Abstract
Purpose
To evaluate pill counts and red blood cell (RBC) membrane fatty acid profiles as measures of compliance with oral omega3 polyunsaturated fatty acids (ω3 PUFAs) and to compare the two techniques.
Methods
Sixteen dry eye disease subjects were given oral ω3 PUFA or placebo for 3 months. Compliance was measured by pill counts and blood tests at baseline and 3 months. The Wilcoxon signed-rank tests and rank-sum tests were used to compare changes from baseline and the difference between the two groups; Spearman correlation coefficients were used to assess the relationship of pill counts to changes in blood FAs.
Results
Pill counts for the ω3 (n=7) and placebo (n=9) groups showed a mean consumption of 4.39 and 4.76 pills per day, respectively. In the ω3 group, the median change from baseline was +1.46% for eicosapentaenoic acid (EPA) (P=0.03), +1.49% for docosahexaenoic acid (DHA) (P=0.08), and −1.91% for arachidonic acids (AA) (P=0.02). In the placebo group, median changes in all measured FAs were small and not statistically significant. The difference in change in FA levels between the two groups was significantly greater for EPA (P=0.01) and AA (P=0.04). The correlations between pill counts and changes in EPA (r=0.36, P=0.43) and DHA (r=0.17, P=0.70) were not strong.
Conclusions
RBC FA analysis can be used to measure compliance in the active group and also monitor the placebo group for nonstudy ω3 intake. Low correlation of pill counts with blood levels suggests that pill counts alone may be inaccurate and should be replaced or supplemented with objective measures.
Introduction
Omega3 (n-3, ω3) polyunsaturated fatty acids (PUFAs) and their anti-inflammatory properties in humans have been a source of considerable interest in recent years. Consequently, they have been the focus for possible treatment of inflammatory ocular conditions such as dry eye disease (DED) and meibomian gland dysfunction.1–3 Although they have shown statistically significant improvements across a broad spectrum of systemic diseases as diverse as IgE-associated disease,4 polycystic ovary syndrome,5 psychiatric disorders,6 infertility,7 cardiac disorders,8 hypertriglyceridemia,9 and obesity,10 their efficacy in DED has not been established.
The results from ω3 studies in DED have been variable, with some studies showing improvement in signs and symptoms; and others showing no significant benefit. Although this could be attributed to the inherent variability of DED, it could also be due to factors related to the study design, such as single-center studies with a small sample size.1,11 Another key issue may be variability in compliance with the dosing regimen, but measurement of compliance typically was not done at all11 or was done by only subjective methods.1,2
Compliance and the ability to monitor compliance are key to determining the efficacy of any new drug. This is particularly significant to ω3 trials,12 as they face unique issues related to compliance. The common problem of subjects in the active arm not taking their medication becomes more relevant in ω3 studies, given the need for chronic treatment with large doses (five pills per day). Furthermore, unlike investigational drugs, ω3 PUFAs are easily available for purchase as over-the-counter (OTC) nutritional supplements, leading to the possibility of subjects in the placebo arm taking the active drug in the form of ω3 supplements. Undocumented use of OTC ω3 may bias the results of the study and lead to lack of significance in interpreting data. Hence, it is essential that future studies incorporate compliance monitoring into their study design, to provide reliable analysis of efficacy.
The purpose of this study was to demonstrate the feasibility of incorporating a minimally invasive objective metric for measuring compliance in a small, randomized, controlled clinical trial of ω3 supplementation in DED. In this feasibility study, we evaluated two tests of compliance: pill counts and red blood cell (RBC) membrane fatty acid analysis for monitoring compliance to ω3 PUFAs or the placebo.
Changes in the blood levels of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acids (AA) reflect ω3 intake.13 The ω3 PUFAs are derivatives of essential fatty acids and cannot appear in the body unless ingested. They are derived from, alpha-linolenic acid (ALA; 18:3, n-3) an 18-carbon PUFA.5 Once ingested, the 18-carbon essential fatty acid is desaturated and elongated to 20-carbon fatty acids, EPA, and DHA, which comprise the majority of ω3 PUFAs in the human diet and have widespread anti-inflammatory effects. On the other hand, derivatives of ω6 PUFAs have proinflammatory effects.14 ω6 fatty acids, typically AA, are present in high concentrations in human cell membranes. ω3 and ω6 fatty acids compete for the same desaturation enzymes,15,16 and when consumed, EPA and DHA partially replace AA in the cell membrane. Therefore, in subjects compliant to active supplements, the blood levels of EPA and DHA should rise, and the blood levels of AA should fall. Changes in blood levels should not be seen in the placebo group, unless they are consuming external sources of ω3 PUFAs or changing their diet. Hence, blood tests to monitor EPA, DHA, and AA were used to monitor compliance, both in the treatment group and the placebo group.13,17–22
Methods
The feasibility study design consisted of a single-center, double-blind, randomized controlled clinical trial of ω3 PUFAs for the treatment of DED to determine the feasibility of the clinical trial protocol and to determine subject compliance to ω3 PUFA supplements. The clinical study was conducted according to the principles of the Declaration of Helsinki and approved by the Mount Sinai School of Medicine Program for the Protection of Human Subjects. After a thorough explanation of study procedures and requirements, written informed consent was obtained from all study subjects.
