Abstract
Friedreich ataxia is an autosomal recessive disorder that affects children and young adults. The mutation consists of a homozygous guanine-adenine-adenine trinucleotide repeat expansion that causes deficiency of frataxin, a small nuclear genome–encoded mitochondrial protein. Low frataxin levels lead to insufficient biosynthesis of iron-sulfur clusters that are required for mitochondrial electron transport and assembly of functional aconitase, and iron dysmetabolism of the entire cell. This review of the neuropathology of Friedreich ataxia stresses the critical role of hypoplasia and superimposed atrophy of dorsal root ganglia. Progressive destruction of dorsal root ganglia accounts for thinning of dorsal roots, degeneration of dorsal columns, transsynaptic atrophy of nerve cells in Clarke column and dorsal spinocerebellar fibers, atrophy of gracile and cuneate nuclei, and neuropathy of sensory nerves. The lesion of the dentate nucleus consists of progressive and selective atrophy of large glutamatergic neurons and grumose degeneration of corticonuclear synaptic terminals that contain γ-aminobutyric acid (GABA). Small GABA-ergic neurons and their projection fibers in the dentato-olivary tract survive. Atrophy of Betz cells and corticospinal tracts constitute a second intrinsic CNS lesion. In light of the selective vulnerability of organs and tissues to systemic frataxin deficiency, many questions about the pathogenesis of Friedreich ataxia remain.
Keywords: Dentate nucleus, Dorsal root ganglion, Frataxin, Friedreich ataxia, Iron, Spinal cord, Sural nerve
INTRODUCTION
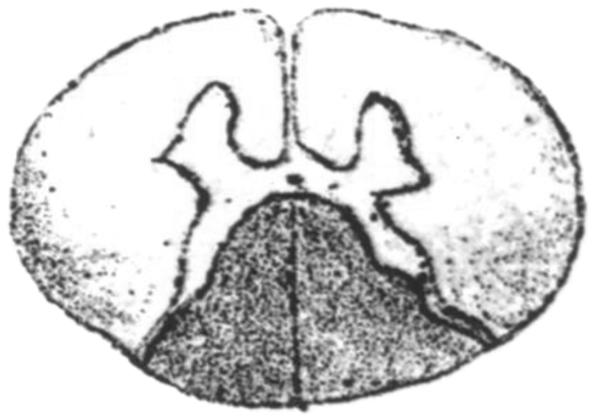
In a post scriptum (Nachtrag) to his 4 previous articles on ataxia, Friedreich (1825–1882) gave detailed gross and microscopic descriptions of the spinal cord, spinal gray matter, dorsal spinal roots, and medulla oblongata (1). He made only cursory reference to an intact cerebellum but emphasized the integrity of the cerebrum. Carmine and hematoxylin stains allowed him to conclude that the disease affected the dorsal columns over the entire length of the spinal cord and, to a lesser degree, the anterolateral columns. He also recognized that nerve fibers in dorsal roots were abnormally thin, including the intramedullary portions. Given this insightful observation, it is surprising that he did not recognize the small size of dorsal root ganglia (DRG). He was aware of severe neuronal loss in Clarke columns and spent considerable effort in the study of the medulla oblongata. He found thinning of the afferent fibers and abnormally small neurons in the gracile and cuneate nuclei. His interpretation that only a developmental delay could explain this constellation of abnormalities reveals his remarkable concept of the pathogenesis of the disease that now bears his name. Friedreich illustrated the spinal cord, but consistent with publishing practice at that time, his figures were bundled with those of other authors at the end of the volume (1). A portion of the upper lumbar spinal cord is reproduced here (Fig. 1). Friedreich’s illustrations of the spinal cord had an extraordinary impact on generations of neurologists and neuropathologists starting in the late 19th century and lasting through most of the 20th century. Friedreich ataxia (FRDA) originated as a disorder of the spinal cord, and many books and articles illustrated the degenerating long tracts as the critical abnormality. This review honors the contribution by Friedreich but departs from the emphasis on the spinal cord. At this time, the clinico-anatomic correlation must consider the combined developmental and degenerative processes of DRG (2) and sensory nerves (3). Progressive destruction of the dentate nucleus (DN) (4–8), atrophy of Betz cells (4), and degeneration of the corticospinal tracts are truly intrinsic lesions of the CNS.
FIGURE 1.
Reproduction of Figure 1d of the spinal cord made by Friedreich in 1877 (1). Figure 1 was reproduced from Friedreich (1), under license by Copyrights Clearance Center on behalf of Springer Science and Business Media. Note the “upside-down” rendition in comparison with current practice. Friedreich used a mixture of carmine and hematoxylin for sections of the spinal cord but also discussed favorable results with gold chloride. He noticed the much more severe involvement of the dorsal columns than the anterolateral columns and suggested that the lesions were actually continuous across the gray matter of the dorsal horns. This drawing also seems to identify the lack of Clarke columns that are normally visible on low-power cross sections at this level.
