Abstract
Ferritins are a family of large (10–12 nm diameter), self-assembled, protein cages that reversibly synthesize Fe2O3•H2O with up to 4500 iron atoms in a central cavity, 65 or 270 nm3; the protein cages without mineral are sometimes called apoferritin. Fe2O3•H2O synthesis depends on controlled Fe2+ entry though four or eight ion channels, directed transport to multiple Fe2+/O oxidoreductase (“ferroxidase”) sites and, in the case of eukaryotic ferritins, guided nucleation and extrusion through channels connecting the active sites to the mineral growth cavity; passage of the diferric oxo catalytic products through the nucleation/extrusion channels allows the eukaryotic ferritin protein cage to influence order in the bulk mineral. Ferritin Fe2+ion channels also control reduction, dissolution, and exit of Fe2+ from the mineral with gated pores on the cytoplasmic surface of ferritin cages. Found in anaerobic and aerobic organisms, from archaea and bacteria to higher plants and animals, ferritins are required for life. They provide metabolic iron concentrates for protein cofactor synthesis, and antioxidant activity after stress. Current applications of ferritin nanocages include clinical measurements of trace amounts released into serum, nutritional sources of concentrated iron, nanomaterial templates, biological delivery of nanosensors, and nanocatalysts. Future applications can exploit the nucleation/ extrusion channels and other metal-protein sites in ferritins.
Introduction
Ferritins are finding increasing use in nanotechnology, but what are they? An ancient family, found in contemporary archaea, bacteria, plants and animal, ferritins are large (8–12 nm diameter), cage proteins (Figure 1) with an interior mineral, Fe2O3•H2O. The caged minerals contain different amount of phosphate, with ratios of Fe:P ~1:1 (plants, bacteria) and Fe:P ~1:8 (animals); caged ferritin minerals are oxidant traps (antioxidants).1 Protein-based catalysis, at multiple ferritin cage sites, use 1–2 Fe2+ waters of coordination,2, 3 and O2 or H2O2 as substrates. The catalytic coupling reactions initiate ferritin synthesis of hydrated iron oxide nanominerals that are metabolic iron concentrates. Ferritin mineral sizes are heterodisperse in natural tissue ferritins, with average sizes varying from ~30–50% of the 4500 Fe maximum in eukaryotic ferritins. Apoferritin is a commonly used name for ferritin protein cages without the mineral; apoferritin can be isolated from recombinant growth, or after dissolving the mineral and chelating the iron under physiological conditions.
Figure 1.
A ferritin protein cage (eukaryote). Drawn from the eukaryotic model, ferritin frog M, crystallized from 1 M MgCl2, 50 mM Bicine buffer, pH 9.0 (PDB file 3KA4) using Discovery Studio Visualizer 2.0. Left: The exterior surface helices of one of the eight Fe2+ ion channels is shown in dark blue; each of the three subunits contributes helix segments to the channel; green dot, top of a line of four Mg2+ ions in the channel. Middle: a cutaway view of the interior of a ferritin protein nanocage showing the internal mineral growth cavity viewed toward the 4-fold axes; four Fe3+O nucleation/ extrusion channels, ~20 Å long, end around the fourfold symmetry axis in the centre; charge densities: red, negative; blue, positive; dots: green, Mg2+; red, water. Right: Drawing of one of the eight Fe2+ ion entry and exit channels, ~15 Å long, from XRD data22; the three arrows at the cell interior end of the pore represent the distribution of entering Fe2+ to the active sites in each of the three subunits that contribute to the channel structure; the three black striped ellipsoids represent the cytoplasmic gates, a network of H and ionic bonds within each subunit that include the conserved residues of the flexible N-terminal peptide.10
The biological story of ferritins likely began with the need to manage effectively environmental variations in iron availability together with certain peculiarities of the chemistry of Fe2+ and O, from which the need to avoid the side effects of free radical reactions and insoluble “rust” arises. The presence of ferritin in anaerobic organisms illustrates the importance of the reversible synthesis of caged iron minerals. Ferritins could have been a key feature for the survival of organisms using dioxygen-evolving photosynthesis and in others facing the increasing presence of atmospheric dioxygen from life-based photosynthesis. In contemporary mammals, deletion of a ferritin gene is lethal. In the battle for iron between humans and invading microbial pathogens, ferritin genes in each of the combatants play key roles.