Eighteen subjects, 15 female, 3 male, ages 31–78 (mean age: 56±14 years) with previous diagnoses of DED were randomly assigned to one of the two groups; the treatment group receiving ω3 PUFA pills for 3 months, and the placebo group receiving placebo pills (olive oil) for 3 months. Each patient was instructed to take five soft gel capsules per day. The total daily dose of ω3 PUFAs from the five pills was 3,000 mg of ω3 PUFAs. The active and the placebo pills were identical in size, shape, color, and taste, to ensure masking. Subjects were asked to maintain a stable diet with respect to fish oil and nutritional supplement consumption during the study. Two subjects dropped out of the study before completion due to reasons unrelated to the study procedures and treatment.
Compliance to the dosing regimen was monitored by two methods: pill counts and blood tests.23 Pill counts were made by dispensing a fixed number of pills to patients and asking them to return any unused pills at their next follow-up visit. Pill counts were done at the 1-month and the 3-month follow-up visits. Blood samples were drawn at baseline and then at 3 months.
Blood analysis
Blood was collected in 4-mL tubes containing EDTA and shipped overnight at room temperature, without further processing, to the Peroxisomal Diseases Laboratory at the Kennedy Krieger Institute in Baltimore, Maryland, for analysis. Within 48 h of collection, the RBCs were separated from the blood samples, washed with phosphate-buffered saline and stored in a −80° freezer until analysis. RBC membrane levels of EPA, DHA levels, and AA were determined using capillary column-negative ion mass spectrometry and expressed as percentage of total fatty acids, as previously described.23
Statistical analyses
Statistical analyses focused on values of EPA, DHA, and AA (% total FA). The Wilcoxon signed-rank tests and rank-sum tests were used to compare changes from baseline and to compare the differences between treatment groups; nonparametric Spearman correlation coefficients were used to assess the relationship of pill counts to changes in RBC membrane fatty acids.
Results
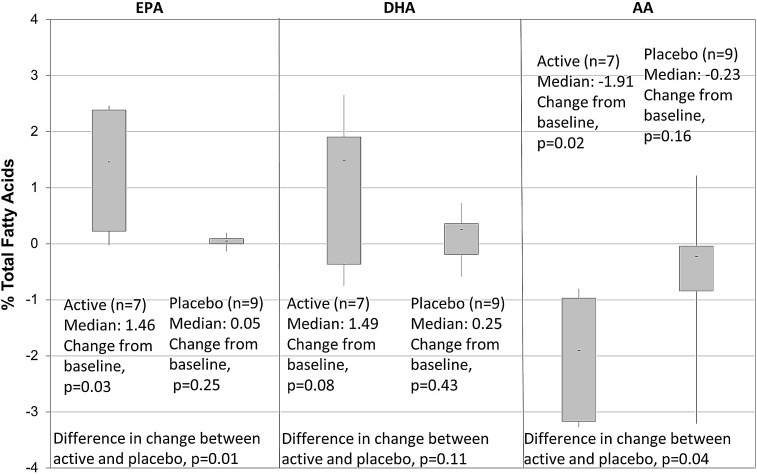
Pill counts and blood tests were completed as per protocol on all 16 subjects. Pill counts for the ω3 group (n=7) and placebo group (n=9) showed a mean consumption of 4.39 and 4.76 pills per day, respectively, that is, the compliance rate by pill counts was 87.8% in the active group and 95.2% in the placebo group. RBC membrane FA analysis in the active group revealed a statistically significant rise in EPA and a statistically significant fall in AA levels, 3 months after supplementation with ω3 PUFAs. The median change from baseline to the 3-month follow-up visit was +1.46% for EPA (P=0.03), +1.49% for DHA (P=0.08), and −1.91% for AA (P=0.02) (Fig. 1). In the placebo group, median changes in all measured FAs were small and not statistically significant (EPA: 0.05%, P=0.25; DHA: 0.25%, P=0.43; AA: −0.23%, P=0.16). Comparing the change in FA levels in the placebo group to the change in the active group showed that the increase in EPA (P=0.01) and the decrease in AA (P=0.04) after treatment was significantly greater in the ω3 group than in the placebo group. There was no statistical correlation between the number of pills taken and changes in blood EPA (r=0.36, P=0.43) and DHA (r=0.17, P=0.70) (Fig. 2). For instance, one subject assigned active treatment had an average pill count of 3.68, yet contrary to the expected increase there was decrease in blood EPA and DHA. Another subject with a high average pill count of 5.16 showed only a modest increase in EPA and a fall in DHA, whereas a subject with a similar pill count of 5.05 showed a high rise in both EPA and DHA (Table 1).