CLINICAL FEATURES AND GENETICS OF FRDA
Friedreich ataxia is a severe autosomal recessive disease of the CNS and peripheral nervous system. Clinical descriptions of large series of FRDA patients before the identification of the mutation in 1996 (9) are still valuable for practicing physicians and trainees (10–12). The mean ages of onset and death are approximately 11 and 38 years, respectively (10). Signs include gait ataxia; dysmetria; dysarthria; dysphagia; severe proprioceptive and superficial sensory loss; weakness and atrophy of the extremities; loss of muscle tone, spasticity, or combinations of both; depressed reflexes; extensor plantar reflexes; complex oculomotor disturbances; visual loss; and hearing deficit. Approximately 85% of FRDA patients succumb to cardiomyopathy (13), and approximately 25% have diabetes mellitus because of a combination of insulin deficiency and resistance (14). During the course of their illness, most FRDA patients develop kyphoscoliosis, and many require surgical correction. Progression of the neurologic disorder is variable. In view of the great complexity of neurologic manifestations, the decline of nervous system function is difficult to measure, but several rating scales have been successfully applied in clinical trials. All patients ultimately require wheel-chairs. In contrast to the elusive neurologic rating scales, echo-cardiography and, more recently, cardiac magnetic resonance imaging have become reliable techniques to quantify the progression of FRDA cardiomyopathy. Friedreich made detailed descriptions of the heart at the time of autopsy (15), but cardiomyopathy did not become an integral part of the recognized FRDA phenotype until the 1940s (16, 17). This review of the pathology of FRDA does not include heart or pancreatic β cells.
In most patients with FRDA, the mutation consists of a pathogenic homozygous guanine-adenine-adenine (GAA) trinucleotide repeat expansion in intron 1 of the frataxin gene (FXN) on chromosome 9q21 (9). The consequence of the mutation is deficiency of frataxin protein. All patients with FRDA have at least some frataxin. In mouse models of FRDA, the total absence of this small mitochondrial protein is lethal to the embryo (18). Very few patients with FRDA, estimated as 2% to 4%, are compound heterozygotes with a GAA expansion on 1 allele and a point mutation or deletion on the other (19). The clinical phenotype is very similar to that of homozygous GAA expansions, although optic atrophy may be more common (19). Genetic testing has become readily available, and clinicians are now able to diagnose FRDA in patients with late onset and long survival (20). Occasionally, the diagnosis is not suspected during life and is confirmed only by examination of deoxyribonucleic acid (DNA) extracted from autopsy tissues (21). Age of onset and death, and other measurements of the clinical phenotype in FRDA, bear a relationship to the length of the shorter GAA expansion in homozygotes (13,20), but various linear and nonlinear regression equations yielded a relatively low R2 (0.4–0.5). The most benign course seems to occur in patients with short GAA expansions on both alleles, and it is more likely that only the level of residual frataxin in affected tissues provides a reliable correlate with disease progression (21).
NEUROPATHOLOGY OF FRDA
This report is based on the systematic study of CNS and peripheral nervous system of 35 patients with FRDA. Tissues of 32 patients became available through nationwide donation programs of the National Ataxia Foundation and Friedreich’s Ataxia Research Alliance during a 19-year period. Collaborating neuropathologists provided samples of 3 additional cases. In 34 patients, the mutation was confirmed either during life or after extraction of DNA from frozen autopsy samples. In 1 patient, the diagnosis was based on clinical features and characteristic autopsy findings. The patient was not tested during life, and all harvested organs were fixed by formalin at the time of autopsy, precluding extraction of suitable DNA. The sex distribution was 23 female/12 male. Age of onset ranged from 2 to 50 years (mean ± SD, 13.4 ± 9.4 years); age of death ranged from 10 to 83 years (mean ± SD, 40.8 ± 20 years).
The mean disease duration was 27.7 ± 14.7 years. In all genotyped patients, the mutation was a homozygous GAA trinucleotide repeat expansion. Means were 805 ± 239 for the longer allele and 624 ± 251 for the shorter allele. The shortest expansion was 160; the longest was 1,200. In some cases, the expansions were identical, and the terms “long” and “short” do not apply. Brain and spinal cord specimens were available in all cases. Dorsal root ganglia were successfully harvested in only 17 FRDA patients, reflecting the difficulties with the identification of DRG because of their small size and distorted skeletal landmarks in kyphoscoliosis. Sural nerves were available from the autopsies of 10 FRDA patients, and 2 specimens were deemed fit for electron microscopy.
Dorsal Root Ganglia, Dorsal Spinal Roots, and Dorsal Column Nuclei
Two well-recognized features of DRG in FRDA are a reduction in the overall size of nerve cells and the presence of residual nodules (Fig. 2). Although the lack of large neurons is apparent on routine stains (Fig. 2A), conclusions about the mechanism of atrophy or hypoplasia are more difficult to achieve (13). In a systematic quantitative study, neuronal areas in DRG of 11 patients with juvenile-onset FRDA were 1,721 ± 776 μm2 (mean ± SD). In DRG of 5 patients with late-onset FRDA, the neuronal area was 2,142 ± 1,255 Km2. Juvenile onset was defined as onset before the age of 20 years and a disease duration of 20 years or longer. Late onset was defined as onset after age 20 years and a duration of 20 years or longer (21). The difference between the groups was not significant, although each group showed significantly smaller mean neuronal sizes than their control group (21). Neuronal sizes in the FRDA cases were not normally distributed, which confirmed the skewed distribution of nerve cell diameters in a single previously reported case (22).
