Abstract
OBJECTIVE
Osteomyelitis in the diabetic foot is a major risk factor for amputation, but there is a limited understanding of early-stage infection, impeding limb-preserving diagnoses. We hypothesized that bone composition measurements provide insight into the early pathophysiology of diabetic osteomyelitis.
RESEARCH DESIGN AND METHODS
Compositional analysis by Raman spectroscopy was performed on bone specimens from patients with a clinical diagnosis of osteomyelitis in the foot requiring surgical intervention as either a biopsy (n = 6) or an amputation (n = 11).
RESULTS
An unexpected result was the discovery of pathological calcium phosphate minerals in addition to normal bone mineral. Dicalcium phosphate dihydrate, also called brushite, and uncarbonated apatite were found to be exclusively associated with infected bone.
CONCLUSIONS
Compositional measurements provided a unique insight into the pathophysiology of osteomyelitis in diabetic foot ulcers. At-patient identification of pathological minerals by Raman spectroscopy may serve as an early-stage diagnostic approach.
Osteomyelitis of the diabetic foot, herein called diabetic osteomyelitis, is a major cause of lower-extremity amputation, yet an understanding of the pathophysiology and technologies enabling early diagnosis of this serious infection are lacking. Clinical and imaging tests show that whole-tissue properties of bone, including hardness and mineralization, are directly affected by diabetic osteomyelitis (1,2). We hypothesized that compositional changes to bone mineral and collagen matrix accompany clinically observable alterations in bone hardness and mineralization. However, no studies to our knowledge have reported on the chemical composition of bone in diabetic osteomyelitis. The objective of the present study was to measure bone composition in diabetic osteomyelitis with the use of Raman spectroscopy.
RESEARCH DESIGN AND METHODS
Clinical study
This is an ongoing translational study performed at the University of Michigan Health System (UMHS) and the Ann Arbor Veterans Affairs (AAVA) Hospital and has been reviewed and approved by their respective institutional review boards. Bone was obtained from 17 patients with a clinical diagnosis of diabetic osteomyelitis requiring surgical intervention to collect a bone biopsy specimen (n = 6) or to amputate (n = 11). No patients were treated with bone cements. Bone fragments were prepared separately for microbiological and histopathological analyses. All patients had bone cultures performed, and some had additional soft tissue and exudates cultured. For microbiology analysis, bone fragments were stored in an ESwab Collection and Transport system (Becton Dickinson, Sparks, MD) and analyzed through standard hospital procedure. Fragments for histopathology were prepared by the UMHS Tissue Procurement Core or the AAVA pathology laboratory. Only otherwise-to-be-discarded bone fragments were used for research purposes.
Bone fragment preparation
Bone fragments for Raman spectroscopic analysis were transported and stored in gauze soaked with PBS enriched with protease inhibitor (0.1% volume for volume) and sodium azide (0.005% weight for volume) to prevent enzymatic or bacterial digestion of bone collagen and stored at −20°C until examination. Most specimens were examined by Raman spectroscopy within 24 h of the biopsy or amputation surgery and thawed at room temperature immediately before analysis. The average size of the biopsy specimens was <5 mm3, and the average size of the amputation specimens was >1 cm3. Raman spectra were collected with microscopy instrumentation adapted for Raman microspectroscopy as described elsewhere (3).
