Abstract
Background
H. pylori (Hp) infection is a main risk factor in gastric carcinogenesis leading to epithelial mutagenesis, and may affect gastric epithelial stem cells.
Aims
To characterize the expression of Lgr5, a marker of epithelial stem cells in human gastric mucosa, to determine whether Hp infection affects Lgr5-positive epithelial cells (LPECs), and whether LPECs are susceptible to DNA damage associated with Hp infection.
Methods
Lgr5 expression was characterized in non-neoplastic gastric mucosa from 52 patients [34 with and 18 without gastric cancer (GC); 21 Hp-positive (Hp+) and 31 Hp-negative (Hp−)] by immunohistochemical and immunofluorescence stains. To determine the extent of DNA damage in LPECs, nuclear 8-hydroxydeoxyguanosine (8OHdG), a marker of DNA damage associated with oxidative stress, was measured by quantitative spectral image analysis.
Results
LPECs were primarily present in gastric antrum. Higher numbers of LPECs were seen in Hp+ than in Hp− non-neoplastic mucosa of GC patients, P=.006, but not in patients without GC. 8OHdG levels in LPECs were significantly higher than in Lgr5-negative epithelial cells in Hp+ GC patients (P=.012) but not in Hp− cases (P=.414), whereas no difference was seen between Hp+ and Hp− mucosa of patients without GC.
Conclusions
The Lgr5-positive epithelial stem cell pool is expanded in Hp-associated gastritis in the antrum of patients with GC. In GC patients with active Hp infection, LPECs may be more susceptible to DNA damage than Lgr5-negative epithelial cells, suggesting that Hp infection may contribute to GC risk by affecting epithelial stem cells in the human stomach.
Keywords: Helicobacter pylori, Gastric, DNA damage, 8OHdG, Lgr5, Stem Cells
Introduction
Helicobacter pylori infection of the stomach leads to chronic gastritis, intestinal metaplasia and increased risk of gastric cancer (GC)1–5. Gastric cancer is the fourth most common cancer in the world 6, and 75% of GC cases are attributable to H. pylori infection5. We have previously demonstrated that H. pylori infection leads to reduced DNA mismatch repair and enhanced mutagenesis in gastric epithelial cells7–9. However, how these induced mutagenic and regulatory alterations are transmitted to cell progeny and potentially result in cancer development remains poorly understood. It is likely that critical genomic and epigenetic changes caused by H. pylori infection affect gastric epithelial stem cells and epithelial progenitor cells, affecting their primary role of re-populating the gastric glandular units and programming them to a neoplastic pathway, consistent with the cancer stem cell hypothesis10.
Few specific markers of gastric stem cells are available, limiting our ability to address their role in H. pylori associated carcinogenesis. The leucine rich repeat containing G protein coupled receptor Lgr5, also known as GPR49, is an orphan G protein-coupled receptor and a Wnt target gene that was shown to be expressed in adult stem cells in the stomach and intestinal epithelium in a mouse model and recently, in human stomach11, 12. Using lineage tracing in mice, Barker et al11 showed that Lgr5 was expressed at the base of the gastric glands and that Lgr5-positive cells had the capacity of self-renewal and could build long-lived gastric antral units in vivo, thus meeting the criteria for stem cell (self-renewal and multipotency defined as the ability to give rise to daughter cells that differentiate into multiple lineages)10. In mice, Lgr5 expression was observed in a limited set of adult tissues including the intestine and antral epithelium of adult stomach13, 14. Therefore, Lgr5 may be used as a marker of gastric epithelial stem cells. Our hypothesis is that Lgr5-positive gastric epithelial stem cells may be important in the response to H. pylori infection and associated inflammation.
To date there is limited data regarding the characterization of Lgr5-positive cells in gastric disease, namely the features of Lgr5-positive cells in human gastric mucosa and the genomic changes of this stem cell population in response to enhanced oxidative stress in the milieu of H. pylori gastritis.
8-Hydroxydeoxyguanosine (8OHdG) is considered to be one of the main DNA modifications induced by reactive oxygen species (ROS), namely in inflammatory conditions such as H. pylori gastritis 15, 16. Interestingly, the levels of 8OHdG in gastric mucosa were shown to significantly decrease after eradication of H. pylori infection15, 16. 8OHdG may also be responsible for DNA base mutations, since the adducts can accumulate and may be only partially repaired through enzyme pathways that may in turn cause further DNA damage 17.
In this study, we characterized the expression of Lgr5 in human gastric mucosa and determined 8OHdG levels in LPECs to determine: a) whether the Lgr5-positive stem cell population is susceptible to oxidative damage in the setting of chronic H. pylori infection and b) whether patients with gastric cancer vs. those without gastric cancer show different responses.
MATERIALS AND METHODS
Cases
Gastric tissue samples from fifty two patients were included in the study. Thirty four patients had gastric cancer and 18 did not (Supplement Table 1). Cases were selected consecutively from archived formalin fixed-paraffin embedded (FFPE) gastrectomy specimens from 2003–2008, at the Hospital of the University of Pennsylvania, Philadelphia, PA. Gastric tissue samples from nine patients with H. pylori gastritis without gastric cancer were from the Department of Laboratory Medicine, Shinshu University School of Medicine, Matsumoto, Japan. Representative tissue blocks containing gastric antral mucosa and/or oxyntic mucosa were selected when available from each individual gastrectomy. In each case, gastric mucosa with none or small areas of intestinal metaplasia were selected for the study (Supplement table 2).
Immunohistochemistry and Immunofluorescence of Stomach Tissues
FFPE tissue sections cut at 5 μm thickness were used. Antigen retrieval was performed with Target Retrieval Solution (DAKO North America, Inc., Carpinteria, CA) by boiling the slides for 20 minutes followed by cooling at room temperature for 20 minutes. The sections were then incubated with Protein Block Serum Free solution (DAKO North America, Inc., Carpinteria, CA) at room temperature for 1 hour. The individual primary antibodies to detect Lgr5, H. pylori, 8OHdG, CD34, CD45, Cytokeratin AE1/AE3, CD44, vimentin and GFP (Supplement Table 3) were applied and incubated with the tissue sections for 1 hour at room temperature in a humidified chamber. The slides were then washed in phosphate buffered saline and incubated with secondary antibody using the DAKO LSAB2 System-HRP Kit according to the manufacturer’s instructions (DAKO North America, Inc., Carpinteria, CA).