Ferritin protein in serum, a minor fraction of predominantly intracellular ferritin in the animal body, is routinely measured, immunologically, to diagnose changes in body iron such as iron deficiency anaemia or iron overload in transfusion-treated genetic haemoglobin deficiency (thalassaemias and sickle cell disease), and in genetic abnormalities of intestinal iron absorption (hereditary haemochromatosis, HH). In HH, excess iron is removed by phlebotomy, but in the genetic anaemias, iron cannot be thus removed because of the defects in haemoglobin synthesis. To prevent death from iron poisoning, since iron cannot be excreted, iron chelation therapy is used. While the serum ferritin assay accurately reflects body iron in iron deficiency and normal health, it is not accurate when body concentrations of iron are abnormally high because the ratio of ferritin iron mineral to ferritin/protein cage increases. Thus, during iron overload, complementary analyses are required,6 such as serum iron, serum transferrin receptor or NMR resonance or SQUID imaging, to assess the need for iron chelation.
As the iron mineral in ferritin increases, during iron overload, the protein cage is damaged and mineral surfaces are exposed to cellular reductants, causing damaging free radical chain reactions. Such damaged ferritin is called haemosiderin. The resulting inflammation increases ferritin gene transcription and protein synthesis4, 5 to reverse the ferritin protein shortage. During normal health and development, Nature uses ferritin iron as a source in animal and plant development; high ferritin concentration in legume seeds is a unique, nutritional source of iron for animals and humans.7
Ferritin structure
Protein cage assembly occurs spontaneously from 12 or 24 polypeptides (mass ~20 kDa) called subunits. After biosynthesis and before cage assembly, ferritin subunits fold into a common protein motif called a 4-α-helix bundle; in the assembled cage, the boundaries of each subunit are obscured (Figure 1). In addition to full cages, in solution studies the next most common species is a complex of two subunits; although it does not appear to be a crucial intermediate. Dissociation of cages by amino acid substitution appears to be more facile in ferritin from bacteria than from higher organisms.8, 9 N- and C-terminal terminal peptide extensions from the ferritin subunit polypeptide bundles vary in length and sequence and, in the assembled cages, have distinctive functions such as gating of Fe2+ exit (eukaryotic maxiferritins)10 or DNA binding and protection (microbial miniferritins).11 Ferritin cages are hollow, with central mineral growth cavities of ~65 nm3 in miniferritins and ~270 nm3 in maxiferritins, or ~24–30% of the protein volume. The protein helices from each polypeptide are arrayed with extraordinary symmetry in ferritin cages, 432 in 24-subunit maxiferritins or 32 in 12-subunit miniferritins. Interfaces between the 4-helix bundle subunits are sufficiently similar to helix–helix interactions within the subunit bundles that from a structural viewpoint the cage appears to be a gigantic array of polypeptide helices (Figure 1).
The cage structure of ferritins is unusually stable, with the cages of eukaryotic ferritins surviving temperatures up to 80 °C in neutral aqueous solutions and high concentrations of chaotropes such as 6 M urea or guanidine. Nevertheless, localized regions of the ferritin cages are less stable than the cage and can be selectively unfolded by lower temperatures, low (mM) concentrations of chaotropes,12 and selected peptides.13 Structural studies show that residues distributed from one end of the subunit, from the 3-fold axes to the 4-fold axes, have long range functional effects,14 suggesting that the minimum functional ferritin structure is the nanocage itself.