RESULTS
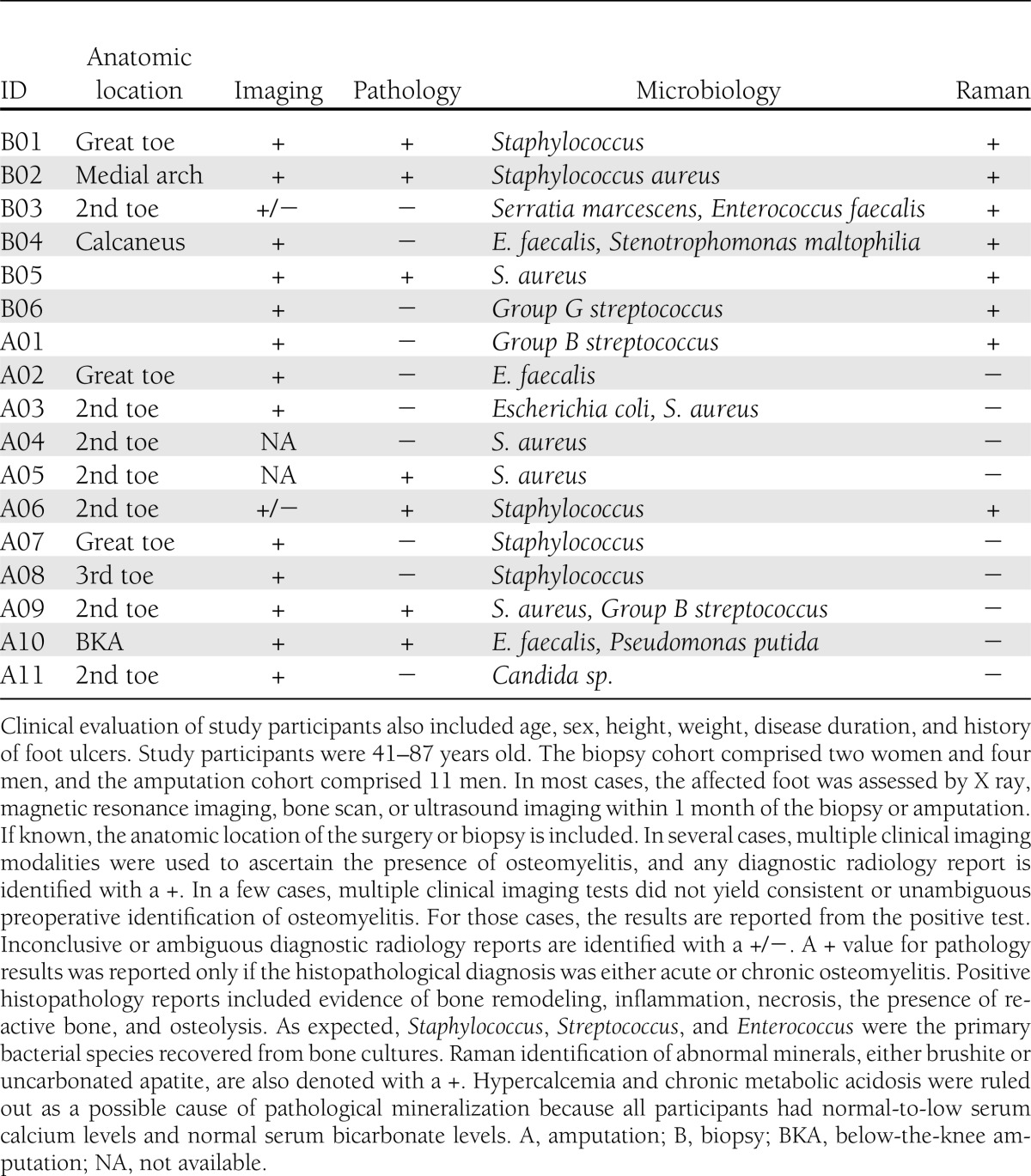
Table 1 shows the clinical imaging, pathology, microbiology, and Raman spectroscopy data for all study participants. In most cases, multiple clinical imaging modalities (magnetic resonance imaging, X ray, ultrasound, or bone scan) were used for preoperative identification of osteomyelitis. Pathology data on a range of pathophysiological states were reactive, active remodeling, necrotic, or osteomyelitic bone. Additional histopathological findings of acute inflammation or fibrosis were found in a few participants in the amputation group. As expected, bone cultures revealed a mixed population of gram-positive bacteria, with Staphylococcus, Streptococcus, or Enterococcus as the dominant species. Raman spectroscopy of the bone fragments revealed the presence of pathological minerals in addition to normal bone mineral. Two pathological minerals were identified: brushite and uncarbonated apatite. A + for Raman spectroscopic results was reported if brushite or uncarbonated apatite was detected. Raman spectra of control bone specimens were consistent with normal bone composition and did not show evidence of pathological mineralization. Storage in enriched PBS did not affect induced compositional changes in a control study of healthy bone fragments.
Table 1.