Immunohistochemistry for H. pylori was performed with a rabbit polyclonal antibody (Supplement Table 3) using Bond Polymer Refine Detection reagents as recommended by the manufacturer (Vision Biosystems, Buffalo Grove, IL).
For immunofluorescence, after the sections were incubated with the primary antibody, the slides were washed in PBS and incubated with anti-rabbit antibody labeled with Alexa Fluor 488 or anti-mouse antibody labeled with Alexa Fluor 568 (Invitrogen, Carlsbad, California, USA) for 1 hour at room temperature in the dark. For negative controls, the primary antibodies were omitted. The slides were then washed in PBS and mounted using Vectashield (Vector Laboratories, Burlingame, USA). The immunofluorescence images and quantitative multispectral data were obtained using an argon laser (420 to 720 nm) connected to an inverted fluorescence microscope (Nuance TRIO, CRI, San Francisco, CA, USA). Images were captured using a 40X objective lens. Digital image overlays were processed using CRI Maestro systems (CRI, San Francisco, CA, USA).
Histology and Scoring of Immunohistochemical and Immunofluorescence stains
The number of Lgr5-positive cells in each case was scored based on the highest number of Lgr5-positive cells per high power field (400X) in gastric epithelium, examined in the entire tissue section. The intensity of Lgr5 stain was reported as three grades as follows: no stain (0); intermediate stain (1); and strong stain (2). Grades 1 and 2 were considered as positive Lgr5 expression. H. pylori immunohistochemistry was scored as positive or negative. The inflammatory grade, including neutrophils and mononuclear inflammatory cells in the gastric mucosa, and extent of intestinal metaplasia were scored using the updated Sydney system (Houston classification) of gastritis, with scores ranging from 0 to 34.
To determine the 8OHdG maximum fluorescence intensity in Lgr5-positive vs. negative epithelial cells, at least 5 cells including both Lgr5-positive and negative epithelial cell were counted in each image. Similarly, in lamina propria, at least 5 non-epithelial cells including both Lgr5-positive and negative cells were counted in each image. Therefore, data from at least 10 Lgr5-positive and 10 Lgr5-negative epithelial and lamina propria cells were obtained from each case. The 8OHdG maximum fluorescence intensities of Lgr5-positive cells were compared with those of Lgr5-negative cells using the image analysis software.
To determine the co-localization of CD45, CD34, and cytokeratin in the Lgr5-positive cells in gastric antral epithelium and lamina propria, at least 10 Lgr5-positive cells from each the glandular epithelium and lamina propria were counted. To determine whether there is co-localization of vimentin (a mesenchymal cell marker) and CD44 (one of a number of gastric stem cell markers 12) in Lgr5-positive cells, immunofluorescence was performed as described above.
Immunofluorescence Stains for Lgr5 in Mouse Stomach and Gastric Cell Lines
Heterozygous Lgr5-EGFP-IRES-CreERT2 mice in a C57BL/6 genetic background (Lgr5 mice) harbor a Lgr5-EGFP-IRES-CreERT2 “knock-in” allele that abolishes Lgr5 gene function and expresses green fluorescent protein (EGFP) and CreERT2 fusion protein from the Lgr5 promoter/enhancer elements. In Lgr5 mice EGFP fluorescence is observed in crypt base cells in mouse small intestine and colon and in epithelial stem cells of the stomach11, 13, 18.
Heterozygous Lgr5 mice were obtained from Jackson Laboratories (Bar Harbor, ME). For immunofluorescence studies, 5 micron thick sections from FFPE mouse stomach were used. Sections were treated with Protein Block Serum Free solution (DAKO North America, Inc., Carpinteria, CA, USA) at room temperature for 1 hour. The slides were then incubated with a goat anti-GFP antibody for 1 hour (Supplement Table 3). The slides were covered with VECTASHIELD HardSet mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Dako NP Universal Negative Control Mouse IgG (NP015) was used as negative control.
The gastric cell lines AGS (CRL-1739, ATCC, Manassas, VA, USA), and SNU638 were grown in RPMI-1640 medium containing 10% fetal bovine serum, penicillin and streptomycin (GIBCO BRL, Gaithersburgh, MD, USA)8, 19. The cell lines were plated in Lab-Tek II slide chambers (Thermo Fisher Sicentific Inc, Rochester, NY, USA), with 1×105 cells per well and grown for 24 hours to reach 90% confluence. The culture medium was removed, and the cells were washed with PBS and then fixed with 2% paraformaldehyde in PBS. The slides were then stained with anti-Lgr5 antibody, followed by incubation with Alexa Fluor 488 conjugated goat anti-rabbit secondary antibody (Invitrogen Inc, Carlsbad, CA) and covered with VECTASHIELD HardSet mounting medium with DAPI.
Statistical Analysis
Statistical analysis used the two-tailed T-test or Mann-Whitney rank sum test, multiple group comparison analyses with ANOVA and pairwise correlations by Pearson product moment correlation. Analyses were performed with the Sigmaplot 11.2 software (Systat Software Inc., Point Richmond, CA, USA). P values <.05 were considered significant.