Ferritin protein cages vary in more than size and amino acid sequence.1 The cage location of ferritin Fe2+/O catalytic sites and transit sites to the catalytic sites differ among most miniand maxiferritins, and between some bacterial and eukaryotic maxiferritins; the postoxidation paths taken by mineral precursors within the cages will, thus, also differ. Iron binding amino acids at the active sites also vary, even in very similar eukaryotic ferritins, causing differences in the catalytic reaction rates and mineralization rates and possibly in mineral crystallinity. Other factors that change ferritin mineral crystallinity include the local concentration of phosphate during mineral formation (plant vs animal ferritins) and the number of catalytically inactive (animal L-type) subunits, which depends on the specific tissue (such as liver or heart).1, 15
Any or all of the natural ferritin variations: such as cavity size, surface amino acid composition, ion channel gates and catalytic mechanism can be tailored to nanotechnological goals using other metals with accessible redox potentials for coupling and nucleation. The entry and extrusion channel sizes could also be selectively modified to create nanoparticles with different compositions and crystallinities, in order to expand the applications of ferritins in nanotechnology.
Functional ferritin metal-protein sites
Most applications to date of ferritin protein cages in nanotechnology have exploited the natural cage as a nanomaterial template or have modified amino acids to bind specific metals, such as introducing sulfur (cysteine) or aromatic nitrogen-containing (histidine) amino acid side chains to bind palladium-containing complexes, to create monodisperse polymers.16–20
The overall ferritin reaction is:
2 Fe2+ + O2 (H2O2) + 2 H2O + 2 OH− → [Fe3+-O-O-Fe3+] → Fe2(OH)6 (caged solid) + 2 H+
The ferritin reaction is reversible when reductants are added to reduce the hydrated ferric oxide, wherupon the oxo/hydroxo bridges between iron atoms of the mineral are rehydrated and cleaved. While the surface-limited, mineral dissolution reaction can be initiated by a biological reductant such as NADH/FMNH2, or a chemical reductant such dithionite, it is clear that the protein cage controls the rates, based on effects of amino acid substitutions that alter cage structure.10, 21, 22 Dithionite, while chemically simpler than NADH/FMNH2, has the disadvantage of modifying the iron binding ligands at the active sites (T. Tosha, J. Schwartz, E.I. Solomon and E.C. Theil, unpublished observations); sulfite is retained in the protein.23
Few of the ferritin metal sites involved in the reversible synthesis of caged, ferritin iron oxide mineral are currently used in nanotechnology design, partly because structural information on some of the sites (e.g., the Fe2+ ion channel cytoplasmic gates or the eukaryote-specific Fe3+O nucleation and extrusion channels) is incomplete and still being extended.10, 14, 21, 22 However, nature has been “tweaking” the metal–protein interactions in ferritins for thousands of millions of evolutionary years, providing a vast, underutilized nanodesign resource. The ferritin metal sites and structures, which can be manipulated for novel nanotechnologies, are: (1) Fe2+ ion entry and exit channels; (2) the catalytic oxidoreductase (ferroxidase) sites; and (3) the Fe3+O mineral nucleation/extrusion channels.
1. Metal sites in ion entry/exit channels (all ferritins): Analogous to membrane ion channels, but part of the water-soluble ferritin cage, ferritin ion channels connect the external surface of the protein cage to the interior. Constructed from helical sections of three ferritin subunits (Figure 1), the channels are at the threefold symmetry axes of the water-soluble, protein cages. Both hydrophobic and ionic interactions stabilize the ferritin ion channel structure. In maxiferritins the 24-subunit cages have as many as 24 active sites and 8 entry channels, while in miniferritins there are 12 active sites and 4 ion channels.
Fe2+ is directed into the ion channels, which are 15 Å long with a constriction in the centre, and into ferritin protein cages by highly conserved carboxylate groups in both large and small ferritins1 (Figure 1). At the internal channel exits, on the interior surface of the ferritin cages, there is a cluster of conserved carboxylate groups, which appear to direct Fe2+ to each of three active sites; additional carboxylate side chains guide the ions away from channel exits and toward the catalytic sites, which couple 2 Fe2+ with O, to synthesize di-Fe3+O mineral precursors. The selection mechanism that matches an Fe2+ leaving the channel with a particular catalytic site is unidentified. However, protein–protein interactions involved in Fe2+ binding at the active site have a Hill coefficient (cooperativity value) of 3.2 In addition, crosslinks between protein subunits slow iron oxidation.24 The ion channels and Fe2+ distribution sites in ferritin cages have the potential for influencing nanoparticles of other elements synthesized in modified ferritin cages.
