Abstract
OBJECTIVE
Clamp studies have shown that the absorption and action of rapid-acting insulin are faster with injection by a jet injector than with administration by conventional pen. To determine whether these pharmacokinetic changes also exist in patients with diabetes and benefit postprandial glucose control, we compared the pharmacologic profiles of insulin administration by jet injection versus conventional insulin pen after a standardized meal in patients with type 1 or type 2 diabetes.
RESEARCH DESIGN AND METHODS
In a randomized, double-blind, double-dummy crossover study, 12 patients with type 1 diabetes and 12 patients with type 2 diabetes received insulin aspart either by jet injection or by conventional pen, in both cases followed by a standardized meal. Blood was sampled for 6 h for determination of glucose and insulin levels to calculate pharmacologic profiles.
RESULTS
Insulin administration by jet injection resulted in shorter time until peak plasma insulin level (51.3 ± 6.4 vs. 91.9 ± 10.2 min; P = 0.003) and reduced hyperglycemic burden during the first hour (154.3 ± 20.8 vs. 196.3 ± 18.4 mmol · min · L−1; P = 0.041) compared with conventional administration. Jet injection did not, however, significantly reduce the hyperglycemic burden during the 5-h period thereafter. There was no indication that the jet injector performed differently in patients with type 1 and type 2 diabetes.
CONCLUSIONS
The considerably more rapid insulin absorption after administration by jet injector translated to a significant if modest decrease in postprandial hyperglycemia in patients with type 1 and type 2 diabetes. The improved early postprandial glucose control may specifically benefit patients who have difficulty in limiting postprandial glucose excursions.
The pharmacologic profile of rapid-acting insulin analogs, although considerably faster than regular insulin, is still relatively slow compared with the profile of endogenous insulin release. As a consequence, patients with type 1 diabetes or insulin-requiring type 2 diabetes who use these analogs still face the risk of immediate postprandial hyperglycemia and late postprandial hypoglycemia. In particular, postprandial hyperglycemia has been recognized as an important contributor to suboptimal glucose control (1), which may explain why the introduction of rapid-acting analogs has had little effect on HbA1c in people with diabetes (2). Some have therefore suggested that these analogs should be injected at least 15 min before meals (3); however, this seems impractical to implement in daily practice.
Poor adherence to insulin therapy because of injection-related anxiety may be another, often neglected, reason for failure to reach glycemic targets with current rapid- and long-acting insulin analogs (4). A sizable proportion of insulin users admit to at least occasionally skipping insulin injections or restricting the number of daily injections (4). Although true needle phobia is rare, many patients with diabetes perceive insulin injections as painful or experience some form of anxiety with injections (5,6), the presence of which is strongly associated with nonadherence and poorer glycemic control (7).
Jet injectors for insulin administration provide a needle-free alternative to the use of pens or syringes and were originally developed for patients with needle phobia. Administration by jet injection significantly accelerates absorption of rapid-acting insulin from the subcutaneous area into the systemic circulation (8). Jet injectors deliver insulin at a high velocity (typically >100 m/s) directly across the skin in the subcutaneous tissue and dispense the insulin over a larger area than does injection by syringe (9). With the euglycemic clamp technique, we recently showed in healthy volunteers that administration of insulin aspart by jet injection reduced both the time until peak plasma insulin levels and the time to maximal glucose-lowering effect by approximately 50% when compared with insulin administered by conventional insulin pen (10).
Although the euglycemic clamp technique is a reliable method to investigate the pharmacodynamics of therapeutic insulin, it cannot be used to predict the glucose-lowering effect of insulin when injected before a meal, particularly in patients with diabetes. The aim of the current study was therefore to investigate the pharmacology of insulin injected by jet injector before a standardized meal in patients with type 1 diabetes and insulin-requiring type 2 diabetes. We also wanted to investigate whether patients would perceive insulin administration with the current jet injector device as more or less painful as insulin injection by pen.
RESEARCH DESIGN AND METHODS
Written, informed consent was obtained from 12 patients with type 1 diabetes and 12 patients with insulin-treated type 2 diabetes. Participants were recruited from the outpatient diabetes clinic of the Radboud University Nijmegen Medical Centre and by advertisement in a local newspaper. All patients were at least 18 years of age and had a BMI <32 kg/m2 and HbA1c ≤9%. Patients were excluded if they had experienced a major vascular event (e.g., myocardial infarction, stroke, symptomatic peripheral artery disease, coronary artery bypass surgery, or percutaneous coronary or peripheral artery angioplasty) in the previous 6 months; used immunosuppressant agents, nonsteroidal anti-inflammatory drugs, anticoagulant therapy, or oral antidiabetic drugs other than metformin for type 2 diabetic patients; or had symptomatic diabetic neuropathy. Pregnancy was excluded where appropriate. The study was approved by the institutional review board of the Radboud University Nijmegen Medical Centre.
