Abstract
Triple-negative breast cancers (TNBCs) are heterogeneous cancers that present tumors without the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Because of the absence of these receptors, there are currently no known specific molecular targets for treatment, and although TNBC tumors are chemosensitive, prognosis is poor because this type of cancer relapses more frequently and more aggressively than hormone receptor-positive cancers. The mechanisms by which TNBCs escape control by chemotherapy are not clear, and it is crucial to identify novel molecular drivers that can be targeted in order to develop more efficient therapeutic approaches. We recently highlighted a pleiotropic role for parathyroid hormone-related protein (PTHrP) in all stages of breast cancer, and used our neutralizing anti-PTHrP monoclonal antibody (mAb M158) to efficiently inhibit progression and metastasis of human breast cancer xenografts in athymic mice. In the present study, we present evidence for a strong in vitro anti-proliferative effect of our blocking anti-PTHrP mAb M158 as a single agent on TNBC lines of various subtypes that are known to express PTHrP (MDA-MB-231, BT-549, MDA-MB-435). The same mAb is inactive in a TNBC line without detectable PTHrP expression (MDA-MB-468). In in vitro combination studies, the mAb enhances the effect of the chemotherapeutic drugs taxol and doxorubicin in PTHrP-positive TNBC cells in an additive manner. When combined with the bisphosphonate zoledronate, M158 can act in additive or antagonistic fashion in vitro depending on the cell line. Our observations identify PTHrP as a novel target against TNBC cell proliferation, and suggest that combination therapies that include an anti-PTHrP approach might increase treatment efficacy in patients with PTHrP-positive TNBC.
Keywords: Breast cancer cell lines, PTHrP, TNBC, zoledronate, doxorubicin, paclitaxel, neutralizing antibody
Introduction
Standard cancer therapies cause breast tumor regression because the majority of cancer cells are sensitive to the administered drugs or to chemo- and radiotherapy. However, it is very common to observe a recurrence of tumors accompanied by a metastatic invasion of distal organs that generally proves fatal. This repopulation phenomenon is due to the fact that solid tumors are genetically diverse and contain small numbers of naturally-resistant tumor cells that become selected by the treatment and clonally expand after administration of therapy [1,2]. For any given treatment or drug, there likely is a resistant population with the potential to give rise to eventual metastases [1]. It is therefore nearly-impossible for drugs given as single agents to provide long-term disease control, and the use of combinations of anti-cancer agents has been adopted in clinics to improve therapeutic effectiveness [2,3]. However, survival enhancement due to dual therapy can be minimal and toxicity problems frequently arise [2]. Consequently, new combinations of current drugs as well as combinations involving novel metabolic targets are actively sought.
Triple-negative breast cancers (TNBCs) preferably strike young patients and constitute 12-24% of all diagnosed breast cancer cases. They are heterogeneous cancers but share an absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) in their tumor cells [4]. Because of the absence of these receptors, there are currently no known specific molecular targets for TNBC treatment. Tumor characteristics of TNBCs include rare histologies, high grade, elevated mitotic count, tumor necrosis, pushing margins of invasion, larger tumor size and axillary node involvement [5]. TNBCs have recently been classified into basal-like, mesenchymal-like and luminal androgen receptor (LAR) subtypes, and the susceptibility of each sub-type to chemotherapy has been defined as follows: basal-like TNBCs show preferential response to cisplatin, mesenchymal-like TNBCs to NVP-BEZ235 (PI3K/mTOR inhibitor) and dasatinib (abl/src inhibitor), and LAR TNBCs to bicalutamide (androgen receptor inhibitor) [6]. However, despite chemotherapy, distant TNBC metastases occur in lung, bone, liver, pleura and brain, and the route of first metastasis correlates with patient survival [7]. Less than 30% of women presenting metastatic TNBC survive 5 years and almost all of them die despite chemotherapy [6]. It is important to note that although the tumors are chemosensitive, prognosis is poor in TNBC because this type of cancer relapses more frequently and more aggressively than hormone receptor-positive cancers [5]. The mechanisms by which TNBCs escape control by chemotherapy are not clear but the identification of molecular drivers that can be targeted is crucial to developing more efficient therapy.
