When we look for our children on the playground, internal representations of these important targets must guide our search through the cluttered and chaotic scene. Several theories of attention propose that we hold target representations (i.e., attentional templates) in visual working memory (VWM) to control perceptual attention (Bundesen, Habekost, & Kyllingsbaek, 2005; Desimone & Duncan, 1995). Although this is a foundational theoretical assumption, there is no direct electrophysiological evidence from humans supporting this proposal and recordings from monkeys have yielded mixed results (Chelazzi, Miller, Duncan, & Desimone, 1993, 2001; Kusunoki, Sigala, Gaffan, & Duncan, 2009). This makes it difficult to rule out the classic hypothesis that visual search may operate like a prepared reflex, unguided by VWM representations (Logan, 1978). In the present study, we tested the attentional-template hypothesis by recording event-related potentials (ERPs) from subjects while they searched for targets in complex scenes.
On each trial, subjects saw a target-cue array followed by a complex search array (see Figure 1A). We focused our analyses on the ERPs following the cue to determine whether the contralateral-delay activity (CDA) was present. The CDA indexes the maintenance of representations in VWM (e.g., Vogel & Machizawa, 2004), thus providing an ideal tool for testing the hypothesis that we maintain attentional templates in VWM during search. That is, unlike imaging studies demonstrating how brain areas modulate under different task demands (e.g., Soto, Humphreys, & Rotshtein, 2007), we can use the CDA component to definitely show that the same VWM mechanisms relied upon in explicit-memory tasks are engaged in maintaining attentional templates. If templates are maintained in VWM, then we should observe a cue-elicited CDA that continues until search is performed. Furthermore, if the cue-elicited CDA directly measures the attentional template, then CDA amplitude measured prior to the search task should predict subsequent performance.
Figure 1.
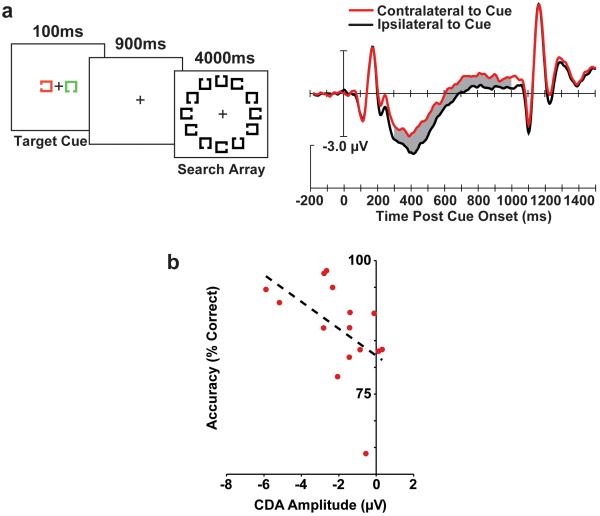
The stimuli, ERP findings, and relationship between the ERPs and behavior. A) Example of the stimulus sequence and the grand-average waveforms from electrodes T5/6, where the effect was maximal, contralateral (red) and ipsilateral (black) to the location of the cue on each trial. The gray region shows the epoch in which the significant CDA was measured. B) Visual search accuracy as a function of the CDA amplitude measured following the cue. Note that a more negative voltage equals a larger CDA. Each point represents the data from an individual subject and the dashed line represents the linear regression.
Method
Participants
Fifteen volunteers (18-35 years-of-age, neurologically normal, normal color vision and acuity) provided informed consent.
Stimuli
The stimuli were viewed on a gray background (54.3 cd/m2) with a black fixation cross (<0.01 cd/m2, 0.4° × 0.4° of visual angle). The two cue stimuli were Landolt squares (0.7° × 0.7°, 0.1° line thickness, with a 0.5° gap, presented 2.2° to the left and right of center), one was green (x = .281, y = .593, 45.3 cd/m2), the other red (x = .612, y = .333, 15.1 cd/m2). The visual search arrays contained 12 black Landolt squares with a gap on the left, right, top, or bottom (<0.01 cd/m2, centered 4.4° from fixation).
Procedure
Figure 1A illustrates the timing of events during each trial. A target containing a gap on the same side as the cued shape was presented on 50% of trials. The cued-target shape (top, bottom, left, or right gap), relevant-cue location (left or right), target presence (present or absent), and the target location, were randomized across trials. Participants responded as fast and accurately as possible to each search array using a hand-held gamepad. Twenty-four practice trials preceded 4 blocks of 192 experimental trials. Across blocks, observers switched between task-relevant red and green cues to prevent physical stimulus confounds (Woodman, in press).
ERP Recording and Analysis
We recorded the electroencephalogram and electrooculogram using standard procedures (Woodman & Luck, 2003; Woodman & Vogel, 2008). A two-step method for artifact and subject rejection (Woodman & Luck, 2003) excluded 16.8% of trials/subject and prompted the replacement of 3 subjects. The CDA was measured 300-1000 ms after cue onset (Vogel & Machizawa, 2004). An ANOVA with the factors of contralaterality (ipsilateral versus contralateral to the cue), hemisphere (left versus right), target presence (present versus absent), and electrode site (PO3/4, O1/2, OL/R, versus T5/6) was used. P-values were Greenhouse-Geisser corrected (Jennings & Wood, 1976). Due to the absence of significant effects, the data were collapsed across cue color.