All participants underwent two standardized meal tests, separated by at least 2 weeks. Patients were requested to reduce the evening dose of insulin or the basal rate of insulin pump administration by 10–20% to avoid nocturnal hypoglycemia and were instructed to consume a low–glycemic index meal on the evenings before the experimental days. On each experimental day, participants were admitted to the research unit at 0730 h in fasting condition and having abstained from smoking, alcohol use, and caffeine use for at least 24 h. Patients with subcutaneous insulin pumps were asked to stop the pump. The experiments were performed with the patients in supine position in a temperature-controlled room (22–24°C). Two catheters were inserted intravenously. One catheter was inserted in retrograde fashion in a dorsal hand vein for blood sampling, with the vein kept patent by placing the hand in a heated box at 55°C (11). The other catheter was placed in an antecubital vein of the contralateral arm for insulin and glucose administration. After baseline variables were obtained, low-dose insulin was infused to achieve normoglycemia, after which insulin infusion was either terminated or, for patients treated with insulin pumps, continued at a rate corresponding to the basal rate of the patient’s insulin pump.
Thirty minutes after achievement of stable normoglycemia, patients received insulin (aspart; Novo Nordisk, Bagsvaerd, Denmark) either by jet injection (Insujet; European Pharma Group bv, Schiphol-Rijk, the Netherlands) or by conventional insulin pen (NovoPen III; Novo Nordisk) and a comparable volume of placebo solution (Test Medium Penfill; Novo Nordisk) by the alternate device in a double-blind fashion, both administered subcutaneously in the abdomen. On the other occasion, the devices containing the insulin and placebo solution were reversed. Thus all participants received both insulin and placebo on the two experimental days (“double dummy”). The dose of insulin was individualized to the patient’s usual prandial insulin requirements and averaged 17.6 ± 3.9 units (8–40 units, 16.3 ± 3.9 units for patients with type 1 diabetes and 18.9 ± 11.1 units for patients with type 2 diabetes; P = 0.44). Two-by-two block randomization was used to randomize the sequence by which the two devices were used for insulin and placebo injections. To ensure blinding, both pen devices were prepared by a nurse who was not otherwise involved in conducting the experiments. Insulin administration with both devices was performed by trained staff only, as described in detail previously (10).
One minute after insulin administration, the participant consumed a standardized meal consisting of three white-bread sandwiches with marmalade and honey and a glass of orange juice (total energy 538 kcal, 108 g carbohydrates, 7 g fat, 11 g protein) in 10–15 min. Plasma glucose measurements were measured at 5-min intervals during the first 3 h of the study and at 10-min intervals for another 3 h. Blood for plasma insulin levels was sampled every 5 min during the first hour, every 15 min for the second hour, and every 30 min thereafter. When plasma glucose values dropped below 4.8 mmol/L, 20% dextrose in water was administered intravenously to maintain normoglycemia. On the second experimental day, patients were asked to rate the amounts of discomfort or pain experienced with the two administration methods on a visual analog scale from 0 to 10 cm and to indicate which device they would prefer should they have a choice.
All pharmacologic parameters were derived from the plasma glucose and insulin levels. With respect to the pharmacokinetics, we calculated the time to maximal insulin concentration (T-INSmax), the maximal insulin concentration (C-INSmax), the area under the insulin concentration curve (C-INSAUC), and the time until 50% of insulin absorption (T-INSAUC50%). We also calculated the times until reaching 50% of the C-INSmax as insulin levels rose and until reaching the same value as insulin levels declined. The pharmacodynamic parameters consisted of the area under the baseline-subtracted plasma glucose concentration–time curve during the first hour (BG-AUC1h) and first 2 h (BG-AUC2h) after insulin injection, representing the initial glycemic load, the maximal glucose excursion (BGmax), the area under the total baseline-subtracted plasma glucose concentration time-curve (BG-AUC6h), and the time until plasma glucose had returned to baseline. Safety parameters included the number of patients requiring exogenous glucose infusion to prevent postprandial hypoglycemia, the amount of exogenous glucose required, and the duration that exogenous glucose was required.