Parathyroid hormone-related protein (PTHrP) is a secreted factor expressed in almost all normal fetal and adult tissues. Its 13 N-terminal amino acids are highly homologous to those of parathyroid hormone (PTH), a characteristic which allows PTHrP to act through a common receptor (PTH1R) [8]. Because the remainder of the PTHrP sequence is unique, the molecule displays properties distinct from those of PTH [9]. PTHrP has growth-promoting and anti-apoptotic properties [9], and is associated with oncologic pathologies such as breast cancer [10,11], lung [12-14], prostate [15-17], renal [18], colorectal [19-21], skin [22,23] and gastric carcinomas [24,25]. Circulating levels of PTHrP generally correlate with the more advanced stages of cancer [19,26-31] and the Pthrp gene has recently been identified in a genomic locus associated with breast cancer susceptibility [32]. We have demonstrated the implication of PTHrP in key steps of breast cancer initiation, progression and metastasis and shown that a neutralizing anti-PTHrP antibody slows the progression and metastasis of human breast cancer xenografts [33]. PTHrP regulates the expression of several tumor-relevant genes [34] and we showed that it is an upstream control of the chemokine receptor CXCR4 [33]. Because overexpression of CXCR4 in TNBC predicts poor clinical outcome [35], we investigate here the putative implication of PTHrP signaling in TNBC and show that our M158 mAb not only results in cytoreductive effects in PTHrP-positive TNBC cell lines of various subtypes, but that the treatment significantly enhances the inhibitory effect of common anti-cancer therapeutic molecules such as taxol and doxorubicin.
Materials and methods
Cell lines and culture conditions
Triple-negative (ER-, PR-, Her2-) human breast cancer cell lines BT549, MDA-MB-231, MBA-MB-435, MDA-MB-468 were from ATCC. Cells were maintained in DMEM medium (Wisent) supplemented with 10% fetal calf serum (Wisent), 100 μg/ml streptomycin and 100 μg/ml penicillin in 37°C humidified incubators with 5% CO2. Cells were passaged every 4 days.
Reagents
Paclitaxel and doxorubicin were purchased from Sigma, zoledronate (Zometa) was from Astra-Zeneca and Herceptin was from Genentech. Drugs were solubilized in culture grade dimethylsulfoxide (DMSO, Sigma, final < 0.1% v/v) and diluted in culture medium immediately before use. Controls using DMSO in culture medium or culture medium alone were identical.
Neutralizing anti-PTHrP monoclonal antibody preparation and treatment
A synthetic human PTHrP peptide (a.a. 1-33) was injected intraperitoneally (25 μg in 50% v/v Freund’s complete adjuvant) in 5-6 week-old female BALB/c mice (Charles River). Mice received a booster dose after 13-15 days, and sera were collected a week later by tail bleeding to confirm the presence of antibodies against PTHrP. Antibody-producing mice were injected with a further 25 μg of the PTHrP peptide. After 72 h, spleen lymphocytes were fused with FO myeloma cells (ATCC, Rockville, MD) and hybridomas selected in HAT medium. Selected clones were adapted to BD cell medium for production of monoclonal antibodies (mAb). The mAb-containing supernatants were centrifuged, filtered and stored at 4 °C. mAb isotype was confirmed with the Bio-Rad Mouse Typer Isotyping Panel kit (Bio-Rad, Mississauga, ON). The mAbs were highly-specific and we observed no cross-reactivity between antibodies and other fragments of the PTHrP molecule. Antibody M158 (IgM) was selected for use in vitro.
Proliferation assays
Cells were trypsinized and plated (5000 cells/well) in 96-wells culture plates (Thermo Fisher) and allowed to adhere overnight. Tests were conducted in triplicates at 30% confluence in 100 μl DMEM growth medium containing 10% FBS. Increasing doses of M158, taxol, doxorubicin and zoledronate were added to the wells as single agents or in combination and the cells incubated in 37 °C humidified incubators (5% CO2) for 72 hours. Presto Blue reagent (InVitrogen/Life Technologies) was used for viability assays: 10 μl of Presto Blue (undiluted) was added to individual wells, re-placed in a 37 °C incubator for 2 hours and the change in fluorescence measured after with a SPECTRA-max Gemini microplate spectrophotometer (Molecular Devices 5) using 544 nm (excitation) and 590 nm (emission) wavelengths.
Isobologram analysis
The isobologram method of Berenbaum was used to determine whether interactions between M158 and various drugs were additive, antagonistic or synergistic [36]. Dose-response curves were obtained for single agents and IC50 values determined. Combination index was calculated by the formula:
CI = d1/Dx1 + d2/Dx2
where Dx1 and Dx2 are the doses of agent 1 and 2 required to produce x percentage of inhibition as a single agent, and d1 and d2 are the doses of agent 1 and 2 needed to produce the same percentage x effect in combination. CI > 1 indicates antagonism, CI = 1 additivity, and CI < 1 synergy.