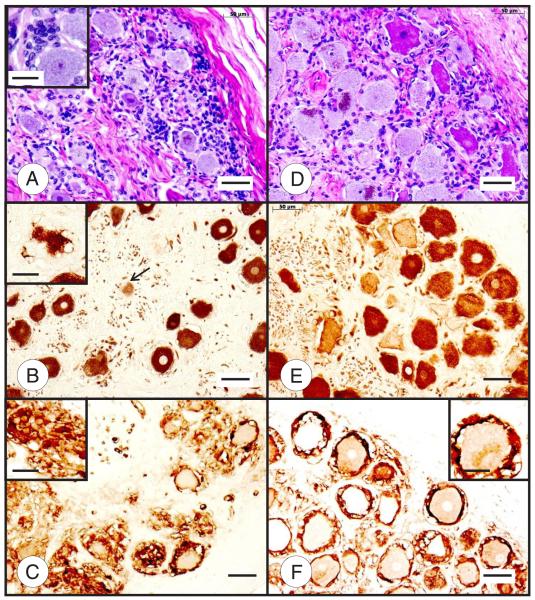
FIGURE 2.
Dorsal root ganglia (DRG) in Friedreich ataxia (FRDA) (A–C) and normal control (D–F). The DRG in FRDA (A) stands out by the smaller size of its neurons and subcapsular hypercellularity. The larger number of nuclei is caused by abundant satellite cells about neurons and in residual nodules ([A] insert). The heterogeneous staining intensity of normal DRG neurons with hematoxylin and eosin (D) and after immunohistochemistry (IHC) with anti–class III β-tubulin (E) is less evident in FRDA (A, B). The section of a DRG ganglion (B) also reveals the highly irregular outlines of several neurons caused by proliferation of satellite cells and ultimate total absorption of the neuron into residual nodules ([B] arrow and inset). The S100α immunostain reveals a single-cell layer of satellite cells around normal neurons in the normal DRG (F). In contrast, the much smaller neurons in FRDA display a highly irregular multilayered rim of these cells and replacement of neurons by nodules ([C] inset). Hematoxylin and eosin (A, D); IHC for class III β-tubulin (monoclonal antibody TUJ-1) (B, E); IHC for S100α (C, F). Scale bars = 50 μm; insets, 20 μm.
Nerve cells in normal DRG do not stain homogeneously with routine stains such as hematoxylin and eosin (Fig. 2D). This heterogeneity is more evident with neuron-specific immunostains, such as for class III β-tubulin (Fig. 2E), and applies to nerve cells across the entire size spectrum. The DRG of most FRDA patients show a degree of hypercellularity that is most prominent beneath the capsule (Fig. 2A). The cytoplasm of these cells is S100α immunopositive, identifying them as derivatives of satellite or Schwann cells (Fig. 2C). S100α-positive cells also make up the “residual nodules” that display traces of class III β-tubulin (Fig. 2B, arrow). It seems that proliferation of satellite cells to 2 or more layers around a neuron (Fig. 2C) precedes an apparent “invasion” of a dying nerve cell (Fig. 2B, inset). The term “residual nodule” is correct when applied to the progressive absorption of a nerve cell. The experiments of Nageotte (23) involving subcutaneous transplantation of rabbit DRG are not relevant to the pathogenesis of FRDA. Although the transplanted DRG underwent a series of pathologic changes in recipient animals, including sprouting of neurites, they obviously had lost all connections to peripheral nerves and spinal cord (23). Thus, the term “nodule of Nageotte,” as a synonym for the clusters of satellite cells in residual nodules, is no longer justified.
The pathology of DRG illustrated in Figure 2 does not support previous interpretations that only large- and intermediatesize neurons are vulnerable to FRDA (22). Size differences, especially in cases of short survival, are more readily explained by neuronal hypoplasia. As the disease progresses, superimposed neuronal destruction becomes manifest by satellite cell proliferation and formation of residual nodules. The nodules are not specific to FRDA, since they also occur in other diseases of DRG or as the result of simple aging.