Summary of clinical data (imaging, histopathology, and microbiology) and experimental Raman spectroscopy data for bone specimens obtained from biopsy specimens or amputations
CONCLUSIONS
In this study, we applied Raman spectroscopy to measuring compositional changes in bone infected by osteomyelitis of the diabetic foot. Bone fragments were examined from patients who underwent either surgical biopsy/debridement or amputation. An unexpected finding was Raman spectral patterns corresponding to dicalcium phosphate dihydrate, also called brushite, and uncarbonated apatite. Compositional changes in bone currently cannot be identified by standard clinical imaging or histopathology but are easily measured by Raman spectroscopy. This study provides insight into the pathophysiology of diabetic osteomyelitis and identified a possible early-stage marker of clinical disease.
Many mechanisms of bone loss in osteomyelitis have been proposed in the literature (4–6). Even though bacterial biofilms are known to form in osteomyelitis, direct bacterial attack on bone is believed to be a negligible mechanism (7–9). The present results suggest that pathological mineralization accompanies bacterial infection, providing insight into the pathophysiology of osteomyelitis. The presence of pathological minerals may also serve as a compositional marker of early-stage bone infection. Brushite is only found in vivo under chronically acidic conditions, such as dental calculus, urinary stones, and chondrocalcinosis. To the best of our knowledge, this is the second report of brushite in mature human bone. Brushite was identified by X-ray absorption and infrared spectroscopy in fibrous dysplasia of the jaw (10). However, this finding has not been reproduced in other studies, and results from only one patient were reported. Poorly carbonated apatite can be found in woven, or immature, bone and is less crystalline than mature bone mineral (11). By contrast, the uncarbonated apatite found in infected bone was more crystalline than immature bone mineral and suggests deposition of a pathological mineral.
Normal serum calcium values in all the participants argue against the possibility that we were observing brushite and uncarbonated apatite as a precursor in normal bone formation or as a nonbone precipitate resulting from systemic hypercalcemia. The likelihood that pathological minerals were formed by an inflammatory response, immune response, or excessive bone remodeling is not supported by our observations and previous studies (12,13). Thus, we hypothesize that a bacteria biofilm is responsible for generating the acidic environment necessary to form brushite. If the localized microenvironment cannot be adequately buffered, then acidic calcium phosphate minerals such as uncarbonated apatite and brushite may precipitate onto the bone surface. This mechanism, although new in its application to diabetic osteomyelitis, is the accepted pathway in microbial degradation of bone postmortem (14).
Associating Raman spectroscopy data with anatomic location was an issue in the measurements and may have had an impact on the rate of identifying pathological minerals. Biopsy specimens were small (<5 mm3) and taken directly from the wound bed, so there was a greater association between the spectroscopy data and the anatomic location of the active infection. Thus, we were able to identify pathological minerals in 100% of the biopsy specimens. However, the amputated tissue was large relative to the recovered fragments. Although we worked closely with the pathology laboratory to obtain bone specimens near the site of suspected infection, obtaining precise anatomic information was a challenge. This challenge was also apparent when we examined the imaging and histopathology data. The lack of correlation between imaging and histopathology data in the amputation cohort underscores the difficulty in identifying osteomyelitis across a large anatomical unit, such as a digit or limb. We suspect that incomplete sampling was primarily responsible for inconsistent Raman spectroscopic identification of pathological minerals in amputated bone. Future translational studies will address developing enhanced anatomic precision with respect to geographic analysis of diabetic wounds.
It is intriguing to conceptualize an at-patient Raman spectroscopic measurement of pathological mineralization. Intraoperative or transcutaneous Raman spectroscopic identification of pathological minerals during biopsy or amputation surgeries may distinguish bone infections from noninfectious bone lesions. Point-of-care measurements are feasible because Raman spectroscopy is amenable to fiber-optic–based instrumentation. Our laboratory has developed portable fiber-optic instrumentation for transcutaneous bone measurements at bedside or in a surgical suite, and our ongoing human studies demonstrate in vivo feasibility and establish a basis for future translational Raman studies of diabetic foot wounds (15).