Statistical analysis
Data are reported as mean ± standard error of means (S.E.M.). Statistical analysis (t-test) was carried out using the GraphPad Prism 6.02 Software (GraphPad Software).
Results
In order to test the extended anti-proliferative action of the blocking anti-PTHrP mAb M158 in triple-negative breast cancer cells in vitro, we selected a panel of human TNBC cells of various sub-types and with different levels of PTHrP expression [37] (Figure 1). The PTHrP-negative line MDA-MB-468 was used as a control throughout. Treatment of breast cancer cells in culture with increasing doses of M158 mAb as a single agent caused a significant inhibition in proliferation of MDA-MB-231, BT-549, and MDA-MB-435 cells, as shown by the top curve in each graph (Figure 2). All of these cell lines express PTHrP (our results, not shown and [37]). The MDA-MB-468 cell line (Figure 2 right) does not express detectable PTHrP and showed little to no inhibition by the antibody, illustrating the specificity of the mAb action. Combination treatment clearly show enhancement of the anti-proliferative effect of doxorubicin by inclusion of the anti-PTHrP mAb for all PTHrP-positive cell lines. As expected, MDA-MB-468 cells were inhibited only by doxorubicin in a dose-dependent manner. Similarly, in combination treatment with M158 and taxol, M158 enhances the anti-proliferation effect of taxol in PTHrP positive cells and again, the mAb is inactive in PTHrP-negative MDA-MB-468 cells (Figure 3). These results show that the anti-PTHrP M158 mAb enhances the anti-proliferation effect of chemotherapeutic drugs doxorubicin and taxol on breast cancer cell lines.
Figure 1.
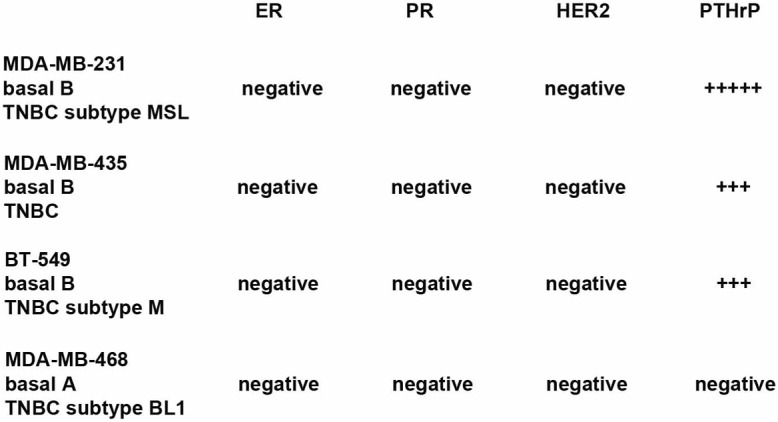
TNBC cell lines subtypes. Top: cell line. Middle: gene cluster. Bottom: triple-negative subtype. MSL: mesenchymal-like; M: mesenchymal; BL1: basal-like type 1. Subtype information from: [6,44].
Figure 2.
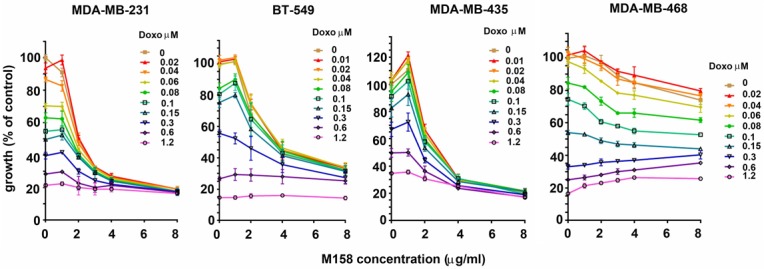
The anti-PTHrP M158 mAb enhances doxorubicin cytoreductive action on TNBC cell lines. Growth curves illustrating combinatorial treatment using increasing doses of mAb M158 and doxorubicin. MDA-MB-231, BT-549 and MDA-MB-435 are PTHrP-positive while MDA-MB-468 is PTHrP-negative. Curves are representative of a set of 3 replicates.
Figure 3.