A gelatinous appearance and thinning of dorsal spinal roots were recognized by Friedreich (1) and by many subsequent observers (2). Figure 3 illustrates the root lesion and its anterograde effects on dorsal columns and Clarke columns in the spinal cord (Fig. 3C–E). The main abnormalities in dorsal roots are lack of large axons (Fig. 3A) and thick myelinated fibers (Fig. 3B). These lesions present a dilemma in interpretation similar to the DRG, that is, is the process hypoplastic, atrophic, or both? Computer-aided histograms confirmed the shift of axonal perimeters to smaller sizes but failed to show a net reduction in the number of axons per unit area (2). Koeppen et al (2) reported a fiber density of 8,966 ± 3,641 axons/mm2 (mean ± SD) in 15 cases of FRDA versus 9,040 ± 973 in 11 normal controls. Despite the obvious lack of myelin in dorsal roots (Fig. 2B), the ratio of myelinated axon to all axons of 0.66 ± 0.16 was higher than that in normal controls (0.55 ± 0.16). Based on immunohistochemistry of S100α and laminin, the authors concluded that myelination of thin axons by fewer than normal Schwann cells caused the unusual density of thinly myelinated dorsal root fibers (2). Hughes et al (24), however, thought that the thin dorsal root fibers remained after larger myelinated axons had disappeared. On longitudinal sections, thin fibers could be traced to the posterior horns of the spinal gray matter. None of the fibers entered the dorsal columns. The authors did not comment on connections to Clarke column. This important study proved that FRDA affects parent neurons in the DRG in a selective manner. Although Goto and Hirano (25) studied only 2 patients with FRDA, their illustration of persistent substance P immunoreactivity in the substantia gelatinosa of the dorsal horns provided compelling evidence that functional fibers arising from DRG continue to make synapses in spinal gray matter. In contrast, connections to the dorsal nuclei of Clarke are lost (Fig. 3C–E). Diminutive size of this gray matter structure and severe neuronal loss are most readily explained by transsynaptic atrophy caused by failed trophic input from DRG during an extended period, presumably beginning during intrauterine life. The absence of neurons in the dorsal nuclei of Clarke also explains the transneuronal ascending degeneration of the dorsal spinocerebellar tract, which is largely uncrossed (Fig. 3C). Fiber loss in this tract blends with the degeneration of the lateral corticospinal tract (Fig. 3C), although the mechanisms of depletion are entirely different. Immunohistochemistry with an antibody to class III β-tubulin also shows a marked lack of axons in the dorsal columns (Fig. 3C). The degeneration of gracile and cuneate fasciculi seems more severe than that of the dorsal spinocerebellar tracts presumably because it is anterograde rather than transneuronal.
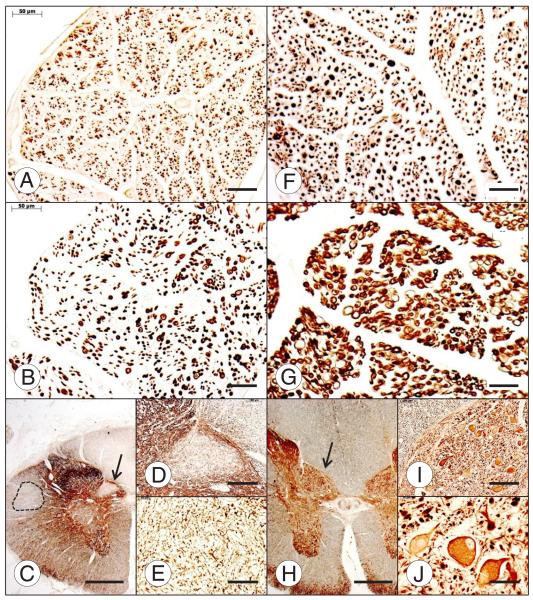
FIGURE 3.
Dorsal spinal roots and dorsal nucleus of Clarke column of the spinal cord in Friedreich ataxia (FRDA) (A–E) and normal control (F–J). In comparison with the normal control (F), the dorsal root in FRDA (A) lacks large axons, but many thin axons remain. Myelinated fibers in the dorsal root of FRDA are abundant (B), but they are much smaller than in the normal (G). The dorsal nucleus of Clarke column is devoid of neurons (C); arrow in (C) indicates fields shown at higher magnification in (D) and (E). There is also long tract degeneration of the dorsal column, dorsal spinocerebellar tract, and corticospinal tract (interrupted line) (C). In the control case (H–J), the spinal cord (H) and the nucleus dorsalis are larger than those in FRDA. The normal nucleus dorsalis displays typical large round nerve cells (H); arrow in (H) indicates fields shown at higher magnification in (I) and (J). Immunohistochemistry (IHC) for phosphorylated neurofilament protein (A, F); IHC for myelin basic protein (B, G); IHC for of class III β-tubulin (C–E, H–J). Scale bars = (A, B, E–G, J) 50 μm; (C, H) 1 mm; (D, I) 200 μm.
A previous study of DRG also included systematic analysis of ventral roots in FRDA (2). Although most observers concluded that ventral (motor) roots in FRDA are normal, some patients with the disease have fibrillation on electromyograms. Therefore, a ventral root lesion would not be surprising. In the cited study, histograms of the anterior roots in 15 FRDA patients showed a mild shift to smaller axons, possibly representing modest anterior horn disease (2).
Friedreich (1) and others commented on the smallness of the medulla oblongata. This thinning is largely caused by shrinkage of the gracile and cuneate nuclei rather than the medullary pyramids. Figure 4 illustrates transsynaptic atrophy of the gracile and lateral cuneate nuclei in selected FRDA cases. The transsynaptic mechanism of the dorsal column nuclei is supported by synaptophysin stains that reveal a great numerical reduction of terminals (25). Neuronal atrophy in gracile and cuneate nuclei is less severe but is otherwise analogous to nerve cell loss in Clarke column. Given the extraordinary impact of DRG disease in FRDA on “downstream” structures of spinal cord and brainstem, it seems appropriate to emphasize that the main damage to the spinal cord is secondary. Only the degenerative changes of the corticospinal tracts should be attributed to an intrinsic lesion of the CNS (see later).
FIGURE 4.