FIG. 1.
Change from baseline to 3 months in RBC membrane %. Total fatty acids: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; RBC, red blood cell.
FIG. 2.
Correlation between average pill counts and change in RBC membrane omega3 polyunsaturated fatty acids over 3 months in active subjects.
Table 1.
Table Showing Average Pills Consumed per Day Over 3 Months and Change in Red Blood Cell Membrane Eicosapentaenoic Acid (ΔEPA) and Docosahexaenoic Acid (ΔDHA) from Baseline to 3 Months for Active Subjects
| Patient ID | Average pill counts | ΔEPA | ΔDHA |
|---|---|---|---|
| 01-101 | 3.68 | −0.023 | −0.747 |
| 01-103 | 3.55 | 0.227 | 1.492 |
| 01-106 | 4.53 | 1.457 | 1.54 |
| 01-114 | 5.16 | 0.801 | −0.363 |
| 01-115 | 5.05 | 2.387 | 2.649 |
| 01-116 | 4.51 | 1.534 | 1.335 |
| 01-122 | 4.26 | 2.456 | 1.904 |
ΔEPA, eicosapentaenoic acid; ΔDHA, docosahexaenoic acid.
Discussion
Compliance is a critical issue in clinical drug trials assessing safety and efficacy of new treatments. It is influenced by a variety of factors including, but not limited to, the efficacy and side effects of the product, the number of medications being taken, the doses per day, and information given by the doctor. In clinical trials of nutritional supplements, OTC availability of the drug adds another confounding factor to compliance since subjects are able to self-medicate. The World Health Organization estimated in 2003 that subjects with chronic diseases in developed countries only take about 50% of their drugs.24 Accurate methods to verify that subjects are taking the study medication are key to reliably establishing the safety and efficacy of new drugs.
Past studies of ω3 have used food frequency questionnaires,25 diaries to register major deviations from the usual diet,26 self-reported compliance,27 and pill counts28–30 to measure compliance. However, all of them rely on the accuracy of the information that the subject provides and therefore their reliability is uncertain. For instance, in our study, pill counts indicated that all subjects took their pills (about 88% compliance), but blood levels in fact showed a decrease in ω3 in 29% of the subjects in the active arm, clearly demonstrating that pill counts do not always correctly reflect changes in blood levels of ω3. Poor correlation between pill counts and blood levels of ω3 are likely due to inaccuracies in pill counts or failure to follow the protocol dosing schedule. Previous research by Witte et al. demonstrated a significant linear correlation between systemic ω3 intake and increase in the proportion of ω3 fatty acids in RBC membrane.13 Therefore, all subjects in the active arm, if they were compliant as their pill counts suggested, would have a significant increase in blood EPA and DHA levels. Although for most people there is a direct correlation between intake and changes in blood levels of ω3, it has been reported that some people may have a genetic variation in the metabolism of ω331 and may require a higher intake of ω3 to increase RBC membrane levels of EPA and DHA. Whether the lack of rise in the RBC membrane is due to poor compliance or a variation in metabolism, in either case, the key to efficacy of ω3 is getting it into the systemic system, that is, blood, as indicated in the RBC fatty acid changes. Thus, including minimally invasive objective metrics of ω3 changes in the blood would clearly demonstrate efficacy or nonefficacy of treatment and eliminate many confounding variables of pill counts per subject questionnaire by evaluating the outcome measures as a function of in vivo ω3 levels.