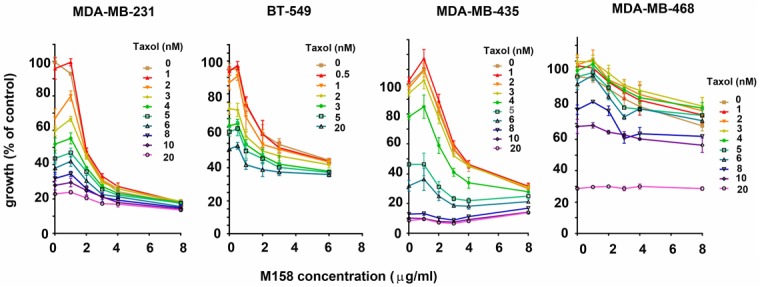
The anti-PTHrP M158 mAb enhances taxol cytoreductive action on TNBC cell lines. Growth curves illustrating combinatorial treatment using increasing doses of mAb M158 and taxol. MDA-MB-231, BT-549 and MDA-MB-435 are PTHrP-positive while MDA-MB-468 is PTHrP-negative. Curves are representative of a set of 3 replicates.
Bisphosphonates have been shown to have growth-inhibitory effects on tumor cells in vitro [38,39]. All breast cancer cells in our panel were incrementally inhibited by increasing doses of Zometa, but addition of the anti-PTHrP mAb enhanced the anti-proliferation effect of the bisphonate in MDA-MB-231 cells while antagonizing the bisphosphonate effect in BT-549 and MDA-MB-435 (Figure 4). These results confirm a biological role for bisphosphonates that is tumor cell-targeted and independent of skeletal osteolytic events, and but indicate a variable outcome to inclusion of anti-PTHrP mAb in combination experiments.
Figure 4.
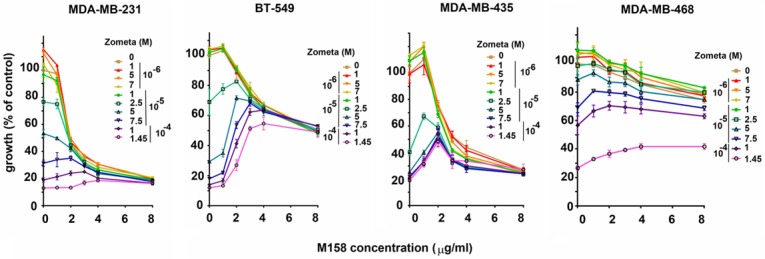
The anti-PTHrP M158 mAb enhances or inhibits zoledronate inhibition of cell proliferation depending on TNBC cell lines. Growth curves illustrating combinatorial treatment using increasing doses of mAb M158 and Zometa. MDA-MB-231, BT-549 and MDA-MB-435 are PTHrP-positive while MDA-MB-468 is PTHrP-negative. Curves are representative of a set of 3 replicates.
The combination index (CI) was calculated for combination treatments [36,40] and the interactions qualified as antagonistic for combinations of M158 and zoledronate in BT-549 and MDA-MB-435, or additive (in all other combinations and cells) (Table 1).
Table 1.
IC50 and CI values for mAB M158, taxol, doxorubicin and zoledronate on PTHrP+ and PTHrP- triple-negative breast cancer cell lines (3 experimental replicates, means ± SEM)
| M158 IC50 | Taxol IC50 | Doxo IC50 | Zoledronate IC50 | M158 + Taxol CI | M158 + Doxo CI | M158 + Zol CI | |
|---|---|---|---|---|---|---|---|
| MDA-MB-231 PTHrP+ | 2.0 ± 0.03 μg/ml | 4.1 ± 0.1 nM | 0.15 ± 0.06 μg/ml | 6.0 ± 1.1 x 10-5 M | 1.37 | 1.23 | 1.03 |
| BT-549 PTHrP+ | 4.2 ± 2.72 μg/ml | 2.8 ± 0.1 nM | 1.65 ± 1.2 μg/ml | 3.38 ± 0.8 x 10-5 M | 1.07 | 1.06 | 3.42 |
| MDA-MB-435 PTHrP+ | 3.1 ± 0.47 μg/ml | 3.6 ± 1.1 nM | 0.50 ± 0.05 μg/ml | 5.26 ± 0.99 x 10-5 M | 1.23 | 1.11 | 3.33 |
| MDA-MB-468 PTHrP- | ------------- | 5.9 ± 4.0 nM | 0.12 ± 0.01 μg/ml | 8.5 ± 3.3 x 10-5 M | ------------- | ------------- | ------------- |
IC50s and CIs values for single agent and combination experiments with M158 anti-PTHrP mAb, taxol, doxorubicin and zoledronate. No values for M158 as a single agent or in combinations can be obtained for MDA-MB-468 cells which do not express PTHrP.