Iron exit from ferritins is a slow process for at least two reasons: limits imposed by the caged mineral surface and by the flexible protein pores at the ion channel surfaces; they are mostly closed, likely to prevent cellular reductants from dissolving the ferritin mineral until iron is needed. Ferritin cage pores and channels are the selectively flexible parts of the cages, which undergo localized unfolding after amino acid substitution, or in the presence of very low chaotrope concentrations such as 1 mM urea, or in response to highly selected peptides. 10, 13, 25 Recently the N-terminal extensions of animal ferritin subunits were revealed as cytoplasmic gates on the ion channels, stabilized through a network of interactions among conserved amino acids that link the N-terminal peptides to the channel entrances.10 The N-terminal sequences are likely a target for natural cytoplasmic molecules that regulate ferritin iron mineral dissolution and Fe2+ exit as well as for manipulated targeting of ferritin-coated nanoprobes and for changing access to the large, central cavity for other nanotechnological applications.
2. Metal binding at or near ferritin oxidoreductase (“ferroxidase”) sites. In ferritins, catalysis couples 2 Fe with O to form the diferric oxo/hydroxo mineral precursors. The sites can be in the middle of each 4-helix bundle subunit in maxiferritins and a few miniferritins1 or, as in most miniferritins, at between two subunits on the inner surface of the protein cage.11
The internal active sites of ferritin in eukaryotes and bacteria resemble those of cofactor sites in diiron oxygenases such as iron ribonucleotide reductase, methane monooxygenase, fatty acid desaturase and myoinositol oxygenase.1 However, in ferritins the iron ligands in one of the two iron-binding sites are weaker than in the dioxygenases, facilitating release of the diferric oxo product. Histidine, present at both iron sites in the diiron oxygenases, is absent at the weaker of the two diiron sites in ferritin, and when introduced by protein engineering, blocks catalytic activity.22 Except for the diferric peroxo intermediate observed in eukaryotic ferritins, details of the catalytic mechanism remain unknown and may differ depending on the number of turnovers, since at least four turnovers/site are required for the catalytic products to traverse the protein cage and enter the mineral growth cavity.15 In contrast are the bacterioferritin (BFR) active sites, where the iron-binding amino acids are very similar to those in diiron oxygenases with histidine in each site. A third, nonhaem iron site appears to restore the cofactor site iron atoms to the appropriate oxidation state in BFR, but where the substrate Fe2+ and O2 bind and what is the reaction pathway remain unknown.9 BFR proteins also contain haem, one per two subunits, but the haem appears to participate in mineral dissolution and Fe2+ exit.26 Still other variations in the oxidoreductase site structure and mechanism occur in other ferritins such as the one from the thermophilic archaeon, P. furiosis.27
Oxidoreductase sites in most of the 12-subunit miniferritins of bacteria and archaea (Dps proteins) differ from those in the 24-subunit ferritins, not only in location on the inner surface of the cage, but also in the oxidation mechanism. Hydrogen peroxide rather than dioxygen is the preferred oxidant in many, but not all, miniferritin proteins, The binding affinities of Fe2+ to each of the two diiron sites are so large that in a number of miniferritins only one Fe2+ binds in the absence of oxidant,11 leading to the idea that oxidant binding preceeds binding of the second Fe2+. Since the catalytic metal–oxo coupling sites in miniferritins are on the mineral growth cavity surface, except for size mineral growth in miniferritins will be protein-independent. Thus miniferritin (Dps) ferritin protein cages would useful for nanotechnology if rapid, less ordered growth of the bulk particle is desired.