Plasma glucose was measured with the glucose enzymatic-amperometric method (Biosen C-line GP+; EKF-diagnostic GmbH, Barleben, Germany) during the experiments. Blood sampled for determination of plasma insulin levels was collected in lithium-heparin tubes. After centrifugation, the supernatant was stored at −20°C. Plasma insulin was measured by radioimmunoassay (12).
Power calculation and statistical analyses
All data are expressed as mean ± SEM unless otherwise indicated. Mean outcomes for all study end points and most safety end points were tested with paired t tests. A χ2 test was used to compare the numbers of patients requiring exogenous glucose with the two injection devices. Continuous data were tested for normal distribution (Shapiro-Wilk test and Kolmogorov-Smirnov test) and subsequently analyzed with repeated measures ANOVA (Wilks Λ test), with the device as between-subjects factor. P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (Statistical Package for the Social Sciences, version 18.0; IBM Corporation, Armonk, NY).
RESULTS
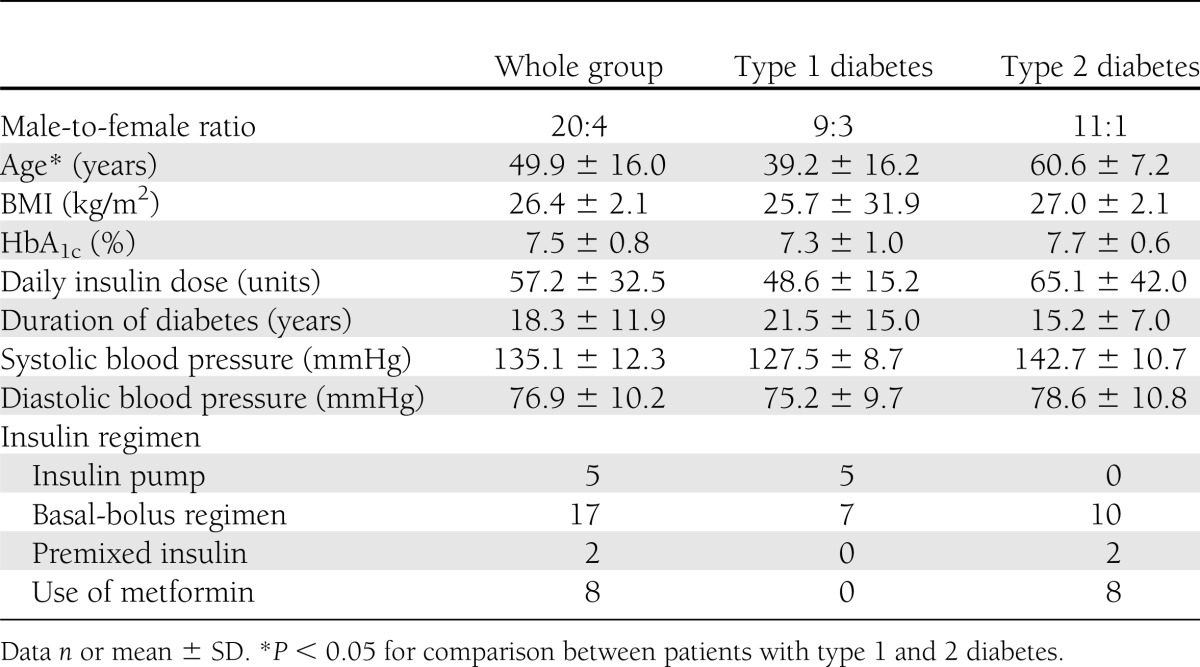
Table 1 summarizes the baseline characteristics of the participants. All 24 subjects completed the meal tests. The first test had to be rescheduled in two cases. In one instance, the insulin dose administered was erroneously calculated too low, and in the other, the spring of the jet injector released prematurely, so that it could not be assessed how much insulin (or placebo), if any, had actually crossed the skin. That jet injector was subsequently returned to the manufacturer and replaced.
Table 1.