Discussion
In the search to optimize cancer therapeutics, significant attention is starting to be directed towards rational combinations of agents targeting complementary survival pathways in the tumor cells [41]. Dual targeting is of particular importance in the context of oncogene addiction, a concept which holds one or a few genes responsible for maintenance of the malignant phenotype and cell survival and where complementary signaling pathways can become activated if the transforming oncogene is blocked by monotherapy [42]. The concurrent use of inhibitory agents, especially those targeting novel pathways is consequently essential to trial design for better anti-cancer therapies. PTHrP has only recently been identified as a factor with a breast cancer-promoting role distinct from its canonical involvement in tumor cell-induced skeletal osteolysis [43], and we expand here our previous observations on the use of an anti-PTHrP mAb which has proven efficient against human breast cancer cell xenografts [33]. A panel of cells was chosen to cover several subtypes of TNBCs: MDA-MB-231 is a mesenchymal stem-like line (MSL), BT-549 is a mesenchymal-like line (M), MSA-MB-435 is another basal B TNBC line, and MDA-MB-468 is a basal-like type1 (BL1) TNBC from the basal A gene cluster [6,44]. The first three cell lines were chosen for their PTHrP positivity, with MDA-MB-468 as a PTHrP-negative control.
As a single agent, M158 showed efficacy against all PTHrP-positive cells tested here, and our previous observation that this mAb carries little toxicity in experimental mice suggests feasible use as a single agent in PTHrP-positive TNBC human therapeutics [33]. More interesting is the enhancement of the anti-proliferative effect of taxol and doxorubicin, currently-used chemotherapy drugs whose dosage could be reduced with the concurrent addition of an anti-PTHrP blocking antibody.
The mechanisms by which bisphosphonates act against bone resorption are well-known [45] but their direct action on tumor cells is a more recent observation [38,39,46-48]. Like other nitrogen-containing bisphosphonates, zoledronate inhibits protein prenylation, a process essential for normal cell function [47]. Here, in MDA-MB-231 cells, combination of zoledronate and M158 is additive suggesting that these inhibitors address two different pathways to cell proliferation, only one of which is PTHrP-driven. In BT-549 and MDA-MB-435, the interaction is antagonistic and suggests both inhibitors address the same pathway, potentially the Ras prenylation activation of PTHrP which leads to proliferation [49]. In MDA-MB-468 cells (without PTHrP), only the zoledronate-sensitive pathway would be active.
In summary, anti-PTHrP monoclonal antibodies are potent proliferation inhibitors in PTHrP-positive TNBC cells and potentiates the effects of chemotherapeutic drugs. The mAb/zoledronate interactions are more complex as they appear to involve a common signaling pathway, however, a clear additive relationship is observed in the MDA-MB-231 cell type. Our observations on TNBC agree with collective data covering chondrosarcoma, thyroid cancer, medulloblastoma, adrenocortical cancer, oral squamous and renal, colon and prostate cancer cells which suggests that modulation of PTHrP levels is a promising approach for anti-cancer strategies [43].
Acknowledgements
This study was supported by the financial help of Canadian Institutes for Health Research (CIHR) grant MOP 10839 and the Susan G. Komen Foundation grant KG 100766 to R.K.
Disclosure of conflict of interest
None.