3. Metal–protein interactions in mineral nucleation/extrusion channels: protein-guided mineralization (eukaryotic ferritins only) Diferric oxo catalytic products in eukaryotic ferritins are destined for the mineral growth cavity. Until catalytic intermediates such as diferric peroxo were studied in the 1990s, the reaction of Fe2+ with O2 and iron oxide mineral growth were considered to be largely protein-independent. The crystallinity of ferritin minerals from animals in general is higher than from plants or bacteria. While the high phosphate content of plant and bacterial ferritin minerals is sufficient to explain the more amorphous structure,28–30 it does not explain differences in iron mineral crystal order among animal ferritins isolated from normal human tissues such as liver and spleen or from diseased tissue.31 The protein cage in animal ferritins plays a significant role in guiding bulk mineral growth and extruding mineral precursors.15 Whether an analogous phenomenon occurs in other ferritin cages is not yet known. The subunit structures of plant32 and animal ferritin cages, contrasting with bacterial ferritins, are specific combinatorial mixtures of slightly different subunits with a range of catalytic activities but the effects on mineral crystallinity are just beginning to be considered.
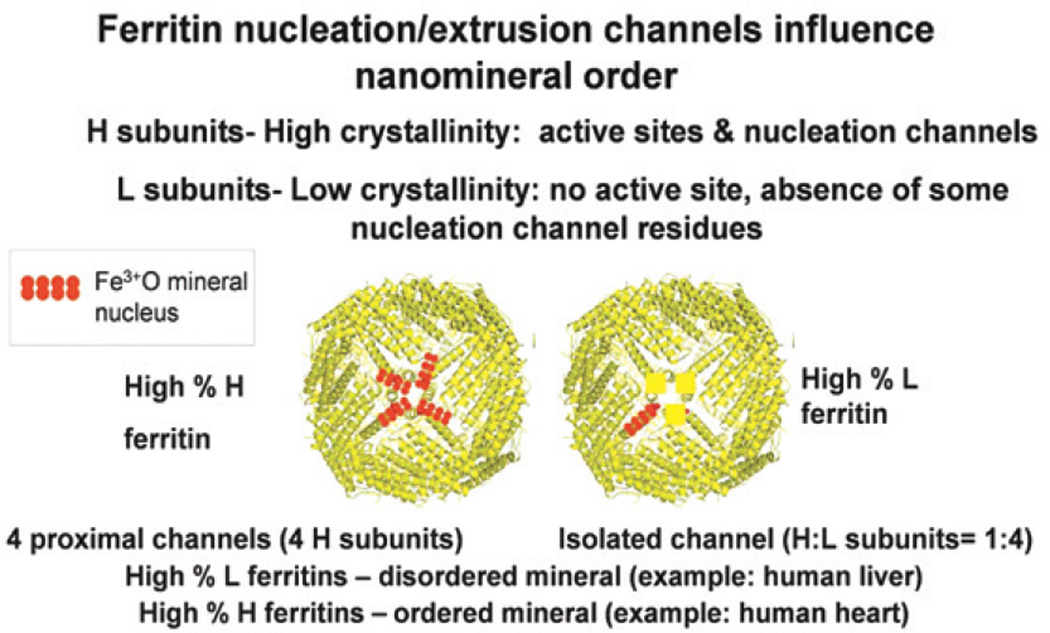
An explanation of the range of crystallinity observed in normal animal ferritins isolated from different tissues lies in the postoxidation events recently observed15 (Figure 2). In animal ferritins, the diferric oxo complex products of protein-based Fe2+–O2 catalytic reactions are propelled into channels between the active sites and the mineral growth cavity; there, mineral nucleation among the diferric oxo complexes occurs.
Figure 2.