RESULTS
Expression of Lgr5 in Gastric Mucosa
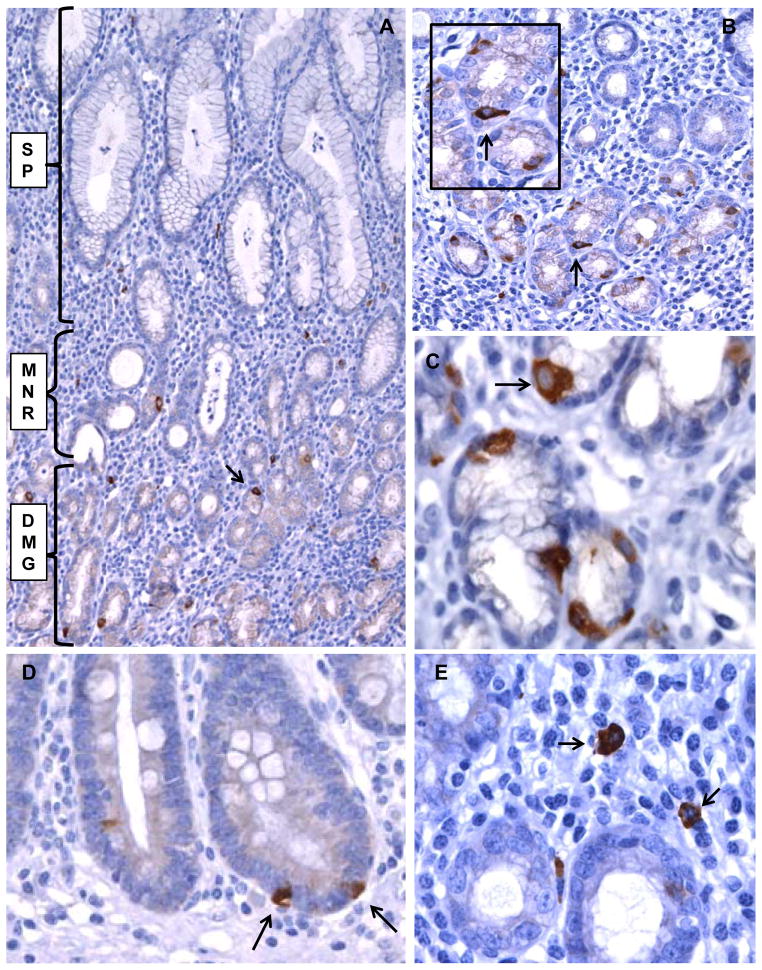
Lgr5 expression was examined in non-neoplastic gastric mucosa from 52 patients. Thirty four patients had gastric adenocarcinoma (mean age 68.6 years) and eighteen did not (mean age of 49.6 years), Supplement Table 1. Overall Lgr5-positive cells were predominantly identified as rare epithelial cells in gastric mucous glands, with fewer positive cells present in the mucous necks of the glands, while no Lgr5-positive cells were found on the surface or gastric pit epithelium (Figure 1). Lgr5 appeared as a diffuse cytoplasmic stain and Lgr5-positive epithelial cells often showed somewhat triangular shapes, with occasionally laterally elongated ends, and were located in the outer aspect of the glands, closer to the lamina propria rather than toward the lumen of the glands (Figure 1).
Figure 1. Lgr5 expression in gastric mucosa detected by immunohistochemistry.
A. H. pylori positive gastric antral mucosa with multiple Lgr5-positive cells in the gastric glands (arrow). The gastric pit and surface epithelium (SP), gland mucous neck region (MNR), and deep mucous gland region (DMG) are indicated. B. Lgr5-positive epithelial cells in gastric antrum from another case (arrow). C. Lgr5-positive cells showing triangular shape and basal orientation (arrow) in the glandular profiles. D. Lgr5-positive cells (arrows) in a focus of intestinal metaplasia. E. Rare Lgr-5 positive cells in the lamina propria (arrows). A and B (original magnification 200X), C, D, E (original magnification 400X).
In addition to Lgr5-positive epithelial cells we also found rare Lgr5-positive cells in the lamina propria, characterized by plasmacytoid morphology (Figure 1).
Overall, 33 antral and 23 oxyntic mucosa samples were examined (Table 1). The mean number of Lgr5-positive epithelial cells was 11.4 and 3.7 in antral and oxyntic mucosa, respectively (P=.001), Table 1.
Table 1. Lgr5-positive epithelial cell numbers in the various groups.
Patients with GC (GC+); patients without GC (GC−).
| Groups |
a Age Mean (Std Dev) |
b Number of samples | Lgr5+ Epithelial cells Mean (Std Dev) |
Test P value |
|---|---|---|---|---|
|
| ||||
| Mucosa Type: | ||||
| cAll Antrum | 58.6 (17.9) | 33 | 11.4 (7.0) | P=.001 |
| dAll Oxyntic | 65.2 (13.8) | 23 | 3.7 (6.4) | |
|
| ||||
| H. pylori status | ||||
| All Positive | 57.4 (19.6) | 21 | 12.0 (6.4) | P=.001 |
| All Negative | 65.1 (14.1) | 35 | 6.0 (7.7) | |
|
| ||||
| GC+ | ||||
| H. pylori Positive | 66.4 (11.1) | 12 | 11.7 (8.1) | P=.006 |
| H. pylori Negative | 69.7 (12.9) | 22 | 4.4 (4.9) | |
| GC− | ||||
| H. pylori Positive | 45.3 (22.5) | 9 | 12.3 (3.7) | P=.314 |
| H. pylori Negative | 53.8 (10.3) | 13 | 8.7 (10.6) | |
|
| ||||
| GC+ (Antrum mucosa) | ||||
| H. pylori Positive | 69.0 (10.2) | 10 | 13.5 (7.5) | P=.036 |
| H. pylori Negative | 64.4 (14.6) | 8 | 6.1 (5.7) | |
| GC+ (Oxyntic Mucosa) | ||||
| H. pylori Positive | a53.5 (3.5) | 2 | 2.5 (0.7) | P=.87 |
| H. pylori Negative | 72.8 (11.3) | 14 | 3.4 (4.3) | |
There were no statistically significant differences between the age groups (P>.05), except for the mean ages of H. pylori-positive vs. H. pylori-negative patients in the sub-group GC+ (Oxyntic Mucosa), P<.05.
When available, separate samples of antral and oxyntic mucosa from each individual patient were studied.
Include H. pylori positive and negative mucosa from patients with and without gastric cancer.
Focal intestinal metaplasia was present in 17 gastric antrum samples with a mean score of 0.85, out of possible scores ranging from 0 to 3 (Supplement Table 2). In 11 cases, intestinal metaplasia was present in the tissue sections used for Lgr5 immunohistochemistry. In the foci of intestinal metaplasia, few positive Lgr5-positive cells were identified in the base of intestinalized glands (Figure 1), with a mean number of Lgr5-positive cells of 1.5, ranging from 1–4 Lgr5-positive cells per 400X field.