Abbreviations
- BL1
basal-like type 1
- CI
combination index
- DMSO
dimethylsulfoxide
- ER
estrogen receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- IC
inhibitory concentration
- M
mesenchymal-like
- mAb
monoclonal antibody
- MSL
mesenchymal stem-like
- PR
progesterone receptor
- PTHrP
parathyroid hormone-related protein
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TNBC
triple-negative breast cancer
References
- 1.Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, Moon YS, Yaqubie A, Kelly N, Le DT, Lipson EJ, Chapman PB, Diaz LA Jr, Vogelstein B, Nowak MA. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013 Jun 25;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Nan A. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv. 2012;2012:915375. doi: 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalenc F, Giamarchi C, Petit M, Poirot M, Favre G, Faye JC. Farnesyl-transferase inhibitor R115, 777 enhances tamoxifen inhibition of MCF-7 cell growth through estrogen receptor dependent and independent pathways. Breast Cancer Res. 2005;7:R1159–67. doi: 10.1186/bcr1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 5.Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36:206–15. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng LM, Hsu NC, Chen SC, Lu YS, Lin CH, Chang DY, Li H, Lin YC, Chang HK, Chao TC, Ouyang F, Hou MF. Distant metastasis in triple-negative breast cancer. Neoplasma. 2013;60:290–4. doi: 10.4149/neo_2013_038. [DOI] [PubMed] [Google Scholar]
- 8.Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–78. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 9.Maioli E, Fortino V. The complexity of parathyroid hormone-related protein signalling. Cell Mol Life Sci. 2004;61:257–262. doi: 10.1007/s00018-003-3233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucht E, Rong H, Pernow Y, Nordqvist AC, Eriksson E, Rankin W, von Schoultz E, Burtis WJ, Granberg B, Falkmer UG, Burton DW, Deftos LJ. Parathyroid hormone-related protein in patients with primary breast cancer and eucalcemia. Cancer Res. 1998;58:4113–4116. [PubMed] [Google Scholar]
- 11.Kohno N, Kitazawa S, Fukase M, Sakoda Y, Kanbara Y, Furuya Y, Ohashi O, Ishikawa Y, Saitoh Y. The expression of parathyroid hormone-related protein in human breast cancer with skeletal metastases. Surg Today. 1994;24:215–220. doi: 10.1007/BF02032890. [DOI] [PubMed] [Google Scholar]
- 12.Brandt DW, Burton DW, Gazdar AF, Oie HE, Deftos LJ. All Major Lung Cancer Cell Types Produce Parathyroid Hormone-Like Protein: Heterogeneity Assessed by High Performance Liquid Chromatography. Endocrinology. 1991;129:2466–2470. doi: 10.1210/endo-129-5-2466. [DOI] [PubMed] [Google Scholar]
- 13.Kitazawa S, Fukase M, Kitazawa R, Takenaka A, Gotoh A, Fujita T, Maeda S. Immunohistologic evaluation of parathyroid hormone-related protein in human lung cancer and normal tissue with newly developed monoclonal antibody. Cancer. 1991;67:984–989. doi: 10.1002/1097-0142(19910215)67:4<984::aid-cncr2820670421>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Nishigaki Y, Ohsaki Y, Toyoshima E, Kikuchi K. Increased serum and urinary levels of a parathyroid hormone-related protein COOH terminus in non-small cell lung cancer patients. Clin Cancer Res. 1999;5:1473–1481. [PubMed] [Google Scholar]
- 15.Dougherty KM, Blomme EA, Koh AJ, Henderson JE, Pienta KJ, Rosol TJ, McCauley LK. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 1999;59:6015–6022. [PubMed] [Google Scholar]
- 16.Iwamura M, di Sant’Agnese PA, Wu G, Benning CM, Cockett AT, Deftos LJ, Abrahamsson PA. Immunohistochemical localization of parathyroid hormone-related protein in human prostate cancer. Cancer Res. 1993;53:1724–1726. [PubMed] [Google Scholar]
- 17.Kremer R, Goltzman D, Amizuka N, Webber MM, Rhim JS. ras Activation of human prostate epithelial cells induces overexpression of parathyroid hormone-related peptide. Clin Cancer Res. 1997;3:855–9. [PubMed] [Google Scholar]
