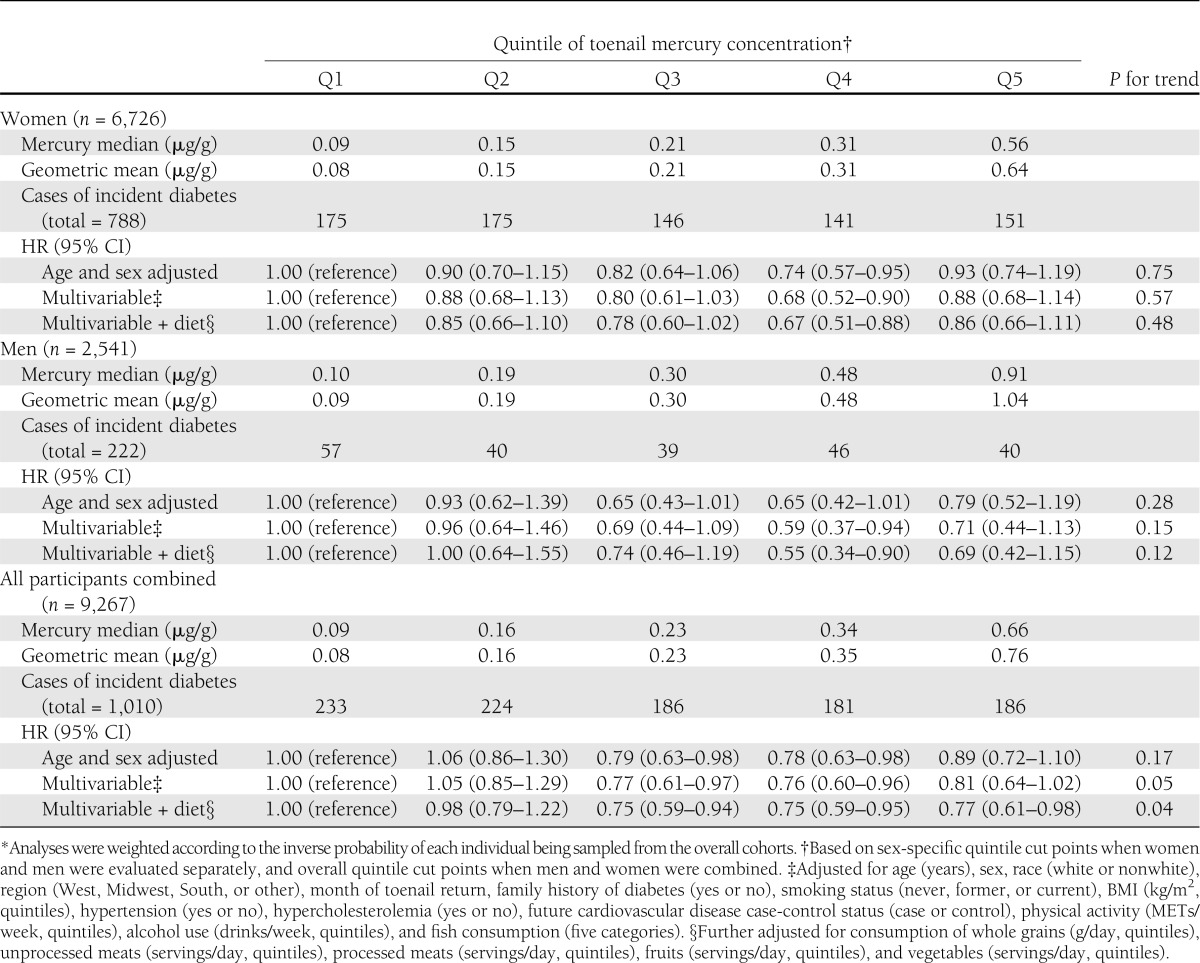
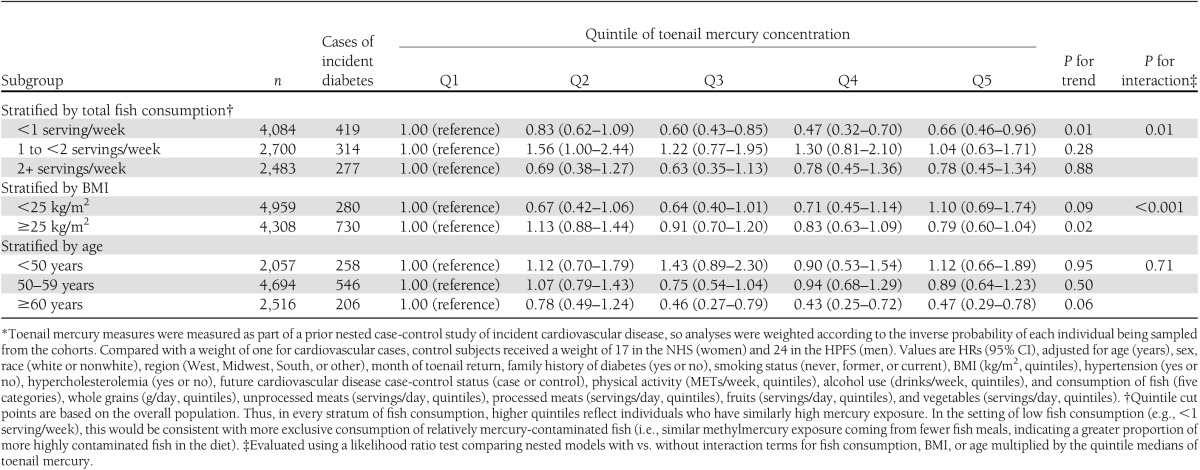
Findings were not altered with further adjustment for total energy intake or toenail selenium concentrations or if we adjusted for estimated dietary long-chain omega-3 fatty acids rather than fish consumption (data not shown). Higher risk of diabetes with higher mercury exposure was also not seen in analyses stratified by fish consumption, BMI, or age (Table 3). Indeed, trends toward lower incidence of diabetes with higher mercury exposure were seen when fish consumption was low among overweight or obese subjects and among older subjects.
Table 3.
Multivariable-adjusted risk of incident diabetes according to mercury exposure among 9,267 U.S. men and women in two separate prospective cohorts, by subgroup*
Findings were also similar across a broader dose response of deciles of toenail mercury (Supplementary Table 1), and in sensitivity analyses excluding early cases of diabetes (within the first 2 years) or late cases (after the first 10 years) (Supplementary Table 2). Results were also similar or even more strongly inverse when restricted to symptomatic cases (48.7% of all cases) reporting at least one classic symptom at the time of diagnosis (Supplementary Table 3).
In prior analyses in these cohorts, self-reported fish and dietary long-chain omega-3 consumption were associated with a modestly higher incidence of diabetes (26). For example, compared with participants consuming fish less than once a month, the pooled multivariate relative risk (95% CI) among those consuming fish five or more times per week was 1.22 (1.08–1.39). Similarly, across quintiles of long-chain omega-3 fatty acids, the pooled multivariate relative risk (95% CI) was 1.24 (1.09–1.40). When we reevaluated these associations in the present subset of cohort participants who also had mercury measures, the findings were very similar: both self-reported fish and omega-3 consumption were associated with a higher incidence of diabetes (data not shown). In exploratory analyses, we evaluated