Expression of Lgr5 in Gastric Cancer Cell Lines and in the Mouse Stomach
Immunofluorescence stains were used to determine the expression of Lgr5 in cultured gastric cancer cell lines. We identified a population of Lgr5-positive cells (5.0%) in the SNU638 cell line, Supplement Figure 1. There were only rare Lgr5-positive cells in the AGS cell line (0.42%). Therefore, the SNU638 cell line may be useful as a model to study the response of Lgr5-positive cells to H. pylori and other agents.
Previous studies in mice showed expression of Lgr5 in a limited set of adult tissues including the antrum of the adult stomach20. Since anti-Lgr5 antibodies that recognize mouse Lgr5 are not currently available, we used an anti-EGFP antibody to identify putative Lgr5 positive cells in the stomach of Lgr5-EGFP-IRES-creERT2 mice. In the mouse stomach, we identified EGFP-positive cells in the base of glands in gastric antrum, consistent with reported studies and with our findings in the human stomach described above13 (Supplement Figure 2).
Effect of H. pylori Infection and Inflammation on Lgr5 Expression in Gastric Mucosa
The H. pylori status of gastric mucosa samples was examined by detection of H. pylori on H&E stained sections and by immunohistochemistry. The grades of inflammation in the mucosa were scored by evaluating the grade of chronic inflammation (lymphocytes and plasma cells or other mononuclear inflammatory cells) and activity grade (neutrophils). Overall, when H. pylori positive (n=21) were compared with H. pylori negative mucosa samples (n=35), the number of Lgr5-positive epithelial cells was higher in H. pylori positive cases (P=.001), Table 1. Further analyses showed that gastric cancer patients with H. pylori positive gastritis in the background mucosa had a higher number of Lgr5-positive epithelial cells than H. pylori negative patients (p=.006), whereas in patients without gastric cancer the number of Lgr5-positive epithelial cells was higher in H. pylori positive than H. pylori negative mucosa but did not reach statistical significance (P=.314), Table 1. Additionally, the number of Lgr5-positive epithelial cells in the mucosa of H. pylori positive gastric cancer patients as compared to H. pylori negative gastric cancer patients was significantly higher in the gastric antrum (P=.036) but not in the oxyntic mucosa (P=.87), Table 1.
Interestingly, no significant difference in the numbers of Lgr5-positive epithelial cells in the mucosa of gastric cancer positive vs. gastric cancer negative patients was found when H. pylori status was not factored in (P>.05). These data suggest an interaction of H. pylori with host susceptibility factors that affect the response of Lgr5-positive epithelial cells in the group of gastric cancer patients.
H. pylori infection is always associated with variable degrees of chronic and active inflammation in the gastric mucosa, the latter consisting of neutrophils. The mean acute and chronic inflammation scores were significantly higher in H. pylori positive than in H. pylori negative mucosa samples (P=.017), Supplement Table 4. When the inflammation scores of antrum and oxyntic mucosa were scored separately, significantly higher scores for both neutrophils and mononuclear inflammatory cells were found in H. pylori positive antrum (P=.001 and P=.017, respectively), while in oxyntic mucosa only the neutrophil scores were significantly higher in H. pylori positive mucosa samples (P=.002), Supplement Table 4. Interestingly, the numbers of Lgr5-positive epithelial cells were positively correlated with the acute inflammation scores in gastric antral mucosa (R=0.407, P=.018, Pearson Product Moment Correlation), while other pairwise comparisons of inflammation and Lgr5 epithelial expression showed no significant correlation.
Quantitative Analyses of 8OHdG in Lgr5-positive and Negative Cells in Gastric Mucosa
The finding of increased numbers of Lgr5-positive epithelial cells in H. pylori positive patients, particularly in the gastric cancer patient group, suggests that these cells may biologically respond to H. pylori infection. Therefore, we hypothesized that they may be susceptible to genomic injury associated with oxidative stress induced by the active inflammatory response to H. pylori infection and possibly by the bacterium or its released products. We performed quantitative analyses of 8OHdG as a marker of oxidative DNA damage in the nucleus of Lgr5-positive cells as compared to Lgr5-negative cells in the non-neoplastic antral mucosa, by using quantitative spectral image analysis. Tissue sections were co-stained with immunofluorescence to detect 8OHdG and Lgr5. 8OHdG levels in Lgr5-positive vs. Lgr5-negative epithelial cells in 33 cases including patients with or without gastric cancer and with or without H. pylori gastritis are summarized in Table 2.
Table 2.
Levels of 8OHdG in nuclei of epithelial cells in antral mucosa.
| Epithelial Cells (EP) | 8OHdG Mean levels, Std Dev |
P-value | |
|---|---|---|---|
| * All cases: N=33 | Lgr5+ cells | 264.97 (148.7) | P=.073 |
| Lgr5− cells | 247.44 (136.8) | ||
| Hp+ (GC+ and GC−) N=18 |
Lgr5+ cells | 200.15 (117.5) | P=.087 |
| Lgr5− cells | 179.15 (97.0) | ||
| Hp− (GC+ and GC−) N=15 |
Lgr5+ cells | 342.76 (147.9) | P=.414 |
| Lgr5− cells | 329.39 (134.8) | ||
| GC+ (Hp+ and Hp−) N=16 |
Lgr5+ cells | 313.98 (165.6) | P=.049 |
| Lgr5− cells | 287.72 (157.9) | ||
| GC− (Hp+ and Hp−) N=17 |
Lgr5+ cells | 218.84 (117.8) | P=.525 |
| Lgr5− cells | 209.53 (104.5) | ||
| GC+ and HP+ N=9 |
Lgr5+ cells | 263.59 (126.9) | P=.012 |
| Lgr5− cells | 215.00 (106.5) | ||
| GC− and Hp+ N=9 |
Lgr5+ cells | 136.7 (64.8) | P=.611 |
| Lgr5− cells | 143. (75.9) | ||
| GC+ and Hp− N=7 |
Lgr5+ cells | 378.77 (195.9) | P=.878 |
| Lgr5− cells | 381.22 (170.5) | ||
| GC− and Hp− N=8 |
Lgr5+ cells | 311.25 (284.1) | P=.341 |
| Lgr5− cells | 92.17 (80.0) | ||
All cases include: GC+, GC−, Hp+ and Hp− cases.