Baseline characteristics
Pharmacokinetic end points
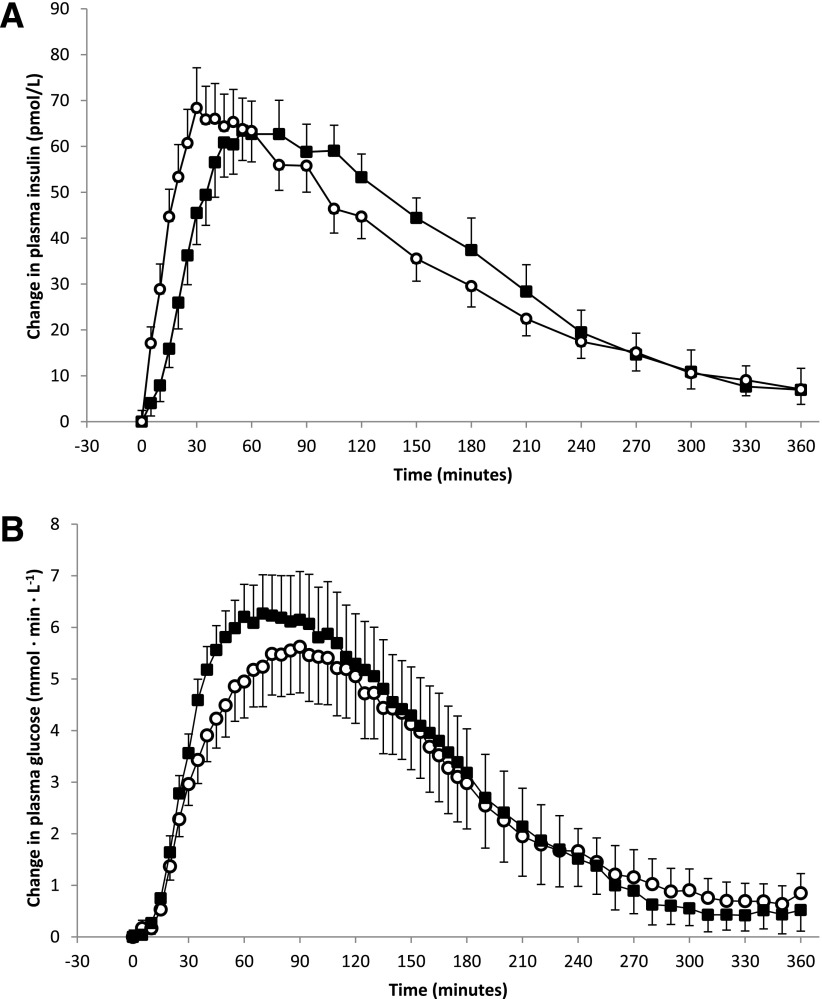
Plasma insulin levels at initiation of insulin injections were slightly higher for the experiments where the conventional pen was used than those for the jet injector (22.8 ± 2.5 vs. 19.4 ± 2.4 mU/L; P = 0.037). Thereafter, plasma insulin levels increased faster when insulin was injected with the jet injector than when injected by conventional pen (Table 2). The time until peak plasma insulin concentrations was advanced from 91.9 ± 10.2 min with the conventional pen to 51.3 ± 6.4 min with the jet injector (P = 0.003), a difference of 40.6 ± 12.3 min (Fig. 1A, Table 2). In addition, T-INSAUC50% and the time for insulin levels to decline after reaching peak values were significantly shorter when insulin was administered by jet injection rather than by conventional pen (Table 2). In contrast, the total amount of insulin absorbed during the entire 6-h period, as reflected by the C-INSAUC, did not differ between the two devices.
Table 2.
Pharmacokinetic and pharmacodynamic parameters for insulin administration with the jet injector and the conventional insulin pen
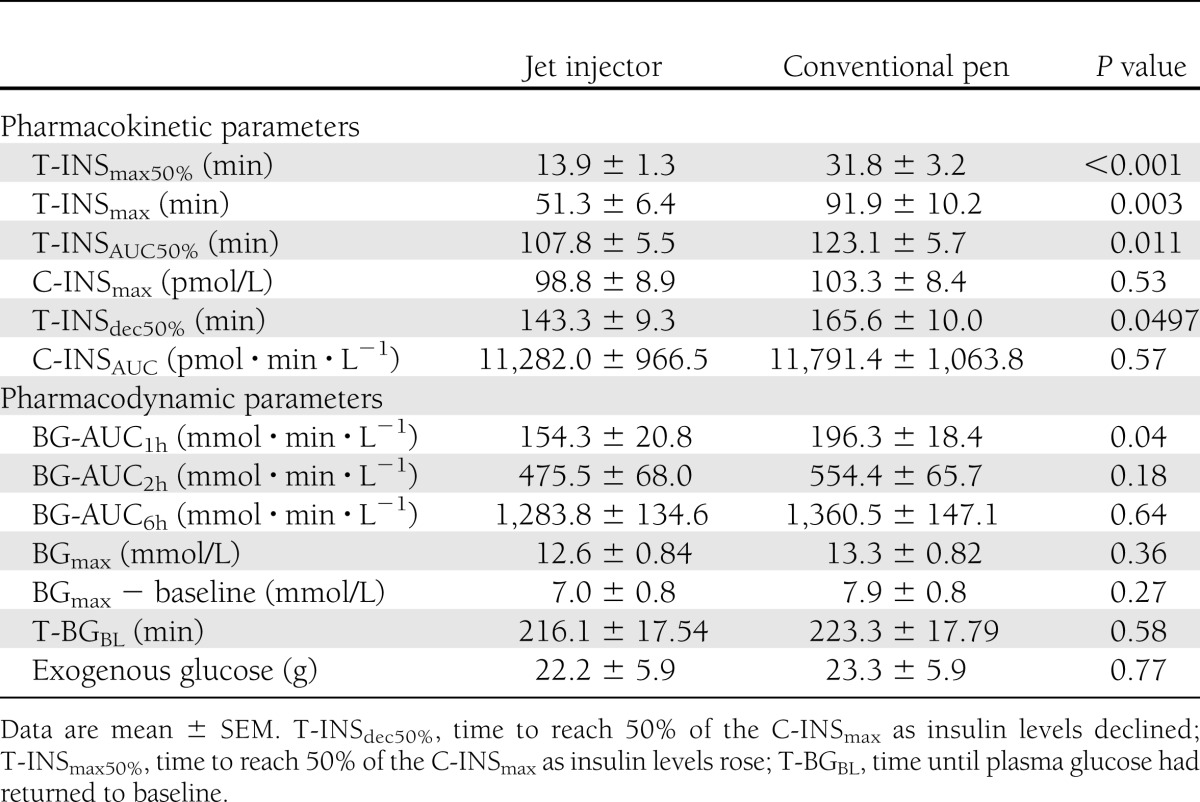
Figure 1.
Changes from baseline during the standardized meal test. A: Changes in plasma insulin levels after insulin administration by jet injector (○) and conventional pen (■). B: Changes in plasma glucose levels after insulin administration by jet injector (○) and conventional pen (■).
Pharmacodynamic end points