RBC fatty acid analysis not only demonstrates the systemic increase in ω3, but is also a stable measure of intake, with low biological variability and measures long-term intake.32 Other objective methods include measuring ω3 PUFAs in swabs of buccal cells,33 plasma phospholipid fatty acid concentrations,33,34 and EPA+DHA proportions in plasma cholesterol esters.30 Although, analyzing buccal cells is less invasive than blood tests, the amount of total lipid on the swabs was very small, and the measurement of ω3 PUFAs was highly variable and unreliable.33 In another study, plasma fatty acid and plasma phospholipid fatty acid pools had a high biological variability and fluctuated with recent meals.32
Given RBC membrane FA analysis is a stable measure with low biological variability, it has been successfully incorporated as a compliance measure into several large-scale randomized clinical trials such as studies on the effect of ω3 PUFAs on bone formation and growth factors in adolescent boys,17 on inflammation and oxidative and nutritional status in patients with lung cancer,18 on gestational diabetes and pre-eclampsia,19 on postpartum depression and neurodevelopmental outcomes in children,20 on symptoms of attention-deficit/hyperactivity disorder21 and atopy in infants.22
The small sample size and fewer men as compared to women enrolled could be a limiting factor for the study. Nonetheless, significant changes were seen in the active group (increase in EPA and decrease in AA), and no corresponding changes were seen in the placebo group. However, in long-term clinical trials, there is always a possibility that subjects may start taking OTC ω3 supplements; this too will be detected by the blood tests. In addition, since the subjects will know that blood tests can determine compliance, this will encourage them to follow the study protocol. When subjects know that these tests can detect pill consumption over a long term, it will encourage them to strictly adhere to their regimen over the course of the clinical trial.
RBC membrane fatty acid analysis reflects long-term ω3 status and is unaffected by recent meals,32 similar to HbA1C measures in diabetes. Any efficacious treatment of DED will need long-term intervention, so it is necessary to have a method to measure long-term compliance. Thus, a long-term measure such as RBC membrane fatty acid analysis is well suited for clinical trials of ω3, which will be used for chronic diseases such as dry eye.
Determining efficacy to a new treatment depends on accurate measures of all aspects of a clinical trial, including compliance with study medication. This feasibility study showed that pill counts do not always accurately measure compliance as defined by the blood levels of ω3 fatty acids. ω3 intervention randomized clinical trials will benefit from including minimally invasive, objective metrics of compliance such as RBC membrane fatty acid analysis that reflect the true long-term ω3 status of subjects. Including a reliable measure of compliance will enhance the ability to accurately determine the efficacy of ω3 supplementation.
Acknowledgments
Supported, in part, by the National Eye Institute/National Institutes of Health (1-R34-EY017626-01A2) as well as by the Martin and Toni Sosnoff Foundation.
Author Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Wojtowicz J.C. Butovich I. Uchiyama E., et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 2.Brignole-Baudouin F. Baudouin C. Aragona P., et al. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89:e591–e597. doi: 10.1111/j.1755-3768.2011.02196.x. [DOI] [PubMed] [Google Scholar]
- 3.Macsai M.S. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Trans. Am. Ophthalmol. Soc. 2008;106:336–356. [PMC free article] [PubMed] [Google Scholar]
- 4.Furuhjelm C. Warstedt K. Fagerås M., et al. Allergic disease in infants up to 2 yr of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr. Allergy Immunol. 2011;22:505–514. doi: 10.1111/j.1399-3038.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 5.Phelan N. O'Connor A. Kyaw Tun T., et al. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2011;93:652–662. doi: 10.3945/ajcn.110.005538. [DOI] [PubMed] [Google Scholar]
- 6.Rondanelli M. Giacosa A. Opizzi A., et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. 2010;29:55–64. doi: 10.1080/07315724.2010.10719817. [DOI] [PubMed] [Google Scholar]