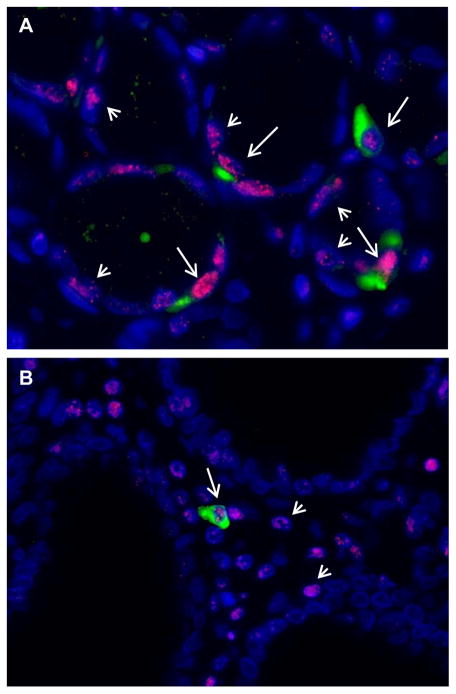
Variable levels of 8OHdG were detected in Lgr5-positive and negative epithelial cells (Figure 2). The overall 8OHdG maximum fluorescence intensity in Lgr5-positive epithelial cells (mean 264.97) was higher than in Lgr5-negative epithelial cells (mean 247.44) but did not reach statistical significance (P=.073), Table 2. In the gastric mucosa of patients with gastric cancer, significantly higher levels of 8OHdG were detected in Lgr5-positive epithelial cells as compared to Lgr5-negative epithelial cells (P=.049), Table 2. Further, among gastric cancer patients with H. pylori gastritis, 8OHdG levels were higher in Lgr5-positive as compared to Lgr5-negative epithelial cells (P=.012), Table 2. The 8OHdG maximum fluorescence intensity in Lgr5-positive epithelial cells was higher in H. pylori positive patients, regardless of gastric cancer status but did not reach statistical significance (P=.087). No significant differences were found for other group comparisons (Table 2). These data further suggest an interaction of H. pylori with host susceptibility factors that affect the response of Lgr5-positive epithelial cells in gastric cancer patients.
Figure 2. Detection of Lgr5 and 8OHdG in epithelial cells of gastric glands by immunofluorescence.
A. In the glandular epithelial cells, 8OHdG fluorescence (red punctate nuclear stain) was detected in nuclei of Lgr5-positive (green cytoplasmic stain, long arrow), and Lgr5-negative cells (short arrow). B. In lamina propria, 8OHdG was detected in nuclei of Lgr5-positive (long arrows) and Lgr5-negative cells (short arrows). Nuclei are stained with DAPI. Original magnification 400X.
In the mucosa of intestinal metaplasia positive cases the difference between 8OHdG maximum fluorescence intensity of Lgr5-positive epithelial cells (mean 289.71, Std Dev 147.24) and 8OHdG maximum fluorescence intensity of Lgr5-negative epithelial cells (mean 268.48, Std Dev 127.91), was not statistically significant (P=.144).
Examples of Lgr5-positive cells in lamina propria showing 8OHdG accumulation are shown in Figure 2. The overall 8OHdG maximum fluorescence intensity in lamina propria Lgr5-positive cells (mean 268.8) was significantly higher than in Lgr5-negative lamina propria cells (mean 247.67), P=.004, Supplement Table 5. Surprisingly, the levels of 8OHdG were higher in Lgr5-positive lamina propria cells in patients who were H. pylori negative and gastric cancer negative.
Co-expression of CD45, Cytokeratin, CD34, CD44 and Vimentin in Lgr5 Positive Cells in Gastric Mucosa
Both the immunohistochemistry as well as immunofluorescence stains of gastric mucosa with anti-Lgr5 antibody showed that in addition to Lgr5-positive epithelial cells, there were rare scattered Lgr5-positive cells in the lamina propria, particularly in patients with H. pylori gastritis. To determine whether the Lgr5-positive cells in the gastric glands and in lamina propria showed features of epithelial, leukocyte or endothelial lineages we evaluated co-expression of CD45, Cytokeratin AE1/AE3, CD34 and Lgr5 in three representative gastric antral mucosa samples of patients with H. pylori gastritis (Table 3).
Table 3.
Co-expression of Cytokeratin AE1/AE3 (CK), CD45, or CD34 in Lgr5-positive cells within glandular epithelium (GE) and lamina propria (LP), detected by immunofluorescence stains. The numbers of CK, CD45 or CD34 positive cells co-expressing Lgr5 were characterized in antral mucosa of three H. pylori positive cases (SR50, SR67 and SR144).
| Co-expression of Lgr5, CK, CD45 (%) | Case SR50 | Case SR67 | Case SR144 |
|---|---|---|---|
| GE: CK+ Lgr5+ cells | 22/22 (100) | 32/32 (100) | 19/19 (100) |
| GE: CD45+ Lgr5+ cells | 0/39 (0) | 0/30 (0) | 0/16 (0) |
| GE: CD34+ Lgr5+ cells | 0/24 (0) | 0/32 (0) | 0/21 (0) |
| LP: CK+ Lgr5+ cells | 0/19 (0) | 0/18 (0) | 0/11 (0) |
| LP: CD45+ Lgr5+ cells | 18/18 (100) | 17/22 (72.3) | 10/10 (100) |
| LP: CD34+ Lgr5+ cells | 0/16 (0) | 0/18 (0) | 0/20 (0) |
In the epithelium of gastric glands, Lgr5-positive cells were always positive for cytokeratin, confirming an epithelial lineage (Table 3 and Figure 3). Further, in gastric epithelium, Lgr5-positive cells did not co-express CD45 (Table 3, Figure 4), CD34, or vimentin (Table 3 and Supplement Figures 3 and 4). To determine whether epithelial Lgr5+ cells co-express other stem cell markers we performed immunofluorescence staining of Lgr5 and CD44 in the same sections (Supplement Figures 5 and 6). We found that most CD44+ epithelial cells were Lgr5−, and essentially all Lgr5+ cells appeared to co-express CD44 (Supplement Figures 5 and 6).