Influence of eukaryotic ferritin cage structure on ordered nanomineral growth. The model of how ferritin cage structure influences nanomineral order is based on the identification of retained oxidation products within the cage over four catalytic (oxidoreductase or “ferroxidase”) cycles,15 formation of dimeric multimers in ferritin with subsaturating amounts of iron34 and tissue-specific variations in natural ferritin minerals.31
Four catalytic cycles at the active sites in each ferritin subunit are required before ferric oxo mineral nuclei emerge at the nucleation channel exits. The exits are at the fourfold axes of the protein cage created by four subunits, and thus four channel exits can be in close proximity (Figures 1 and 2). The nucleation/extrusion channels in eukaryotic ferritin protein cages were first observed using solution NMR to identify residues near Fe3+ and magnetic susceptibility to identify ferric oxo tetramers in the channnels, from the reaction between two diferric oxo catalytic products.15 Conserved amino acids along the channel have selective effects on Fe2+/O2 oxidant and diferric peroxo (DFP) decay to the diferric oxo reaction products.14 A key residue in the process is A2614 between the active sites and the channel entrances. It is part of a group of amino acids always present in catalytically active (H) ferritin subunits that can be identified by covariation analyses33 in comparison with catalytically inactive (L) ferritin.
The proximity of several nucleation/extrusion channel exits to each other (Figure 2) explains why H-rich and L-rich ferritins form iron minerals with more or less order, respectively.31 The physiological significance of the variations in ferritin mineral crystallinity is currently obscure. When a mineral nucleus of 4–8 Fe3+–O is extruded from a ferritin cage channel, in a ferritin with a preponderance of H-subunits, it will be surrounded by a number of nuclei emerging from nearby channels. Since the Fe3+O mineral nucleus will have 4–8 Fe(III) atoms/cluster, when it emerges into the mineral growth cavity near three other channels each with emerging mineral nuclei of the same size, formation of a cluster of 16–32 Fe3+O is facilitated, providing an organized surface for subsequent bulk mineral growth. However, if the protein cage has a preponderance of L subunits, the mineral nucleus from one channel will move randomly through the mineral growth cavity before encountering another mineral nucleus. Mineral growth in L-rich ferritin subunits will, therefore, be more disordered (Figure 2), as is observed in the L-rich ferritin of the liver.31
The spatial relationships among the nucleation/extrusion channel exits, related to the ordered growth of caged ferritin nanoparticles minerals, establishes the functional role for the fourfold symmetry axes in ferritin. Until now the fourfold symmetry axes of maxiferritin cages have been most influential through their strong visual and artistic impact on the viewers of the cage structure. Using eukaryotic ferritin nucleation/extrusion channels for nanoparticle design is a tempting future experimental direction.
Summary and perspective
Ferritin protein nanocages, a complex, ancient family found in all kingdoms of life, have multiple metal–protein interactions, in addition to the large (24–30% of total volume ) central cavity. To date, nanotechnology has mainly exploited the large cavity for nanomaterial and nanodevice templating, with some use of the cage for polymerization catalysis.
Over thousands of millions of years, nature has produced ferritin cages with different surface amino acid compositions (varying 80%), different oxido-reduction mechanisms for Fe2+/ O reactions, different cage sizes and, in higher organisms, ferritins with gated Fe2+ entry/exit channels and nucleation/extrusion channels that appear to guide mineral growth and order. In biology, ferritins are at the centre of Fe/O chemistry, providing metabolic iron concentrates for plant, animal and cell development, and antioxidant protection by scavenging reactive products released (Fe2+ and O) during stress. In some cases specialized ferritins (Dps proteins) protect DNA by direct protein cage binding to the DNA. In medicine, specific features of both host and pathogen ferritins contribute to the battles over iron. In agriculture ferritins concentrate iron for nitrogen fixation and legume seed growth. New uses of ferritin protein cages in nanotechnology only require novel couplings or modifications of nature’s specific ferritin features (ion channels, catalytic coupling sites, and nucleation and extrusion channels) and nanochemistry.
Acknowledgment
Support for the work of the authors described has been supported by the USPHS-NIH (DK20251), the CHORI Foundation and CHRCO Partners. The contributions of Dr Rabindra K. Behera to figure preparation and to discussion of the text are greatly appreciated.