joint associations with incident diabetes of toenail mercury concentrations and self-reported fish consumption (Supplementary Table 4) or estimated long-chain omega-3 consumption (Supplementary Table 5). Compared with the reference group of individuals having both low toenail mercury and low fish (or low omega-3) consumption, no clear pattern of risk was evident. Indeed, participants having both higher toenail mercury and lower fish or omega-3 consumption tended to have lowest incidence of diabetes, whereas participants having both higher toenail mercury and higher fish or omega-3 consumption did not have a significantly different incidence of diabetes.
CONCLUSIONS
In two separate large U.S. prospective cohort studies, we found no evidence that methylmercury exposure was associated with higher risk of diabetes. These findings were robust in a variety of different subgroups and sensitivity analyses. These results provide evidence that at usual levels of exposure seen in these men and women, methylmercury exposure is unlikely to increase the risk of diabetes.
When the cohorts were combined and in some population subgroups, higher mercury levels were associated with trends toward lower incidence of diabetes. Because methylmercury does not induce biological effects that would plausibly protect against diabetes, this observed relationship is likely due to confounding; i.e., links between higher methylmercury exposure and other factors that lower risk of diabetes. Consistent with the major source of exposure, higher toenail mercury was associated with factors linked to higher fish consumption, including slightly lower BMI, modestly higher physical activity, and greater alcohol use. Although we adjusted for these factors and self-reported fish consumption in multivariable models, residual confounding due to imperfect measurements or other unmeasured factors cannot be excluded. These two cohorts also comprised relatively racially and socioeconomically uniform populations, greatly minimizing confounding by race, education, and income.