- 7.Safarinejad M.R. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S. Sutherland F. Wheeler M., et al. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human atrial mechanical function after reversion of atrial arrhythmias to sinus rhythm: reversal of tachycardia-mediated atrial cardiomyopathy with fish oils. Heart Rhythm. 2011;8:643–649. doi: 10.1016/j.hrthm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 9.McCombie G. Browning L.M. Titman C.M., et al. Omega-3 oil intake during weight loss in obese women results in remodelling of plasma triglyceride and fatty acids. Metabolomics. 2009;5:363–374. doi: 10.1007/s11306-009-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sneddon A.A. Tsofliou F. Fyfe C.L., et al. Effect of a conjugated linoleic acid and omega-3 fatty acid mixture on body composition and adiponectin. Obesity (Silver Spring). 2008;16:1019–1024. doi: 10.1038/oby.2008.41. [DOI] [PubMed] [Google Scholar]
- 11.Jackson M.A. Burrell K. Gaddie I.B. Richardson S.D. Efficacy of a new prescription-only medical food supplement in alleviating signs and symptoms of dry eye, with or without concomitant cyclosporine A. Clin. Ophthalmol. 2011;5:1201–1206. doi: 10.2147/OPTH.S22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris W.S. The omega-3 index: clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 2010;12:503–508. doi: 10.1007/s11886-010-0141-6. [DOI] [PubMed] [Google Scholar]
- 13.Witte T.R. Salazar A.J. Ballester O.F. Hardman W.E. RBC and WBC fatty acid composition following consumption of an omega 3 supplement: lessons for future clinical trials. Lipids Health Dis. 2010;9:31. doi: 10.1186/1476-511X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCowen K.C. Bistrian B.R. Essential fatty acids and their derivatives. Curr. Opin. Gastroenterol. 2005;21:207–215. doi: 10.1097/01.mog.0000153361.90653.cb. [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 16.Calder P.C. Dietary modification of inflammation with lipids. Proc. Nutr. Soc. 2002;61:345–358. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 17.Damsgaard C.T. Mølgaard C. Matthiessen J. Gyldenløve S.N. Lauritzen L. The effects of n-3 long-chain polyunsaturated fatty acids on bone formation and growth factors in adolescent boys. Pediatr. Res. 2012;71:713–719. doi: 10.1038/pr.2012.28. [DOI] [PubMed] [Google Scholar]
- 18.Finocchiaro C. Segre O. Fadda M., et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br. J. Nutr. 2012;108:327–333. doi: 10.1017/S0007114511005551. [DOI] [PubMed] [Google Scholar]
- 19.Zhou S.J. Yelland L. McPhee A.J., et al. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am. J. Clin. Nutr. 2012;95:1378–1384. doi: 10.3945/ajcn.111.033217. [DOI] [PubMed] [Google Scholar]
- 20.Makrides M. Gibson R.A. McPhee A.J., et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 21.Milte C.M. Parletta N. Buckley J.D., et al. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: a randomized controlled trial. Nutrition. 2012;28:670–677. doi: 10.1016/j.nut.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.D'Vaz N. Meldrum S.J. Dunstan J.A., et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. 2012;130:674–682. doi: 10.1542/peds.2011-3104. [DOI] [PubMed] [Google Scholar]
- 23.Lagerstedt S.A. Hinrichs D.R. Batt S.M., et al. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 24.Adherence to long-term therapies: Evidence for action. www.who.int/chp/knowledge/publications/adherence_full_report.pdf. [Apr 25;2011 ]. www.who.int/chp/knowledge/publications/adherence_full_report.pdf Online document available at.
- 25.Singh M. Stark P.C. Palmer C.A. Gilbard J.P. Papas A.S. Effect of omega-3 and vitamin E supplementation on dry mouth in patients with Sjögren's syndrome. Spec. Care Dentist. 2010;30:225–229. doi: 10.1111/j.1754-4505.2010.00158.x. [DOI] [PubMed] [Google Scholar]
- 26.Aragona P. Bucolo C. Spinella R. Giuffrida S. Ferreri G. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren's syndrome patients. Invest. Ophthalmol. Vis. Sci. 2005;46:4474–4479. doi: 10.1167/iovs.04-1394. [DOI] [PubMed] [Google Scholar]
- 27.Galan P. Kesse-Guyot E. Czernichow S., et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee L.D. Lester J.L. Cole R.M., et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayer J.G. Harmer J.A. Xuan W., et al. Dietary supplementation with n-3 polyunsaturated fatty acids in early childhood: effects on blood pressure and arterial structure and function at age 8 y. Am. J. Clin. Nutr. 2009;90:438–446. doi: 10.3945/ajcn.2009.27811. [DOI] [PubMed] [Google Scholar]
- 30.van de Rest O. Geleijnse J.M. Kok F.J., et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2008;88:706–713. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- 31.Simopoulos A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. (Maywood). 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- 32.Harris W.S. Thomas R.M. Biological variability of blood omega-3 biomarkers. Clin. Biochem. 2010;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Dangour A.D. Allen E. Elbourne D., et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010;91:1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 34.van der Meij B.S. Langius J.A. Smit E.F., et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 2010;140:1774–1780. doi: 10.3945/jn.110.121202. [DOI] [PubMed] [Google Scholar]