- 18.Agouni A, Sourbier C, Danilin S, Rothhut S, Lindner V, Jacqmin D, Helwig JJ, Lang H, Massfelder T. Parathyroid hormone-related protein induces cell survival in human renal cell carcinoma through the PI3K Akt pathway: evidence for a critical role for integrin-linked kinase and nuclear factor kappa B. Carcinogenesis. 2007;28:1893–901. doi: 10.1093/carcin/bgm106. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara M, Ito M, Tomioka T, Ohtsuru A, Taguchi T, Kanematsu T. Clinicopathological implications of parathyroid hormone-related protein in human colorectal tumours. J Pathol. 1999;187:217–222. doi: 10.1002/(SICI)1096-9896(199901)187:2<217::AID-PATH210>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Nishihara M, Kanematsu T, Taguchi T, Razzaque MS. PTHrP and tumorigenesis: is there a role in prognosis? Ann N Y Acad Sci. 2007;1117:385–392. doi: 10.1196/annals.1402.046. [DOI] [PubMed] [Google Scholar]
- 21.Dihlmann S, Kloor M, Fallsehr C, von Knebel Doeberitz M. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26:1503–1512. doi: 10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- 22.El Abdaimi K, Papavasiliou V, Goltzman D, Kremer R. Expression and regulation of parathyroid hormone-related peptide in normal and malignant melanocytes. Am J Physiol Cell Physiol. 2000;279:C1230–8. doi: 10.1152/ajpcell.2000.279.4.C1230. [DOI] [PubMed] [Google Scholar]
- 23.Henderson J, Sebag M, Rhim J, Goltzman D, Kremer R. Dysregulation of Parathyroid Hormone-like Peptide Expression and Secretion in a Keratinocyte Model of Tumor Progression. Cancer Res. 1991;51:6521–8. [PubMed] [Google Scholar]
- 24.Alipov GK, Ito M, Nakashima M, Ikeda Y, Nakayama T, Ohtsuru A, Yamashita S, Sekine I. Expression of parathyroid hormone-related peptide (PTHrP) in gastric tumours. J Pathol. 1997;182:174–179. doi: 10.1002/(SICI)1096-9896(199706)182:2<174::AID-PATH840>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Han Z, Wu K, Shen H, Li C, Han S, Hong L, Shi Y, Liu N, Guo C, Xue Y, Qiao T, Fan D. Akt1/protein kinase B alpha is involved in gastric cancer progression and cell proliferation. Dig Dis Sci. 2008;53:1801–1810. doi: 10.1007/s10620-007-9824-2. [DOI] [PubMed] [Google Scholar]
- 26.Deftos LJ, Barken I, Burton DW, Hoffman RM, Geller J. Direct evidence that PTHrP expression promotes prostate cancer progression in bone. Biochem Biophys Res Commun. 2005;327:468–472. doi: 10.1016/j.bbrc.2004.11.162. [DOI] [PubMed] [Google Scholar]
- 27.Firkin F, Seymour JF, Watson AM, Grill V, Martin TJ. Parathyroid hormone-related protein in hypercalcaemia associated with haematological malignancy. Br J Haematol. 1996;94:486–492. doi: 10.1046/j.1365-2141.1996.d01-1819.x. [DOI] [PubMed] [Google Scholar]
- 28.Kissin MW, Henderson MA, Danks JA, Hayman JA, Bennett RC, Martin TJ. Parathyroid hormone related protein in breast cancers of widely varying prognosis. Eur J Surg Oncol. 1993;19:134–142. [PubMed] [Google Scholar]
- 29.Kremer R, Shustik C, Tabak T, Papavasiliou V, Goltzman D. Parathyroid-hormone-related peptide in hematologic malignancies. Am J Med. 1996;100:406–411. doi: 10.1016/S0002-9343(97)89515-0. [DOI] [PubMed] [Google Scholar]
- 30.Rankin W, Grill V, Martin TJ. Parathyroid hormone-related protein and hypercalcemia. Cancer. 1997;80:1564–1571. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1564::aid-cncr6>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Truong NU, de BEMD, Papavasiliou V, Goltzman D, Kremer R. Parathyroid hormone-related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003;115:115–121. doi: 10.1016/s0002-9343(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 32.Ghoussaini M, Fletcher O, Michailidou K, Turnbull C, Schmidt MK, Dicks E, Dennis J, Wang Q, Humphreys MK, Luccarini C, Baynes C, Conroy D, Maranian M, Ahmed S, Driver K, Johnson N, Orr N, dos Santos Silva I, Waisfisz Q, Meijers-Heijboer H, Uitterlinden AG, Rivadeneira F Netherlands Collaborative Group on Hereditary Breast and Ovarian Cancer (HEBON); Hall P, Czene K, Irwanto A, Liu J, Nevanlinna H, Aittomäki K, Blomqvist C, Meindl A, Schmutzler RK, Müller-Myhsok B, Lichtner P, Chang-Claude J, Hein R, Nickels S, Flesch-Janys D, Tsimiklis H, Makalic E, Schmidt D, Bui M, Hopper JL, Apicella C, Park DJ, Southey M, Hunter DJ, Chanock SJ, Broeks A, Verhoef S, Hogervorst FB, Fasching PA, Lux MP, Beckmann MW, Ekici AB, Sawyer E, Tomlinson I, Kerin M, Marme F, Schneeweiss A, Sohn C, Burwinkel B, Guénel P, Truong T, Cordina-Duverger E, Menegaux F, Bojesen SE, Nordestgaard BG, Nielsen SF, Flyger H, Milne RL, Alonso MR, González-Neira A, Benítez