Acknowledgments
K.A.E.-W. acknowledges the training grant T32-AR-007080 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a career development grant from the Michigan Institute of Clinical and Health Research (UL1RR024986). This work was supported by grants R01-AR-055222 and R01-AR-047969 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to M.D.M.) and R21-EB-101026 from the National Institute of Biomedical Imaging and Bioengineering (to B.J.R.), National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
K.A.E.-W. contributed to the data analysis and drafted the manuscript. K.A.E.-W. and F.W.L.E.-W. contributed to the data collection. K.A.E.-W., F.W.L.E.-W., C.M.H., M.D.M., and B.J.R. contributed to the revision of the manuscript. K.A.E.-W., C.M.H., M.D.M., and B.J.R. contributed to the study design. K.A.E.-W., C.M.H., and B.J.R. contributed to the study conduct. K.A.E.-W. and M.D.M. contributed to the data interpretation. K.A.E.-W. and B.J.R. approved the final version of the manuscript. K.A.E.-W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at SPIE Photonics West, San Francisco, California, 2–7 February 2013.
The authors thank Jeff Kozlow and Jill Southwick for clinical collaboration at the AAVA Hospital. UMHS Tissue Procurement Core, UMHS Comprehensive Cancer Center (grant CA49652), and Steven Chensue and Lynn St. Dennis, VA Pathology, prepared bone specimens. The authors also thank Kaiser Optical Systems for instrument support; Erin Bigelow, University of Michigan Orthopaedic Research Laboratory, for preparation of cadaveric bone specimens; and Haiping Sun, University of Michigan Electron Microbeam Analysis Laboratory, for technical assistance.
References
- 1.Devendra D, Farmer K, Bruce G, Hughes P, Vivian G, Millward BA. Diagnosing osteomyelitis in patients with diabetic neuropathic osteoarthropathy. Diabetes Care 2001;24:2154–2155 [DOI] [PubMed] [Google Scholar]
- 2.Lavery LA, Armstrong DG, Peters EJG, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 2007;30:270–274 [DOI] [PubMed] [Google Scholar]
- 3.Dehring KA, Crane NJ, Smukler AR, McHugh JB, Roessler BJ, Morris MD. Identifying chemical changes in subchondral bone taken from murine knee joints using Raman spectroscopy. Appl Spectrosc 2006;60:1134–1141 [DOI] [PubMed] [Google Scholar]
- 4.Chihara S, Segreti J. Osteomyelitis. Dis Mon 2010;56:5–31 [DOI] [PubMed] [Google Scholar]
- 5.Henderson B, Nair SP. Hard labour: bacterial infection of the skeleton. Trends Microbiol 2003;11:570–577 [DOI] [PubMed] [Google Scholar]
- 6.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, Hänsch GM. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock 2003;20:503–510 [DOI] [PubMed] [Google Scholar]
- 7.Gristina AG, Oga M, Webb LX, Hobgood CD. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 1985;228:990–993 [DOI] [PubMed] [Google Scholar]
- 8.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 2008;52:13–22 [DOI] [PubMed] [Google Scholar]
- 9.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 1996;64:2371–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto H, Sakae T. Brushite in fibrous dysplasia of the jaw bone. Acta Pathol Jpn 1987;37:1699–1705 [DOI] [PubMed] [Google Scholar]
- 11.Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. J Bone Miner Res 2007;22:1548–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahata M, Maher JR, Juneja SC, et al. Mechanisms of bone fragility in a mouse model of glucocorticoid-treated rheumatoid arthritis: implications for insufficiency fracture risk. Arthritis Rheum 2012;64:3649–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misof BM, Gamsjaeger S, Cohen A, et al. Bone material properties in premenopausal women with idiopathic osteoporosis. J Bone Miner Res 2012;27:2551–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann B, Newesely H. Dekompositionsvorgänge des Knochens unter langer Liegezeit. 1. Die mineralishche Phase. Anthropol Anz 1982;40:19–31 [in German] [PubMed] [Google Scholar]
- 15.Esmonde-White FWL, Morris MD. Validating in vivo Raman spectroscopy of bone in human subjects. In Proceedings of SPIE. Kollias N, Ed. San Francisco, CA, SPIE, 2013, p. 85656K [Google Scholar]