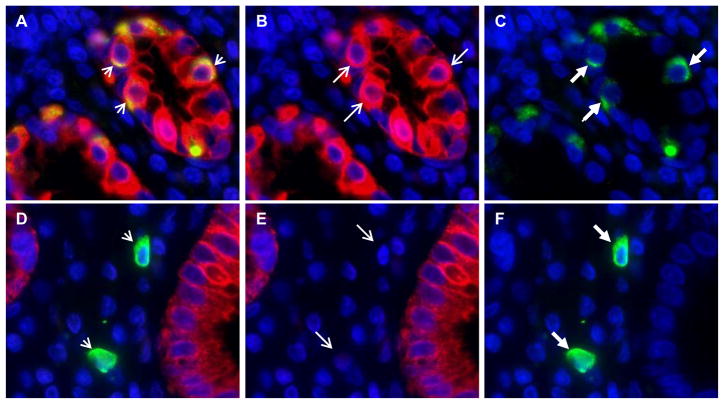
Figure 3. Co-localization of Lgr5 and cytokeratin in epithelial cells of gastric antral glands.
Panel a: Overlapped immunofluorescence images show co-localization of Lgr5 and cytokeratin in epithelial cells (yellow cells, short arrow). Panel a was created by overlapping the cytokeratin AE1/AE3 immunofluorescence stain (shown in panel b), where red stain and the long thin arrow indicate cytokeratin positive cells, with the Lgr5 immunofluorescence stain (shown in panel c), where green stain and the long thick arrow indicate Lgr5-positive epithelial cells. Panel d: Overlapped immunofluorescence images show lack of co-localization of cytokeratin in Lgr5-positive lamina propria cells (short arrows). The overlapped images in panel d were the cytokeratin immunofluorescence stain (shown in panel e), and the Lgr5 immunofluorescence stain (shown in panel f). Two Lgr5-positive lamina propria cells are indicated by the long thick arrows in panel f, and these two cells are negative for cytokeratin (panel e, long thin arrows). Nuclei are stained with DAPI. Original magnification 400X.
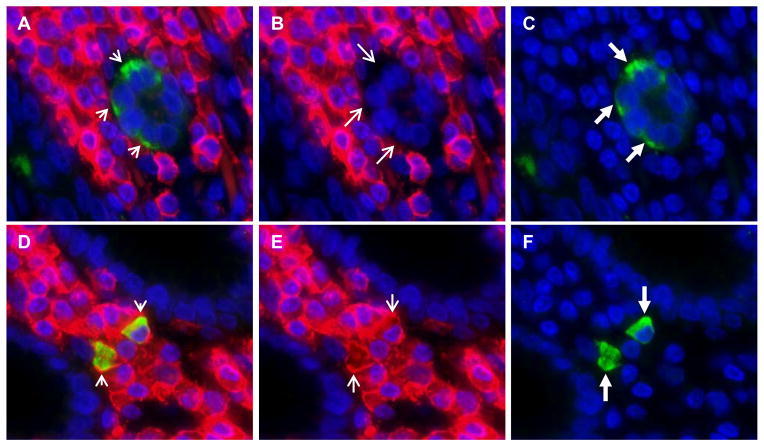
Figure 4. Lgr5-positive cells in lamina propria of gastric antrum co-express CD45.
Panel a. Overlapped immunofluorescence images show lack of co-localization of CD45 in Lgr5-positive epithelial cells (short arrows). Panel a was created by overlapping the CD45 stain (shown in panel b) with the Lgr5 stain (shown in panel c). Lgr5-positive epithelial cells stain green and are indicated by long thick arrows in panel c. The CD45 stain is negative in Lgr5-positive epithelial cells, as indicated by the long thin arrows in panel b. Panel d was created by overlapping the CD45 stain (shown in panel e) with the Lgr5 stain (shown in panel f) and shows co-localization of CD45 in Lgr5-positive lamina propria cells (yellow cells, short arrows). Lgr5-positive lamina propria cells stain green and are indicated by long thick arrows in panel f. The CD45 stain is positive in Lgr5-positive lamina propria cells, as indicated by the long thin arrows in panel e. Nuclei are stained with DAPI. Original magnification 400X.
In the lamina propria, most Lgr5-positive cells co-expressed CD45 indicating they are of leukocyte lineage (Table 3 and Figure 4). This is consistent with their plasmacytoid morphology and localization amidst the inflammatory infiltrate in the lamina propria. In two cases (SR50 and SR54), all lamina propria Lgr5-positive cells co-expressed CD45, whereas in case SR67 a proportion of lamina propria Lgr5-positive cells did not (27.7%), suggesting that there may be another lineage contributing to the Lgr5 cell population in the gastric mucosa (Table 3 and Supplement Figure 7). Lamina propria Lgr5+ cells were found to be vimentin positive, consistent with the leukocyte lineage indicated by the fact that these cells are also CD45+, however most vimentin+ lamina propria cells were Lgr5-negative (Supplement Figure 4).
DISCUSSION
This is the first study characterizing the distribution and oxidative DNA damage of Lgr5-positive epithelial cells, a population of gastric epithelial stem cells in human stomach, and their association with H. pylori gastritis and gastric cancer. A contribution of epithelial stem cells in gastric carcinogenesis, including H. pylori associated carcinogenesis, has been implied from studies in mouse models10, 11, 21, 22 and therefore, a role for epithelial stem cells in the development of human gastric cancer has been proposed23.
In this study, we observed that Lgr5-positive epithelial cells occur almost exclusively in the human gastric antrum, while smaller numbers were observed in oxyntic mucosa of gastric body or fundus. Similar to recently reported data by Simon et al. 12, we identified small numbers of Lgr5 positive cells in mucous glands of gastric mucosa. While we observed higher numbers of Lgr5+ cells in the deep mucous glands than in the neck of these glands, Simon et al. reported higher numbers in the neck regions. This difference in distribution may be explained by the fact that the two studies used different antibodies against Lgr5, which could differentially detect Lgr5 protein variants, or alternatively, by differences in the assessment of the neck region vs. deeper glandular region, as these are not rigidly delimited in the mucosa.