Neuronal atrophy in gracile and lateral cuneate nuclei in Friedreich ataxia (FRDA). Normal gracile nucleus (A) and normal lateral cuneate nucleus (B). FRDA, gracile nuclei are shown in (C) and (E); FRDA, lateral cuneate nuclei are shown in (D) and (F). Panels (C, D) and (E, F) are from 2 different FRDA cases. Immunohistochemistry of nonphosphorylated neurofilament protein. Levels of the medulla oblongata and location of the nuclei were identified based on Olszewski and Baxter (63). Scale bar = 50 μm.
Sensory Peripheral Nerves
Patients with FRDA almost invariably have sensory peripheral neuropathy that is more severe in the legs. Sural nerve biopsies have provided abundant information on sensory peripheral nerves in FRDA (26). Genetic testing, however, has made diagnostic sural nerve biopsy obsolete; the images shown in Figure 5 are based on nerves harvested at autopsy. The parent cell bodies of peripheral sensory nerves and dorsal spinal roots are located in DRG; therefore, it is not surprising that nerve and root lesions display similarities: persistence of thin axons (Fig. 5A), lack of large myelin sheaths (Fig. 5A, C), and greatly reduced numbers of S100α-positive Schwann cells (Fig. 5B). The comparison must take into consideration the much greater distance of the nerve from DRG. There is a major difference in the degree of myelination of sural nerves (Fig. 5A) when compared with dorsal roots (Fig. 3B). Jitpimolmard et al (27) found only a minor reduction of myelinated fibers in the dorsal roots of a single case of FRDA. In contrast, the lack of myelin in the sural nerve at the ankle reached nearly 50%. This extent of myelination is actually high for FRDA and may reflect their patient’s benign course over 52 years. Morral et al (26) performed systematic computer-assisted counts of axons and PMP22-positive myelin sheaths in sural nerves of 6 typical FRDA patients. Mean counts of axons per square millimeter in FRDA (27,464 ± 8,443) did not differ from controls (26,268 ± 5,267), but myelinated axons were significantly reduced in number: FRDA, 2,819 ± 1,046/mm2 versus controls, 9,206 ± 1,927/mm2. In addition to the numerical reduction in myelinated fibers by 69.4%, there was thinning of axons and the remaining myelinated fibers on histograms. Antibodies to 2 other proteins of peripheral nerve myelin (myelin basic protein; peripheral myelin protein P0) gave similar results, thereby militating against a selective failure of myelin sheath assembly caused by lack of one of these proteins (26).
FIGURE 5.
Sural nerve abnormalities in Friedreich ataxia (FRDA). (A, D) Double-label immunofluorescence of peripheral myelin protein P0 (Quantum Red) and axonal class III β-tubulin (Alexa 488, green) of the sural nerve in FRDA (A) shows persistence of axons, although axonal diameters are reduced and myelin is deficient compared with those of the normal (D). Larger myelinated fibers are entirely absent (A). (B, E) Few S100α-immunoreactive Schwann cells remain in the sural nerve of FRDA (B) in contrast to the normal control (E). (C, F) Electron microscopy of the nerve in FRDA confirms lack of thick myelin sheaths and an abundance of thin unmyelinated axons surrounded by tortuous Schwann cell processes ([C] arrow) in contrast to the normal control (F). Scale bars = (A, D) 25 μm; (B, E) 20 μm; (C, F) 5 μm. Dr Benjamin B. Gelman (Galveston, TX) provided the electron micrograph shown in (C).
The lack of S100α-positive cells of sural nerves in FRDA (Fig. 5B) may be more important than heretofore realized. According to Stefansson et al (28), Schwann cells surrounding myelinated and unmyelinated axons express this protein (28). The large discrepancy between class III β-tubulin (Fig. 5A) and S100α (Fig. 5B) points to defective interaction of Schwann cells and axons (26). Morral et al (26) also reported that Schwann cells in sural nerves of FRDA patients express abundant peripheral nerve laminin (laminin 2), implying intact basement membranes. Taken together, the evidence supports lack of myelination and myelin repair beginning in youth and superimposed progression because of axonal disease (29). The most compelling evidence for progression arises from a comparison of sural nerve myelination in the biopsies of 14 young patients with FRDA (mean age, 10.5 years) (30) and the autopsy samples reported by Morral et al (26) (mean age, 37 years). The calculated deficit in myelinated fibers rose from 46% to 69.4%.