There were no differences in plasma glucose values between the two experimental conditions, either at baseline (10.5 ± 0.6 vs. 10.8 ± 0.7 mmol/L; P = 0.65) or directly before the experiments (5.61 ± 0.13 vs. 5.45 ± 0.18 mmol/L; P = 0.49). The time-action curves for the plasma glucose level after meal ingestion were significantly different between the two devices (P = 0.018 by ANOVA). In line with the faster insulin pharmacokinetics, the hyperglycemic burden during the first hour, as reflected by the area under the glucose concentration curve (BG-AUC1h) was significantly reduced when insulin was administered with the jet injector rather than by conventional pen (Fig. 1B and Table 2). This benefit favoring the jet injector relative to the conventional insulin pen was no longer apparent after 2 h (Table 2). Although glucose values tended to be lower in the late postprandial phase after conventional pen administration, there were no significant differences between the two devices with regard to the maximal glucose value, maximal glucose increment, or area under the 6-h glucose concentration curve (Table 2).
Safety
There were no differences between the jet injector and conventional pen with respect to number of patients requiring exogenous glucose to prevent hypoglycemia (17 vs. 18; P = 0.75), the timing of initiation of glucose administration (180 vs. 194 min; P = 0.79), or the amount of glucose administered (21.0 ± 5.5 g vs. 23.7 ± 5.7 g; P = 0.61). Both injection methods were well tolerated and elicited similar experiences of pain or discomfort (visual analog scale 1.96 vs. 1.40; P = 0.14). Of the total of 24 patients, 13 preferred the conventional pen to the jet injector, 4 preferred the jet injector, and 6 remained indifferent. One patient did not complete the questionnaire.