The numbers of Lgr5-positive epithelial cells were increased in the antral mucosa of patients with H. pylori gastritis and gastric cancer but not in patients without gastric cancer. This finding suggests that H. pylori infection associated factors may disturb the normal regulation of Lgr5-positive epithelial stem cells, leading to alteration of the normal pattern of asymmetric vs. symmetric division, with a possible increase in symmetric division. Lineage tracing experiments in the Lgr5-EGFP-IRES-creERT2 mouse model may be useful to address the underlying mechanisms. The presence of multiple Lgr5-positive cells in gastric glands has also been found in mouse stomach and it is still unclear how many of them represent bonafide stem cells. Lgr5 may mark a somewhat broader range of progenitors with proliferative ability, including those with less long-term self-renewal potential 10.
The near absence of Lgr5-positive cells in the gastric body suggests that other stem cell markers are needed to identify the stem cell population of oxyntic glands in the gastric body and fundus. In the mouse model, Barker et al11 showed that Lgr5 was expressed at the base of the gastric glands and that Lgr5-positive cells had the capacity of self-renewal and could build long-lived gastric antral units in vivo. Consistent with the findings reported in the mouse model, we observed that most Lgr5-positive cells were located at the base of gastric antral glands in human gastric mucosa, and confirmed a similar pattern of expression in mouse stomach. We also showed that the gastric cancer cell line SNU638 expresses Lgr5, providing a cell culture model for future studies of Lgr5 roles in gastric carcinogenesis. Future studies to determine whether co-cultures of AGS or SNU638 cells with H. pylori increase the number of Lgr5-postive cells, as was seen in patients infected with H. pylori, as well as whether different virulence types of H. pylori, such as the cagPAI, have differential effects in Lgr5+ cells, are warranted.
Although the association between H. pylori and gastric cancer is well established5, 24, 25, the critical molecular mechanisms and cellular targets that drive the development and progression of gastric cancers remain poorly understood. Factors known to play a role in H. pylori gastric carcinogenesis include the inflammation associated with the infection, increased oxidative DNA damage26, altered DNA repair in the epithelium, and increased mutagenesis of the epithelial genome7, 8, 27. In this study we showed that oxidative DNA damage of Lgr5-positive epithelial cells in gastric mucosa, determined by the levels of nuclear 8OHdG, was increased as compared to Lgr5-negative epithelial cells in patients with gastric cancer and H. pylori infection but not in gastric cancer patients without H. pylori infection. These findings are consistent with data showing that inflammation associated with H. pylori infection increased oxidative DNA damage26, and impaired DNA repair in the epithelium with increased mutagenesis of the epithelial genome7, 8, 27. Further, our data suggest that oxidative damage of Lgr5-positive epithelial cells may be mediated by the interaction of H. pylori with host susceptibility factors that affect the response of Lgr5-positive epithelial cells in gastric cancer patients. Since oxidative damage can lead to pathogenic mutations that may reprogram cells to a neoplastic pathway, the oxidative DNA damage caused by H. pylori infection in Lgr5-positive epithelial stem cells of particularly susceptible individuals may represent a critical mechanism of gastric carcinogenesis. This notion is consistent with reported lineage tracing studies in mice showing that a deletion mutant of the adenomatous polyposis coli gene (APC) in Lgr5-positive gastric progenitor cells was sufficient to give rise to antral tumors, whereas APC deletion in more differentiated cells did not 10, 11.
Of interest, the presence of intestinal metaplasia, which represents a metaplastic cell lineage in the early sequence that leads from H. pylori gastritis to gastric carcinoma, did not result in significant differences between oxidative DNA damage of Lgr5-positive vs. negative epithelial cells.
We also observed that in addition to Lgr5-positive epithelial cells within the gastric glands, there were rare scattered Lgr5-positive cells in the lamina propria, which showed plasmacytoid morphology. These findings are similar to those previously reported in colonic mucosa14, where rare scattered Lgr5-positive cells were observed in the lamina propria, suggesting they may represent hematopoietic lineage derived cells, as Lgr5 has previously been found to express in bone marrow, based on mRNA expression studies28.
To characterize the cellular lineages of Lgr5-positive cells in gastric mucosa we performed co-localization immunofluorescence studies. We showed that all Lgr5-positive cells in the gastric glands co-stained with cytokeratin and did not co-stain with vimentin, indicating their epithelial lineage. Co-immunofluorescence stains with Lgr5 and CD44, another gastric stem cell marker, showed similar findings as reported by Simon et al12, in that most CD44+ epithelial cells were Lgr5−, and essentially all Lgr5+ cells appeared to co-express CD44, consistent with a gastric stem cell lineage. In contrast, most Lgr5-positive cells in the lamina propria co-expressed CD45 and vimentin, indicating a leukocyte lineage, with a few cells revealing absent co-expression of CD45. The rare CD45+Lgr5− cells may represent an additional Lgr5-positive cell lineage, such as of hematopoietic origin. Surprisingly, the levels of 8OHdG in Lgr5-positive cells in lamina propria were higher in patients negative for gastric cancer and H. pylori, contrasting to the response seen in Lgr5-positive cells in gastric glands. The significance of this finding is unclear, however, it is possible that the Lgr5-positive lamina propria cells undergo faster turnover, and thus accumulate less 8OHdG adducts in patients with H. pylori gastritis and gastric cancer, who may show variable degrees of inflammation in the background gastric mucosa.
In summary, this is the first study characterizing the distribution of Lgr5-positive cells in human stomach and implicating Lgr5-positive epithelial stem cells in the adaptive response during H. pylori gastritis. This is characterized by an increase in number of possibly dysregulated Lgr5-positive epithelial stem cells, as they show increased sensitivity to oxidative DNA damage in response to H. pylori infection, particularly in patients with co-existing gastric cancer. Overall our data suggest a potential role for Lgr5-positive epithelial stem cells in H. pylori associated gastric carcinogenesis.