Dentate Nucleus and Its Connections
The major lesion of the CNS in FRDA is located in the DN. Atrophy of the DN and its efferent myelinated fibers is grossly visible on sagittal slices of the cerebellar hemisphere (31). The collapse of the gray matter structure is even more evident when fixed or fresh slices are stained for iron (13). Atrophy of the superior cerebellar peduncles is also evident in slices and stained sections of upper pons and lower midbrain. Several studies have addressed the selective vulnerability of DN neurons to FRDA (8, 21, 31–32). The availability of antibodies to glutamic acid decarboxylase (GAD), vesicular glutamate transporters 1 and 2 (VGluT1; VGluT2), and glycine transporter 2 (GlyT2) has made it possible to examine the relative importance of neurohumoral synaptic transmission by the respective transmitters γ-aminobutyric acid (GABA), glutamate, and glycine in the structures of the cerebellum and inferior olivary nuclei that are of interest in FRDA. The normal DN contains large and small neurons (Fig. 6D), but in FRDA, only large nerve cells disappear as the disease progresses. In contrast to DRG, which are subject to hypoplasia and superimposed atrophy, the DN is probably entirely normal before onset of the disease. Direct evidence of an intact DN during childhood or adolescence is, however, still limited (21). Although subtotal loss of neurons in the DN in FRDA should give rise to transsynaptic degeneration of the contralateral inferior olivary nuclei, the nerve cells in this gray matter ribbon remain intact. The likely explanation for this phenomenon is the resistance of most if not all small neurons of the DN in FRDA (Fig. 6A). They are the origin of crossed dentato-olivary fibers that provide inhibitory GABA-ergic input and putative trophic substances (8). Two thirds of the afferent terminals about large and small neurons in the DN derive from Purkinje cells. Neuronal loss in the DN is accompanied by grumose degeneration, which consists of proliferation of modified GABA-ergic terminals about the dendrites of dying DN neurons (Fig. 6B). Peculiarly, neuronal atrophy in the DN does not generate a retrograde reaction in Purkinje cells, and the cerebellar cortex in FRDA is generally intact. The resistance of Purkinje cells to retrograde atrophy may be caused by persistence of corticonuclear connections to surviving small neurons in the DN or collateral axonal sprouting and synaptic connections with other neurons in the cerebellar cortex. It is remarkable that undercutting of the cerebellar cortex in experimental animals provides insight into the fate of the human cerebellar cortex in FRDA (33). Figure 6C shows severe depletion of frataxin-reaction product in the DN of FRDA. Neurons and their axosomatic and axodendritic terminals are rich in mitochondria, which is evident on immunohistochemical visualization of mitochondrial complex V (F0Fβ-1) (31). Mitochondrial complex V reaction product persists in the clusters of grumose degeneration, although frataxin disappears (31). Therefore, loss of frataxin from synaptic terminals seems closely related to grumose degeneration. Double label immunofluorescence shows clusters of greatly modified terminals with anti-synaptophysin, whereas anti-frataxin yields very little or no reaction product (13). Frataxin deficiency is not the only cause of grumose degeneration. This bizarre modification of terminals is nonspecific and occurs in other disorders of the DN, such as spinocerebellar ataxia type 3 (Machado-Joseph disease) and progressive supranuclear palsy.
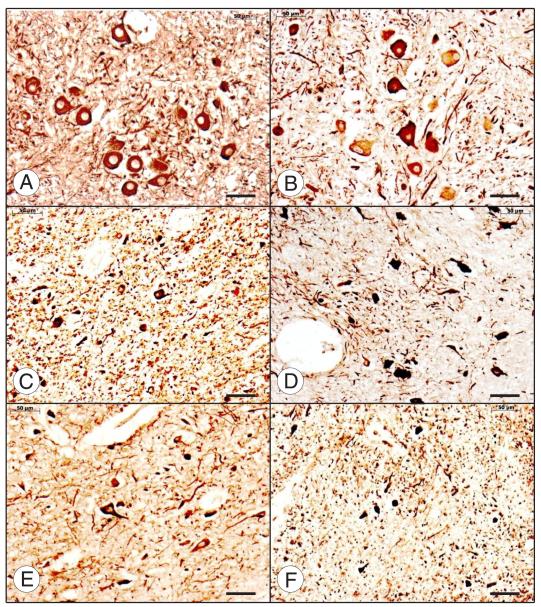
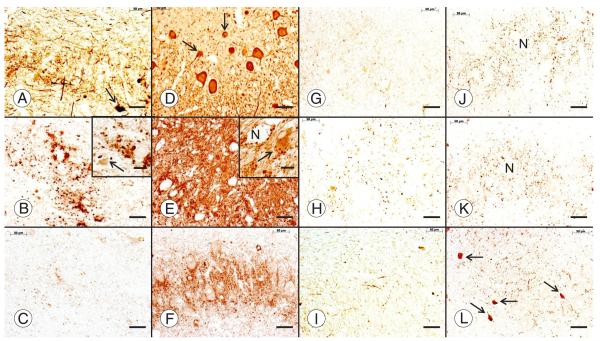
FIGURE 6.
The dentate nucleus (DN) in Friedreich ataxia (FRDA). Friedreich ataxia (A–C, G–I); normal control (D–F, J–L). All stains are immunohistochemical preparations: neuron-specific enolase (A, D); glutamic acid decarboxylase (GAD) (B, E); frataxin (C, F); vesicular glutamate transporter (VGluT1) (G, J); vesicular glutamate transporter 2 (VGluT2) (H, K); glycine transporter 2 (GlyT2) (I, L). Friedreich ataxia causes loss of large neurons (A), whereas small neurons persist ([A, D] arrows). Neuronal loss in FRDA causes an overall reduction of GABA (γ-aminobutyric acid)-ergic terminals in the DN (B) and grumose degeneration of the remaining nerve endings ([B] inset). Small neurons of the normal DN ([E] inset, arrow) and the DN in FRDA ([B] inset, arrow) display pale GAD reaction product. Numerous frataxin-positive axodendritic and axosomatic terminals outline large and small neurons of the normal DN (F). In FRDA, only traces of frataxin reaction product remain (C). Vesicular glutamate transporter 1 and VGluT2 reaction products reveal negative images of large neurons (N in [J] and [K], respectively). Vesicular glutamate transporter 1– and VGluT2-reactive terminals are less abundant in FRDA ([G] and [H], respectively), and negative images of neurons are absent. The normal DN contains GlyT2 reaction product that is as abundant as VGluT1 and VGluT2 (L). Arrows in (L) indicate the somata of 4 small glycinergic nerve cells in the normal DN. In FRDA, GlyT2 (I) is much less prominent than in the control. Scale bars = 50 μm; insets, 20 μm.