Subgroup analysis
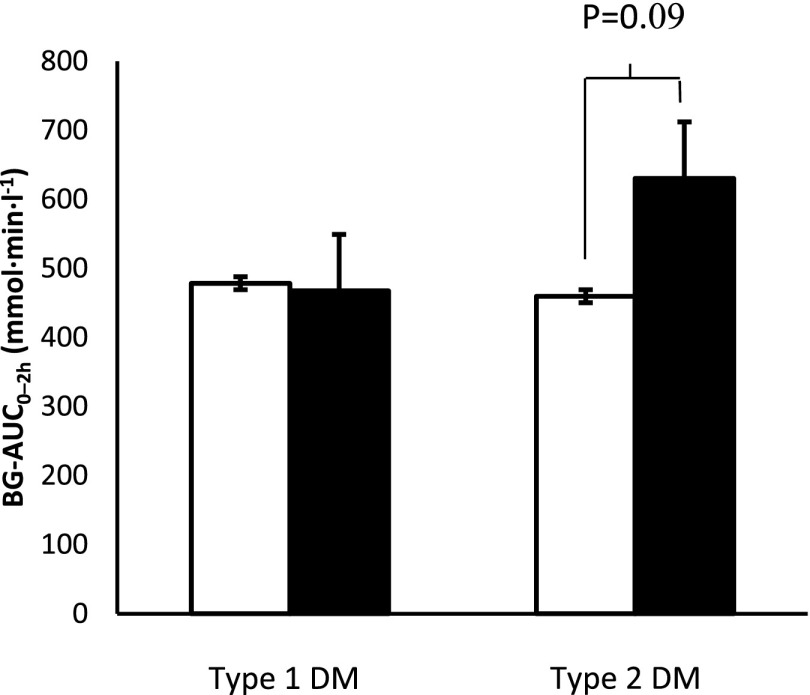
The pharmacodynamic benefits of insulin administration by jet injection tended to be numerically higher in patients with type 2 diabetes; however, the difference was not statistically different between patients with type 1 and type 2 diabetes. For type 1 diabetes, BG-AUC2h after jet injection was 478 ± 117 vs. 500 ± 98 mmol · min · L−1 after conventional administration (P = 0.87); values for type 2 diabetes were 471 ± 82 vs. 662 ± 96 mmol · min · L−1 (P = 0.09) (Fig. 2). There were no differences in subgroups defined by age, sex, or BMI (data not shown).
Figure 2.
The 2-h BG-AUC values for the jet injector (white bars) and conventional pen (black bars) in subgroups according to type of diabetes.
CONCLUSIONS
Previously, we showed in young, healthy volunteers that absorption and action of aspart insulin occurred twice as fast when administered by jet injection as by conventional pen (10). The current study confirms a more rapid absorption of insulin aspart when administered by the current jet injector compared with a conventional pen in patients with type 1 and type 2 diabetes. These pharmacokinetic properties translated into a significant, albeit modest, decrease in early (first 60 min) postprandial hyperglycemia after a standardized meal rich in carbohydrates. Beyond 1 h, the benefit of jet injection on postprandial glycemic burden was no longer statistically significant.
Considering the substantial enhancement of insulin action by jet injection in our clamp study (10) and the current improvement of insulin absorption, we anticipated a sizeable pharmacodynamic benefit of insulin administration by jet injection in the current study. Although the lower early postprandial glucose levels and tendency toward lower glucose levels in the later postprandial phase confirmed the results of the clamp study under conditions more like an actual clinical situation, the effects were rather modest. This partial discrepancy may be explained first by the relatively large variation, both inter- and intraindividually, in glucose excursions after the meal test. Indeed, maximal glucose excursions varied between 5.9 and 22.9 mmol/L among subjects and between 0.1 and 12.9 mmol/L within subjects. Glucose levels at admission at the research unit were similarly variable, although all patients had consumed a low–glycemic index supper on the evening before the experiments. Factors contributing to this large variation may include the heterogeneity of the participants, the suboptimal glycemic control, and use of insulin by all participants, including those with type 2 diabetes.
A second explanation concerns the finding that insulin absorption was much slower in the patients than we had previously observed in healthy volunteers (10). Although jet injection advanced insulin absorption to roughly similar extents in both groups, insulin levels peaked substantially later in diabetic subjects with than in those without diabetes (51.3 vs. 30.6 min; P = 0.012). Because the standardized meal consisted mainly of high–glycemic index carbohydrates, insulin absorption may have been too slow to sufficiently counteract the fast glucose load absorption. Whether jet injection would have performed better for a more usual mixed meal with slower food absorption cannot be determined from our data. Parenthetically, most patients in our study claimed never to consume high–glycemic index carbohydrates in such large quantities, to avoid extreme glucose excursions.