Supplementary Material
Supplement Figure 1.
Supplement Figure 2.
Supplement Figure 3.
Supplement Figure 4. Vimentin co-expression with LGR5
Supplement Figure 5. Co expression of CD44 and lgr5 in epithelial cells in antrum
Supplement Figure 6. Co expression of CD44 and lgr5 in lamina propria in antrum
Supplement Figure 7.
Supplement Table 1. Patients included in the study.
Supplement Table 2. Intestinal metaplasia scores in gastric mucosa. The extent of intestinal metaplasia in gastric mucosa was graded with a scale from 0 to 3. Foci of intestinal metaplasia, representing intestinal metaplasia of grades 1 to 2 were present in 17 of 33 gastric antral samples.
Supplement Table 3. Antibodies used in the study. *Directed against the second cytoplasmic domain of human Lgr5.
Supplement Table 4. Inflammation scores in gastric mucosa. Mononuclear inflammation (Mono).
Supplement Table 5. Levels of 8OHdG in lamina propria cells in antral mucosa. *All cases include: GC+, GC−, Hp+ and Hp− cases.
References
- 1.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Marshall BJ. Helicobacter pylori: the etiologic agent for peptic ulcer. JAMA. 1995;274:1064–1066. doi: 10.1001/jama.274.13.1064. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K. Gastritis and gastric cancer. Asia Gastroenterol Clin North Am. 2000;29:609–21. doi: 10.1016/s0889-8553(05)70133-3. [DOI] [PubMed] [Google Scholar]
- 4.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol; International workshop on the histopathology of gastritis; Houston. 1994; 1996. pp. 1161–81. [DOI] [PubMed] [Google Scholar]
- 5.Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971–6. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Kim JJ, Tao H, Carloni E, Leung WK, Graham DY, Sepulveda AR. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542–53. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Tao H, Park DI, Sepulveda JL, Sepulveda AR. Demonstration and Characterization of Mutations Induced by Helicobacter pylori Organisms in Gastric Epithelial Cells. Helicobacter. 2006;11:272–86. doi: 10.1111/j.1523-5378.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Sepulveda AR, Yao Y, Yan W, Park DI, Kim JJ, Gooding W, Abudayyeh S, Graham DY. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology. 2010;138:1836–44. doi: 10.1053/j.gastro.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Qiao XT, Gumucio DL. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855–65. doi: 10.1007/s00535-011-0413-y. [DOI] [PubMed] [Google Scholar]
- 11.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Simon E, Petke D, Boger C, Behrens HM, Warneke V, Ebert M, Rocken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One. 2012;7:e35486. doi: 10.1371/journal.pone.0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 14.Becker L, Huang Q, Mashimo H. Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. Scientific World Journal. 2008;8:1168–76. doi: 10.1100/tsw.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–6. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahm KB, Lee KJ, Choi SY, Kim JH, Cho SW, Yim H, Park SJ, Chung MH. Possibility of chemoprevention by the eradication of Helicobacter pylori: oxidative DNA damage and apoptosis in H. pylori infection. Am J Gastroenterol. 1997;92:1853–7. [PubMed] [Google Scholar]
- 17.Kuchino Y, Mori F, Kasai H, Inoue H, Iwai S, Miura K, Ohtsuka E, Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–9. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- 18.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–96. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Tao H, Kim JJ, Burkhead B, Carloni E, Gasbarrini A, Sepulveda AR. Alterations of DNA mismatch repair proteins and microsatellite instability levels in gastric cancer cell lines. Lab Invest. 2004;84:915–22. doi: 10.1038/labinvest.3700117. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizawa K, Jelezcova E, Brown AR, Foley JF, Nyska A, Cui X, Hofseth LJ, Maronpot RM, Wilson SH, Sepulveda AR, Sobol RW. Gastrointestinal hyperplasia with altered expression of DNA polymerase beta. PLoS One. 2009;4:e6493. doi: 10.1371/journal.pone.0006493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 22.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–24. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-- First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 25.Gologan A, Graham DY, Sepulveda AR. Molecular Markers in Helicobacter pylori-Associated Gastric Carcinogenesis. Clin Lab Med. 2005;25:197–222. doi: 10.1016/j.cll.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Farinati F, Cardin R, Russo VM, Busatto G, Franco M, Rugge M. Helicobacter pylori CagA status, mucosal oxidative damage and gastritis phenotype: a potential pathway to cancer? Helicobacter. 2003;8:227–34. doi: 10.1046/j.1523-5378.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 27.Sepulveda A, Goyal A. Helicobacter pylori and Gastric Neoplasms. In: Tan DaL G, editor. Advances in Surgical Pathology: Gastric Cancer. Lippincott Williams and Wilkins; 2011. pp. 22–37. [Google Scholar]
- 28.Takeda K, Kinoshita I, Shimizu Y, Matsuno Y, Shichinohe T, Dosaka-Akita H. Expression of LGR5, an intestinal stem cell marker, during each stage of colorectal tumorigenesis. Anticancer Res. 31:263–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1.
Supplement Figure 2.
Supplement Figure 3.
Supplement Figure 4. Vimentin co-expression with LGR5
Supplement Figure 5. Co expression of CD44 and lgr5 in epithelial cells in antrum
Supplement Figure 6. Co expression of CD44 and lgr5 in lamina propria in antrum
Supplement Figure 7.
Supplement Table 1. Patients included in the study.
Supplement Table 2. Intestinal metaplasia scores in gastric mucosa. The extent of intestinal metaplasia in gastric mucosa was graded with a scale from 0 to 3. Foci of intestinal metaplasia, representing intestinal metaplasia of grades 1 to 2 were present in 17 of 33 gastric antral samples.
Supplement Table 3. Antibodies used in the study. *Directed against the second cytoplasmic domain of human Lgr5.
Supplement Table 4. Inflammation scores in gastric mucosa. Mononuclear inflammation (Mono).
Supplement Table 5. Levels of 8OHdG in lamina propria cells in antral mucosa. *All cases include: GC+, GC−, Hp+ and Hp− cases.