Figure 6 also shows the fate of glutamatergic afferents and glycinergic neurons in the DN of FRDA. Vesicular glutamate transporter 1 (Fig. 6G, J)– and VGluT2 (Fig. 6H, K)-positive terminals are thought to derive from collaterals of VGluT2-positive climbing and VGluT1- and VGluT2-positive mossy fibers. Depletion in FRDA (Fig. 6G, H) may be the result of lost targets, namely, the large neurons in the DN.
The presence of GlyT2-positive neurons (Fig. 6L) proves that the group of small nerve cells in the normal DN is heterogeneous, adding even greater complexity to the synaptic circuitry of the normal DN and the DN in FRDA (34). Interpretation of GlyT2 reaction product is difficult because only optimally fixed paraffin-embedded tissue samples provide an adequate reaction product. Nevertheless, it seems that FRDA causes loss of glycinergic nerve cells, varicose axons, and terminals in the DN (Fig. 6I). Glycinergic neurons in the human DN may be similar to those in rodents and represent both interneurons and projection neurons (34). Some may express only glycine; others, glycine and GABA (34).
Corticospinal Tracts
Most patients with FRDA show subtle signs of upper motor neuron disease such as Babinski signs on a background of diffuse weakness and hypotonia. Some patients, however, have spasticity of the legs and hyperreflexia. Wrist extension and distal finger contractures, first illustrated by Spiller (5), are almost always present and should also be interpreted as upper motor disease. Corticospinal tract degeneration is the second purely intrinsic CNS lesion of FRDA but has not been studied recently (4–7). Degenerating fibers are difficult to detect above pontine levels (6–7), but lack of Betz cells in the motor cortex seems invariable (in 11 of 11 cases) (7). There is a deficiency of these cells in layer V of the motor cortex (Fig. 7A, B), a reduction of the size of the medullary pyramid and paucity of myelinated fibers (Fig. 7C and inset), and marked atrophy of the corticospinal tract at the level of the thoracic spinal cord (Fig. 7D). The corticospinal tract in FRDA resembles the upper motor neuron lesion of amyotrophic lateral sclerosis and suggests that a similar “dying back neuropathy” is at work. The uncrossed corticospinal tract may also be affected in FRDA.
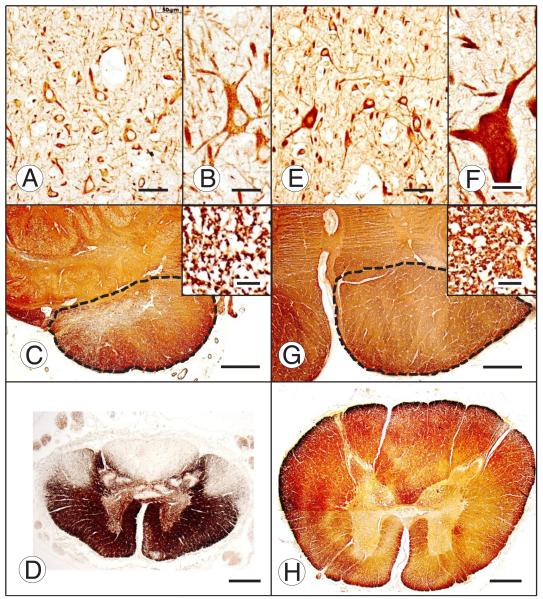
FIGURE 7.
Motor cortex and corticospinal tract in Friedreich ataxia (FRDA). Friedreich ataxia (A–D); normal control (E–H); motor cortex immunostained for nonphosphorylated neurofilament protein (A, B, E, F); medullary pyramids immunostained for myelin basic protein (MBP) ([C], [G], and insets); upper lumbar spinal cord, immunostain for MBP (D, H). Most neurons in layer V of the motor cortex in FRDA (A) are comparable in size to those in the normal (E), but Betz cells are absent. The largest pyramidal neuron in layer V of FRDA is shown in (B). A Betz cell in the normal control is shown in (F). The limits of the medullary pyramids are outlined by interrupted lines. The pyramid in FRDA (C) is diminutive in comparison with the normal control (G), and the density of myelinated fibers is lower (insets in [C] and [G], respectively). Myelin basic protein immunostain of the upper lumbar cord shows the small cross-sectional area in FRDA (D) in comparison with the control specimen (H) and loss of myelinated fibers in dorsal columns, dorsal spinocerebellar tracts, and corticospinal tracts. Scale bars = (A, E) 50 μm; (B), (F) and insets in (C) and (G) 20 μm; (C, D, G, H) 1 mm.