Interestingly, overall fish consumption in these cohorts has been linked to a modestly higher incidence of diabetes (26), whereas toenail mercury concentrations were associated with trends toward lower risk. These differences may partly relate to choices of fish species consumed; associations for overall fish consumption reflect the average intakes of all types of fish consumed, whereas associations for toenail mercury reflect intakes of a limited set of large, predatory fish species. The varying associations could also partly relate to differences in validity or bias of self-reported fish consumption, compared with toenail mercury as an objective biomarker. When we evaluated joint associations of fish or omega-3 consumption and mercury levels, we did not find any clear pattern of joint associations that would suggest offsetting benefits versus risks. Overall, the results provide little evidence that higher methylmercury exposure from fish consumption increases risk of diabetes.
The CARDIA cohort recently reported on the association between toenail mercury concentrations and incident diabetes (9). After adjustment for age, sex, race, BMI, education, smoking, alcohol use, physical activity, and family history, mercury exposure was not significantly associated with incident diabetes. However, after further adjustment for dietary omega-3 intake, dietary magnesium, and toenail selenium, a positive association was seen (P trend = 0.02). These findings suggested that, only after accounting for other beneficial components in fish, the remaining methylmercury exposure might increase risk of diabetes. Interestingly, this positive association also appeared potentially weaker among participants consuming higher amounts of dietary omega-3 fatty acids or magnesium. The reasons for the divergent results between our findings and those in CARDIA are unclear. Both studies were prospective, included U.S. adults, assessed toenail mercury concentrations using the same U.S. biomarker laboratory, adjusted for major confounders including other beneficial components in fish, and included participants with similar overall absolute risk (in CARDIA, 7.2% of subjects developed diabetes during 18 years of follow-up; in our cohorts, 10.9% developed diabetes during ∼25 years of follow-up) and similar ranges of mercury exposure. Among both our cohorts combined, geometric mean mercury exposure in the top quintile was twofold higher than the U.S. EPA reference dose (25), and in the top decile, nearly threefold higher. These ranges of mercury exposure were also similar to exposure levels documented in European populations (27,28) and in nationally representative U.S. surveys (29). Pooling our results with those reported by CARDIA, no significant association was evident (Fig. 1). CARDIA included fewer total participants (3,875 vs. 9,267) and fewer cases of incident diabetes (280 vs. 1,010). In comparison with our participants who were largely middle age at baseline, the CARDIA cohort was younger (20–32 years of age at baseline), raising the possibility that biological effects of methylmercury could influence diabetes risk in youth but not middle age. Conversely, we did not find statistically significant effect modification by age (P interaction = 0.71) or evidence for higher risk at age <50 years even with a similar number of cases in this age stratum as in the entire CARDIA cohort. Differences in the results between these studies could also be due to chance.
Figure 1.
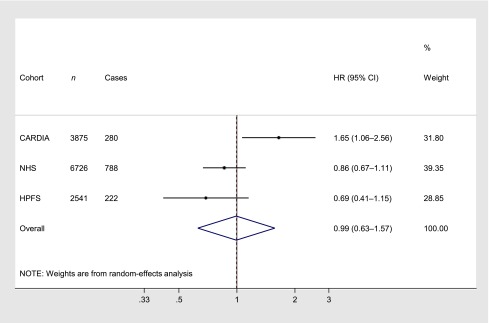
Meta-analysis of three prospective cohort studies evaluating mercury exposure and incident diabetes. Cohort-specific HRs in the top quintile of toenail mercury concentrations, compared with the lowest quintile, are shown and were combined using random-effects meta-analysis with inverse-variance weights. Using fixed-effects meta-analysis, the pooled HR (95% CI) was 0.95 (0.78–1.17). Heterogeneity was evident (I2 75.4%).
We found inverse associations between mercury exposure and incident diabetes among people with low omega-3 intake or who were overweight or obese. Whether the results in these groups represent benefits of fish consumption, for which toenail mercury concentration is a marker, or chance findings requires further study. Given absence of a priori hypotheses about interaction in these subgroups, these findings require confirmation and should be interpreted cautiously.
For sensitive subpopulations, specific guidance exists to balance benefits and risks of fish consumption versus methylmercury exposure to optimize brain development during gestation and infancy (4,30). No corresponding guidelines exist for the general adult population, owing to insufficient evidence for any significant long-term effects of chronic low-level methylmercury exposure in adults. Some early studies suggested that methylmercury exposure may be linked to higher CHD (27,28), but our recent work in these two large prospective cohort studies demonstrated no evidence for increased risk of either CHD or stroke at typical population ranges of chronic methylmercury exposure (12).