References
- 1.Theil EC. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Current Opinion Chem. Biol. 2011;15:304–311. doi: 10.1016/j.cbpa.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz JK, Liu XS, Tosha T, Theil EC, Solomon EI. Spectroscopic definition of the ferroxidase site in M ferritin: comparison of binuclear substrate vs cofactor active sites. J. Am. Chem. Soc. 2008;130:9441–9450. doi: 10.1021/ja801251q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes CM, Theil EC, Raymond KN. Iron uptake in ferritin is blocked by binding of [Cr(TREN)(H2O)(OH)]2+, a slow dissociating model for [Fe(H2O)6]2+ Proc. Natl Acad. Sci. USA. 2002;99:5195–5200. doi: 10.1073/pnas.032089399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc. Natl Acad. Sci. USA. 2005;102:15048–15052. doi: 10.1073/pnas.0505148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari P, Kulkarni H, Dheda S, Betti S, Harrison C, St Pierre TG, Olynyk JK. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2012;6:77–83. doi: 10.2215/CJN.04190510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theil EC, Chen H, Miranda C, Janser H, Elsenhans B, Nunez MT, Pizarro F, Schumann K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 2012;142:478–483. doi: 10.3945/jn.111.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Orner BP. Self-assembly in the ferritin nano-cage protein superfamily. Int. J. Mol. Sci. 2011;12:5406–5421. doi: 10.3390/ijms12085406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Brun NE, Crow A, Murphy ME, Mauk AG, Moore GR. Iron core mineralisation in prokaryotic ferritins. Biochim. Biophys. Acta. 2010;1800:732–744. doi: 10.1016/j.bbagen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Tosha T, Behera RK, Ng HL, Bhattasali O, Alber T, Theil EC. Ferritin protein nanocage ion channels: gating by N-terminal extensions. J. Biol. Chem. 2012;287:13016–13025. doi: 10.1074/jbc.M111.332734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiancone E, Ceci P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim. Biophys. Acta. 2010;1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Jin W, Theil EC. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl Acad. Sci. USA. 2003;100:3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XS, Patterson LD, Miller MJ, Theil EC. Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J. Biol. Chem. 2007;282:31821–31825. doi: 10.1074/jbc.C700153200. [DOI] [PubMed] [Google Scholar]
- 14.Haldar S, Bevers LE, Tosha T, Theil EC. Moving iron through ferritin protein nanocages depends on residues throughout each four α-helix bundle subunit. J. Biol. Chem. 2011;286:25620–25627. doi: 10.1074/jbc.M110.205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turano P, Lalli D, Felli IC, Theil EC, Bertini I. NMR reveals pathway for ferric mineral precursors to the central cavity of ferritin. Proc. Natl Acad. Sci. U S A. 2010;107:545–550. doi: 10.1073/pnas.0908082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann S. Self-assembly and transformation of hybrid nano-objects and nanostructures under equilibrium and non-equilibrium conditions. Nature Materials. 2009;8:781–792. doi: 10.1038/nmat2496. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Hirata K, Ueno T, Morino K, Shimizu N, Yamamoto M, Takata M, Yashima E, Watanabe Y. Polymerization of phenylacetylene by rhodium complexes within a discrete space of apo-ferritin. J. Am. Chem. Soc. 2009;131:6958–6960. doi: 10.1021/ja901234j. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Kumagai S, Zheng B, Uraoka Y, Douglas T, Yamashita I. A watersoluble carbon nanotube network conjugated by nanoparticles with defined nanometre gaps. Chem. Commun. 2011;47:3475–3477. doi: 10.1039/c0cc05503d. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa T, Kosuge H, Uchida M, Dua MM, Iida Y, Dalman RL, Douglas T, McConnell MV. RGD-conjugated human ferritin nanoparticles for imaging vascular inflammation and angiogenesis in experimental carotid and aortic disease. Mol. Imaging Biol. 2012;14:315–324. doi: 10.1007/s11307-011-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terashima M, Uchida M, Kosuge H, Tsao PS, Young MJ, Conolly SM, Douglas T, McConnell MV. Human ferritin cages for imaging vascular macrophages. Biomaterials. 2011;32:1430–1437. doi: 10.1016/j.biomaterials.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellapadrona G, Stefanini S, Zamparelli C, Theil EC, Chiancone E. Iron translocation into and out of Listeria innocua Dps and size distribution of the protein-enclosed nanomineral are modulated by the electrostatic gradient at the 3-fold “ferritin-like” pores. J. Biol. Chem. 2009;284:19101–19109. doi: 10.1074/jbc.M109.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosha T, Ng HL, Bhattasali O, Alber T, Theil EC. Moving metal ions through ferritin-protein nanocages from three-fold pores to catalytic sites. J. Am. Chem. Soc. 2010;132:14562–14569. doi: 10.1021/ja105583d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk F, Lenders JP, Crichton RR, Schneider W. Reductive mobilisation of ferritin iron. Eur. J. Biochem. 1985;152:167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- 24.Mertz JR, Theil EC. Subunit dimers in sheep spleen apoferritin: the effect on iron storage. J. Biol. Chem. 1983;258:11719–11726. [PubMed] [Google Scholar]
- 25.Jin W, Takagi H, Pancorbo B, Theil EC. “Opening” the ferritin pore for iron release by mutation of conserved amino acids at interhelix and loop sites. Biochemistry. 2001;40:7525–7532. doi: 10.1021/bi002509c. [DOI] [PubMed] [Google Scholar]
- 26.Yasmin S, Andrews SC, Moore GR, Le Brun NE. A new role for heme, facilitating release of iron from the bacterioferritin iron biomineral. J. Biol. Chem. 2011;286:3473–3483. doi: 10.1074/jbc.M110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honarmand Ebrahimi K, Hagedoorn PL, Jongejan JA, Hagen WR. Catalysis of iron core formation in Pyrococcus furiosus ferritin. J. Biol. Inorg. Chem. 2009;14:1265–1274. doi: 10.1007/s00775-009-0571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade VJ, Treffry A, Laulhere J-P, Bauminger ER, Cleton MI, Mann S, Briat J-F, Harrison PM. Structure and composition of ferritin cores from pea seed (Pisum sativum) Biochim. Biophys. Acta. 1993;1161:91–96. doi: 10.1016/0167-4838(93)90201-2. [DOI] [PubMed] [Google Scholar]
- 29.Rohrer JS, Islam QT, Watt GD, Sayers DE, Theil EC. Iron environment in ferritin with large amounts of phosphate, from Azotobacter vinelandii and horse spleen, analyzed using extended X-ray absorption fine-structure (EXAFS) Biochemistry. 1990;29:259–264. doi: 10.1021/bi00453a035. [DOI] [PubMed] [Google Scholar]
- 30.Waldo GS, Wright E, Whang ZH, Briat JF, Theil EC, Sayers DE. Formation of the ferritin iron mineral occurs in plastids. Plant Physiol. 1995;109:797–802. doi: 10.1104/pp.109.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Pierre TG, Tran KC, Webb J, Macey DJ, Heywood BR, Sparks NH, Wade VJ, Mann S, Pootrakul P. Organ-specific crystalline structures of ferritin cores in beta-thalassemia/hemoglobin E. Biology of Metals. 1991;4:162–165. doi: 10.1007/BF01141308. [DOI] [PubMed] [Google Scholar]
- 32.Zhao G. Phytoferritin and its implications for human health and nutrition. Biochim. Biophys. Acta. 2010;1800:815–823. doi: 10.1016/j.bbagen.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Suel GM, Lockless SW, Wall MA, Ranganathan R. Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nature Struct. Biol. 2003;10:59–69. doi: 10.1038/nsb881. [DOI] [PubMed] [Google Scholar]
- 34.Jameson GNL, Jin W, Krebs C, Perreira AS, Tavares P, Liu X, Theil EC, Huynh BH. Stoichiometric production of hydrogen peroxide and parallel formation of ferric multimers through decay of the diferric-peroxo complex, the first detectable intermediate in ferritin mineralization. Biochemistry. 2002;41:13435–13443. doi: 10.1021/bi026478s. [DOI] [PubMed] [Google Scholar]