Why rapid-acting insulin absorption is slower in patients with diabetes than in subjects without diabetes is unknown. We previously showed a strong association between BMI and rate of insulin absorption in healthy subjects, arguing a role for greater subcutaneous tissue thickness (13). In the current patient group, however, insulin absorption was unrelated to the BMI or any other measure of body composition. The presence of insulin antibodies, commonly found in patients on long-term insulin therapy, has been suggested to attenuate the absorption of subcutaneous insulin (14); however, insulin antibodies were not measured in our study. Alternatively, subcutaneous adipose tissue blood flow (ATBF) may also affect the absorption of insulin (15). Reduced subcutaneous ATBF has been reported in overweight nondiabetic subjects (16), as well as in patients with type 1 (17,18) and type 2 diabetes (19). In addition, ATBF may fail to increase in response to dietary stimuli in overweight subjects (16) and in patients with type 2 diabetes (19).
Although the pharmacodynamic benefit of jet injection appears small, it is of potential clinical relevance for patients with early postprandial glucose excursions not sufficiently covered by conventional insulin injections. Administration of rapid-acting insulin by this jet injector may represent an especially valuable alternative for patients with type 2 diabetes, in whom the postprandial glycemic benefit of jet injection tended to be more pronounced than in those with type 1 diabetes. Parenthetically, a device that performs at least as well as, and potentially better than, conventional pens may be a good option for any patient who does not tolerate insulin injections by needle or regards these as otherwise uncomfortable. A recent survey showed that 28.6% of patients with type 2 diabetes perceive insulin injections as painful (20), putting such patients at high risk of skipping at least occasional injections (7). When questioned, most patients still preferred the conventional pen to the jet injector; however, these patients were unselected, generally tolerated conventional injections without discomfort, consequently feeling no need to change the mode of administration, and did not handle the jet injector (or the conventional pen) themselves under study conditions. Future research will need to reveal tolerability of the jet injector after personal experience with the device in daily practice for a longer period.
Handling a jet injector may be more cumbersome than handling a conventional insulin pen. It requires sufficient training in air-free filling of the chamber with insulin and correct placement of the injector on the skin to ensure that the entire volume of insulin reaches the subcutaneous compartment. Inadequate contact between injector and the skin has been reported to result in bruises and “wet” injections, leading to discomfort and unpredictable insulin absorption profiles (21). Importantly, the current device has a built-in lock-release system that only releases insulin when proper skin contact has been made and sufficient pressure has been applied to the nozzle of the injector. Other factors that determine optimal insulin delivery relate to jet velocity and nozzle diameter (22–24). Our data are therefore in part specific to the jet injector used and cannot be extrapolated to other jet injectors.
Various strategies are currently under development to enhance absorption and action of subcutaneous insulin. These strategies include local skin heating to stimulate tissue perfusion (25), coadministration with hyaluronidase to break the solidity of the extracellular matrix (26), and destabilizing insulin hexamer formation by addition of EDTA and citric acid (27). The pharmacokinetic and pharmacodynamic benefits of these interventions are more or less comparable to those of jet injection and range from ∼10 to ∼60% advancement of peak insulin levels and maximal glucose-lowering effect (25–27). A difference between jet injection and other developments to enhance insulin absorption is that the former is already available for clinical application, whereas the latter are in still in various stages of development and have not yet been marketed.
In the current study, we found that jet injection accelerated the absorption of insulin aspart in patients with type 1 and insulin-treated type 2 diabetes. This better pharmacokinetic profile was initially followed by a congruent reduction in glucose excursions after a high-glycemic index meal, in particular during but not beyond the first hour. Jet injection may therefore be a good needle-free alternative to conventional insulin pens of at least equivalent pharmacological efficacy for the administration of insulin in patients with diabetes. Future research is needed to determine whether the better pharmacologic properties of insulin jet injection translate into beneficial long-term effects on glycemic control and risk of hypoglycemia in patients with diabetes.
Acknowledgments
European Pharma Group bv, Schiphol-Rijk, the Netherlands, funded the study. No other potential conflicts of interest relevant to this article were reported.
The funder, European Pharma Group bv, was not involved in the design or execution of the study or in the writing of the manuscript.
E.E.C.E. performed the experiments, analyzed the data, interpreted the data, drafted the first version of the manuscript, edited the manuscript, and approved the final version of the manuscript. C.J.T. analyzed the data, interpreted the data, edited the manuscript, and approved the final version of the manuscript. B.E.d.G. designed the study, wrote the study protocol, performed the experiments, analyzed the data, interpreted the data, edited the manuscript, and approved the final version of the manuscript. B.E.d.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Karin Saini, Anja Rasing, and Mariëlle Verstegen (research nurses, Radboud University Nijmegen Medical Centre) for their assistance during the clamps, Petra van de Ven and Sandra Hendriks (diabetes nurses, Radboud University Nijmegen Medical Centre) for preparing the insulin pens, and the patients for their participation in this study. The authors also thank Tim Heise, Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany, for his advice on study methodology and careful reading of the manuscript.