Results
Behavior
Subjects were more accurate on target absent than target-present trials (92.2% versus 79.3% correct, respectively), F(1,14) = 6.68, η2 = 0.21, p < .001, suggesting that performance was limited by an inability to maintain the target representation on a subset of trials. When correct, RTs were faster on target present than target-absent trials (1300 versus 1965 ms, respectively), F(1,14) = 108.69, η2 = 0.63, p < .001.
ERPs
Figure 1A shows that we found a significant cue-elicited CDA. This resulted in significant effects of contralaterality, F(1,14) = 14.67, η2 = 0.002, p < .01, electrode site, F(3,42) = 7.27, η2 = 0.04, p < .001, and contralaterality X electrode site, F(3,42) = 15.85, η2 = 0.13, p < .0001, due to the expected CDA scalp distribution. No other main effects or interactions were significant (ps > .25).
The difficulty of the search task created a range of behavioral performance that allowed us to determine whether the cue-elicited CDA amplitude predicted how well search was performed. As Figure 1B shows, subjects with larger cue-elicited CDAs also had more accurate search performance (r2 = .26, p < .05). The relationship between amplitude and RT was not significant (r2 = .07 p > .20). The latter is not surprising given that search RTs are influenced by the speed of attentional shifts and categorization, as well as thresholds for deciding target absence (Bundesen, 1990; Chun & Wolfe, 1996). Our ability to predict search accuracy using the cue-elicited CDA supports our conclusion that we directly measured the VWM representations that drove the attention demanding search process using ERPs.
Conclusions
Here we show that our VWM representations guide attention when performing tasks where our target switches from moment-to-moment, as we typically do in the real world (e.g., searching for your son in the pool, then your daughter near the swings). Our findings validate a critical assumption of several prominent theories of attention (e.g., Bundesen et al., 2005; Desimone & Duncan, 1995). Seemingly contradictory findings from monkey neurophysiology (Chelazzi et al., 2001; Kusunoki et al., 2009) and human behavioral studies (Woodman & Luck, 2007; Woodman, Vogel, & Luck, 2001) had raised doubt about the attentional-template hypothesis. However, these previous studies most likely minimized the contribution of VWM by using a small, well-learned set of stimuli (Kusunoki et al., 2009; Woodman, Luck, & Schall, 2007).
Our findings also have implications for determining the locus of cognitive impairments in clinical disorders. Specifically, an inability to represent attentional templates in VWM could be mistaken for attention deficits during tasks requiring strong top-down control (Gold, Fuller, Robinson, Braun, & Luck, 2007; Zubin, 1975). The present technique may provide a way to define specific phenotypes within a variety of clinical diagnoses where VWM malfunctions could masquerade as attention deficits (Hill, Harris, Herbener, Pavuluri, & Sweeney, 2008; Walshaw, Alloy, & Sabb, 2010).
Acknowledgments
G.F.W. is supported by NEI and NSF. Deborah Pardo aided in data collection.
References
- Bundesen C. A theory of visual attention. Psychological Review. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Bundesen C, Habekost T, Kyllingsbaek S. A neural theory of visual attention: Bridging cognition and neurophysiology. Psychological Review. 2005;112:291–328. doi: 10.1037/0033-295X.112.2.291. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. Responses of neurons in macaque area V4 during memory-guided visual search. Cerebral Cortex. 2001;11:761–772. doi: 10.1093/cercor/11.8.761. [DOI] [PubMed] [Google Scholar]
- Chun MM, Wolfe JM. Just say no: How are visual searches terminated when there is no target present? Cognitive Psychology. 1996;30(1):39–78. doi: 10.1006/cogp.1996.0002. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Harris MSH, Herbener ES, Pavuluri M, Sweeney JA. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2008;34:743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Sigala N, Gaffan D, Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cerebral Cortex. 2009;19:2522–2534. doi: 10.1093/cercor/bhp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. Attention in character classification tasks: Evidence for the automaticity of component stages. Journal of Experimental Psychology: General. 1978;107:32–63. [Google Scholar]
- Soto D, Humphreys GW, Rotshtein P. Dissociating the neural mechanisms of memory-based guidance of visual selection. Proceedings of the National Academy of Sciences. 2007;104:17186–17191. doi: 10.1073/pnas.0703706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004 Apr 15;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Walshaw PD, Alloy LB, Sabb FW. Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: In search of distinct phenotypic profiles. Neuropsychology Review. 2010;20:103–120. doi: 10.1007/s11065-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF. A brief introduction to the use of event-related potentials (ERPs) in studies of perception and attention. Attention, Perception & Psychophysics. doi: 10.3758/APP.72.8.2031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Do the contents of visual working memory automatically influence attentional selection during visual search? Journal of Experimental Psychology: Human Perception and Performance. 2007;33:363–377. doi: 10.1037/0096-1523.33.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ, Schall JD. The role of working memory representations in the control of attention. Cerebral Cortex. 2007;17:i118–i124. doi: 10.1093/cercor/bhm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK. Selective storage and maintenance of an object’s features in visual working memory. Psychonomic Bulletin & Review. 2008;15:223–229. doi: 10.3758/pbr.15.1.223. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ. Visual search remains efficient when visual working memory is full. Psychological Science. 2001;12:219–224. doi: 10.1111/1467-9280.00339. [DOI] [PubMed] [Google Scholar]
- Zubin J. Problem of attention in schizophrenia. In: Sutton S, Zubin J, editors. Experimental Approaches to Psychopathology. Academic Press; New York: 1975. pp. 139–166. [Google Scholar]