The Institute of Medicine, the U.S. Dietary Guidelines, and the World Health Organization have each concluded that regular fish consumption is a recommended part of a healthy diet and that among adults, health benefits far outweigh potential risks (31–33). Our findings provide further credence to these conclusions, providing little evidence that methylmercury exposure from fish consumption increases diabetes risk.
Supplementary Material
Acknowledgments
This research was supported by R01-ES014433 from the National Institute of Environmental Health Sciences, National Institutes of Health (NIH), as well as by NIH research grants HL-34594, HL-088521, HL-35464, DK-58845, CA-87969, CA-55075, and CA-167552.
No potential conflicts of interest relevant to this article were reported.
The funding sources had no role in the study design; the collection, analysis, or interpretation of the data; the writing of the report; or the decision to submit the manuscript for publication.
D.M. obtained funding, collected data, conceived analyses, interpreted findings, and wrote the manuscript. P.S. performed statistical analyses, interpreted findings, and reviewed and edited the manuscript. J.S.M. collected data, interpreted findings, contributed to discussion, and reviewed and edited the manuscript. P.G., D.S.S., D.S., and F.B.H. interpreted findings, contributed to discussion, and reviewed and edited the manuscript. D.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all of the participants and support staff in the NHS and HPFS, without whom this research would not have been possible.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0894/-/DC1.
References
- 1.Chen YW, Huang CF, Tsai KS, et al. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes 2006;55:1614–1624 [DOI] [PubMed] [Google Scholar]
- 2.Chen YW, Huang CF, Tsai KS, et al. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol 2006;19:1080–1085 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. What you need to know about mercury in fish and shellfish [article online], 2004. Available from http://www.fda.gov/food/resourcesforyou/consumers/ucm110591.htm Accessed 16 April 2013
- 5.Futatsuka M, Kitano T, Wakamiya J. An epidemiological study on diabetes mellitus in the population living in a methyl mercury polluted area. J Epidemiol 1996;6:204–208 [DOI] [PubMed]
- 6.Serdar MA, Bakir F, Hasimi A, et al. Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J of Diabetes Dev Ctries 2009;29:35–40 [DOI] [PMC free article] [PubMed]
- 7.Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J Hazard Mater 2011;185:749–755 [DOI] [PubMed] [Google Scholar]
- 8.Wu JHY, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(Suppl. 2):S214–S227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA trace element study. Diabetes Care 2013;36:1584–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002;287:1815–1821 [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Ascherio A, Hu FB, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Shi P, Morris JS, et al. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med 2011;364:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Shi P, Morris JS, et al. Mercury exposure and risk of hypertension in US men and women in 2 prospective cohorts. Hypertension 2012;60:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi A, Douglass CW, Kim HD, et al. The relationship between amalgam restorations and mercury levels in male dentists and nondental health professionals. J Public Health Dent 2003;63:52–60 [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health 2009;6:1894–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900 [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA 2000;283:2961–2967 [DOI] [PubMed] [Google Scholar]
- 21.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 22.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 23.Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr 2008;88:1618–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer JL. Analysis of Incomplete Multivariate Data. New York, Chapman and Hall, 1997 [Google Scholar]
- 25.U.S. Environmental Protection Agency. Integrated Risk Information System. Methylmercury (MeHg) (CASRN 22967-92-6): reference dose for chronic oral exposure (RfD) [Internet], 2001. Available from http://www.epa.gov/iris/subst/0073.htm Accessed 24 May 2013
- 26.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009;90:613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guallar E, Sanz-Gallardo MI, van’t Veer P, et al. Heavy Metals and Myocardial Infarction Study Group Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 2002;347:1747–1754 [DOI] [PubMed] [Google Scholar]
- 28.Virtanen JK, Voutilainen S, Rissanen TH, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 2005;25:228–233 [DOI] [PubMed]
- 29.McDowell MA, Dillon CF, Osterloh J, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ Health Perspect 2004;112:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercury in fish: Cause for concern? [article online], 1995. Available from http://www.fda.gov/ohrms/dockets/ac/02/briefing/3872_advisory%207.pdf Accessed 16 April 2013
- 31.Institute of Medicine of the National Acadamies Seafood Choices: Balancing Benefits and Risks. Washington, DC, The National Academies Press, 2007 [Google Scholar]
- 32.U.S. Department of Agriculture U.S. Department of Health and Human Services: Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC, U.S. Government Printing Office, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food and Agriculture Organization of the United Nations World Health Organization: Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption. Rome, Italy; Geneva, Switzerland, Food and Agriculture Organization of the United Nations, World Health Organization, 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