J, Anton-Culver H, Ziogas A, Bernstein L, Dur CC, Brenner H, Müller H, Arndt V, Stegmaier C Familial Breast Cancer Study (FBCS); Justenhoven C, Brauch H, Brüning T Gene Environment Interaction of Breast Cancer in Germany (GENICA) Network; Wang-Gohrke S, Eilber U, Dörk T, Schürmann P, Bremer M, Hillemanns P, Bogdanova NV, Antonenkova NN, Rogov YI, Karstens JH, Bermisheva M, Prokofieva D, Khusnutdinova E, Lindblom A, Margolin S, Mannermaa A, Kataja V, Kosma VM, Hartikainen JM, Lambrechts D, Yesilyurt BT, Floris G, Leunen K, Manoukian S, Bonanni B, Fortuzzi S, Peterlongo P, Couch FJ, Wang X, Stevens K, Lee A, Giles GG, Baglietto L, Severi G, McLean C, Alnaes GG, Kristensen V, Børrensen- Dale AL, John EM, Miron A, Winqvist R, Pylkäs K, Jukkola-Vuorinen A, Kauppila S, Andrulis IL, Glendon G, Mulligan AM, Devilee P, van Asperen CJ, Tollenaar RA, Seynaeve C, Figueroa JD, Garcia-Closas M, Brinton L, Lissowska J, Hooning MJ, Hollestelle A, Oldenburg RA, van den Ouweland AM, Cox A, Reed MW, Shah M, Jakubowska A, Lubinski J, Jaworska K, Durda K, Jones M, Schoemaker M, Ashworth A, Swerdlow A, Beesley J, Chen X kConFab Investigators; Australian Ovarian Cancer Study Group. Muir KR, Lophatananon A, Rattanamongkongul S, Chaiwerawattana A, Kang D, Yoo KY, Noh DY, Shen CY, Yu JC, Wu PE, Hsiung CN, Perkins A, Swann R, Velentzis L, Eccles DM, Tapper WJ, Gerty SM, Graham NJ, Ponder BA, Chenevix-Trench G, Pharoah PD, Lathrop M, Dunning AM, Rahman N, Peto J, Easton DF. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44:312–8. doi: 10.1038/ng.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest. 2011;121:4655–69. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dittmer A, Vetter M, Schunke D, Span PN, Sweep F, Thomssen C, Dittmer J. Parathyroid hormone-related protein regulates tumor-relevant genes in breast cancer cells. J Biol Chem. 2006;281:14563–14572. doi: 10.1074/jbc.M510527200. [DOI] [PubMed] [Google Scholar]
- 35.Chu QD, Panu L, Holm NT, Li BDL, Johnson LW, Zhang S. High Chemokine Receptor CXCR4 Level in Triple Negative Breast Cancer Specimens Predicts Poor Clinical Outcome. J Surg Res. 2010;159:689–95. doi: 10.1016/j.jss.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Berenbaum MC, George K, Sidney W. Advances in Cancer Research. Academic Press; 1981. Criteria for Analyzing Interactions between Biologically Active Agents; pp. 269–335. [DOI] [PubMed] [Google Scholar]
- 37.Guise TA, Kozlow WM, Heras-Herzig A, Padalecki SS, Yin JJ, Chirgwin JM. Molecular mechanisms of breast cancer metastases to bone. Clin Breast Cancer. 2005;5:S46–53. doi: 10.3816/cbc.2005.s.004. [DOI] [PubMed] [Google Scholar]
- 38.Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15:2211–21. doi: 10.1359/jbmr.2000.15.11.2211. [DOI] [PubMed] [Google Scholar]
- 39.Ural AU, Avcu F, Candir M, Guden M, Ozcan MA. In vitro synergistic cytoreductive effects of zoledronic acid and radiation on breast cancer cells. Breast Cancer Res. 2006;8:R52. doi: 10.1186/bcr1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berenbaum MC. A Method for Testing for Synergy with Any Number of Agents. J Infect Dis. 1978;137:122–30. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 41.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008;118:3003–6. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–57. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 43.Luparello C. Parathyroid Hormone-Related Protein (PTHrP): A Key Regulator of Life/Death Decisions by Tumor Cells with Potential Clinical Applications. Cancers. 2011;3:396–407. doi: 10.3390/cancers3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 46.Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia. 1998;12:220–9. doi: 10.1038/sj.leu.2400892. [DOI] [PubMed] [Google Scholar]
- 47.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 48.Ural AU, Avcu F. Evolving therapeutic role of bisphosphonates in multiple myeloma. Br J Cancer. 2005;93:267–8. doi: 10.1038/sj.bjc.6602694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aklilu F, Park M, Goltzman D, Rabbani SA. Induction of parathyroid hormone-related peptide by the Ras oncogene: role of Ras farnesylation inhibitors as potential therapeutic agents for hypercalcemia of malignancy. Cancer Res. 1997;57:4517–22. [PubMed] [Google Scholar]