Footnotes
Clinical trial reg. no. NCT01438632, clinicaltrials.gov.
References
- 1.Monnier L, Colette C, Owens D. Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes Technol Ther 2011;13(Suppl. 1):S25–S32 [DOI] [PubMed] [Google Scholar]
- 2.Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006;19:CD003287. [DOI] [PubMed] [Google Scholar]
- 3.Rassam AG, Zeise TM, Burge MR, Schade DS. Optimal administration of lispro insulin in hyperglycemic type 1 diabetes. Diabetes Care 1999;22:133–136 [DOI] [PubMed] [Google Scholar]
- 4.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care 2010;33:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gale EA. Two cheers for inhaled insulin. Lancet 2001;357:324–325 [DOI] [PubMed] [Google Scholar]
- 6.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care 1997;20:585–590 [DOI] [PubMed] [Google Scholar]
- 7.Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther 2012;14:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malone JI, Lowitt S, Grove NP, Shah SC. Comparison of insulin levels after injection by jet stream and disposable insulin syringe. Diabetes Care 1986;9:637–640 [DOI] [PubMed] [Google Scholar]
- 9.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov 2006;5:543–548 [DOI] [PubMed] [Google Scholar]
- 10.Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care 2011;34:1804–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981;30:936–940 [DOI] [PubMed] [Google Scholar]
- 12.de Galan BE, Tack CJ, Lenders JW, et al. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes 2002;51:790–796 [DOI] [PubMed] [Google Scholar]
- 13.de Galan BE, Engwerda EE, Abbink EJ, Tack CJ. Body mass index and the efficacy of needle-free jet injection for the administration of rapid-acting insulin analogs, a post hoc analysis. Diabetes Obes Metab 2013;15:84–86 [DOI] [PubMed] [Google Scholar]
- 14.Radermecker RP, Renard E, Scheen AJ. Circulating insulin antibodies: influence of continuous subcutaneous or intraperitoneal insulin infusion, and impact on glucose control. Diabetes Metab Res Rev 2009;25:491–501 [DOI] [PubMed] [Google Scholar]
- 15.Vora JP, Burch A, Peters JR, Owens DR. Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care 1992;15:1484–1493 [DOI] [PubMed] [Google Scholar]
- 16.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010;59:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebrandt P. Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand Suppl 1991;603:41–45 [PubMed] [Google Scholar]
- 18.Vora JP, Burch A, Peters JR, Owens DR. Absorption of radiolabelled soluble insulin in type 1 (insulin-dependent) diabetes: influence of subcutaneous blood flow and anthropometry. Diabet Med 1993;10:736–743 [DOI] [PubMed] [Google Scholar]
- 19.Tobin L, Simonsen L, Bülow J. The dynamics of the microcirculation in the subcutaneous adipose tissue is impaired in the postprandial state in type 2 diabetes. Clin Physiol Funct Imaging 2011;31:458–463 [DOI] [PubMed] [Google Scholar]
- 20.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab 2012;9999:1081–1087 [DOI] [PubMed] [Google Scholar]
- 21.Schramm J, Mitragotri S. Transdermal drug delivery by jet injectors: energetics of jet formation and penetration. Pharm Res 2002;19:1673–1679 [DOI] [PubMed] [Google Scholar]
- 22.Stachowiak JC, Li TH, Arora A, Mitragotri S, Fletcher DA. Dynamic control of needle-free jet injection. J Control Release 2009;135:104–112 [DOI] [PubMed] [Google Scholar]
- 23.Schramm-Baxter J, Mitragotri S. Needle-free jet injections: dependence of jet penetration and dispersion in the skin on jet power. J Control Release 2004;97:527–535 [DOI] [PubMed] [Google Scholar]
- 24.Linn L, Boyd B, Iontchev H, King T, Farr SJ. The effects of system parameters on in vivo injection performance of a needle-free injector in human volunteers. Pharm Res 2007;24:1501–1507 [DOI] [PubMed] [Google Scholar]
- 25.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther 2009;31:980–987 [DOI] [PubMed] [Google Scholar]
- 26.Muchmore DB, Vaughn DE. Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Tech 2010;4:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiner S, Hompesch M, Pohl R, et al. A novel insulin formulation with a more rapid onset of action. Diabetologia 2008;51:1602–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]