The small size of the spinal cord stands out in contrast to the normal profile (Fig. 7D, H). Koeppen (35) presented a correlation of cross-sectional areas of the thoracic spinal cord and age of onset. The areas were small in all FRDA patients, irrespective of age of onset (2–37 years), measuring only 17.4 ± 4 mm2 in FRDA versus 36 ± 5 mm2 in controls (mean ± SD). It is unlikely that acquired degeneration of dorsal columns and dorsal spinocerebellar and corticospinal tracts, and shrinkage of the dorsal nuclei of Clarke are sufficient to explain the dramatic thinning of the spinal cord. Friedreich’s interpretation of developmental delay may now receive belated confirmation (1).
PATHOGENESIS OF FRDA
Frataxin Deficiency, GAA Triplet Repeat Expansion, and the Complex Pathologic Phenotype
There is a large gap between the seemingly straightforward deficiency of a small protein, frataxin, and the peculiar selective vulnerability of the nervous system, heart, and endocrine pancreas. Although frataxin is encoded by the nuclear genome, FRDA is a mitochondrial disease. Frataxin must be imported into the mitochondrial interior and processed to a mature form of 14.2 kDa through a series of steps (36). Frataxin has been the focus of interest in FRDA research for several years, and its major normal function is the generation of iron-sulfur clusters (37–39). Iron-sulfur clusters are needed for the operation of mitochondrial complexes I, II, and III, aconitase, ferrochelatase, and several other enzymes.
The GAA trinucleotide repeat expansion in FRDA causes a transcriptional block due to formation of triplex DNA and DNA-ribonucleic acid (RNA) hybrids (40). Beyond impaired transcription, epigenetic mechanisms have been established or proposed. These mechanisms are entrapment of the frataxin gene (FXN) in heterochromatin caused by histone hypoacetylation (41, 42) and abnormal DNA methylation in CpG islands of the gene upstream and downstream of the GAA triplet expansion (43, 44). An exciting new hypothesis may lead to the understanding of why symptoms of FRDA are delayed to childhood or adolescence (45). Under this concept, somatic GAA triplet repeat expansion occurs throughout life. For the neural phenotype of FRDA, the remarkable expansion in DRG is especially noteworthy (45). Although frataxin levels were not measured, it may be assumed that progressive impairment of transcription of FXN and frataxin deficiency may indeed follow the somatic expansion. Control of the expansion also seems dependent on the activity of mismatch repair enzymes (46, 47). It is possible that one or more of these enzymes actually promote somatic GAA expansion, triggering the onset of FRDA (47).
Iron and Other Metals in the Pathogenesis of FRDA
Many unresolved issues remain about the role of iron-mediated oxidative injury to vulnerable tissues in FRDA. Campuzano et al (9) recognized the relationship of frataxin to iron homeostasis at a time when the accumulation of tiny iron-reactive granules in the heart of patients with FRDA was well established (48). In 1 case of FRDA, iron-positive inclusions were present in a cardiac biopsy sample at the age of 9 years, although myocardial fibrosis was absent (49). In the autopsy specimen collected 17 years later, the abundance of iron was similar, but the cardiac lesion had advanced to more significant fiber hypertrophy and fibrosis (49). Therefore, iron is an early player in the pathogenesis of FRDA, at least in the cardiomyopathy of the disease complex. It is widely held that iron excess occurs only in mitochondria, perhaps at the expense of cytosolic iron (50). Possible oxidative injury is the rational basis for antioxidant therapy (51), although Bayot et al (52) considered iron accumulation late and inconsistent. Electron microscopy of an FRDA heart after enhancement of ferritin by bismuth subnitrate localized reaction product to mitochondria; and an antibody to mitochondrial ferritin revealed reactive granules in a small percentage of fibers (49). More recent studies from the same laboratory on FRDA hearts, using quantitative X-ray fluorescence and ferritin immunohistochemistry, revealed that the measurable ironexcess is cytosolic rather than mitochondrial (53). These observations do not invalidate mitochondrial iron excess in FRDA but suggest that iron excess in the cytosol contributes to oxidative damage outside of mitochondria.
The vulnerability of the DN to FRDA was thought to be related to its high iron content, although other iron-rich regions of the CNS escape damage (31). A reexamination of iron in the DN by X-ray fluorescence and ferritin immunohistochemistry showed the bulk of iron in the white matter of DN hilum and fleece of Stilling (32). In contrast, X-ray fluorescence detected copper and zinc in close association with the gray matter ribbon of the normal DN. In FRDA, the 3 metals became widely colocalized, raising the possibility of combined metal toxicity, especially because of the iron-copper combination. The available evidence does not exclude iron in the pathogenesis of FRDA in the DN. Instead, the role of iron may be more complex than being a reactant in a Fenton-type reaction (54).
Transgenic Mouse Models of FRDA
Generating mouse models emulating human FRDA has proven difficult. The models must generate frataxin deficiency without completely knocking out the murine frataxin gene (fxn). At the present time, 8 mouse models of FRDA are available from Jackson Laboratories, Bar Harbor, ME (55). Martelli et al (56) recently reviewed their advantages and disadvantages. As in FRDA of humans, murine models must retain some frataxin to survive beyond gestation (18), but moderate expansions of GAA trinucleotide repeats do not cause an equivalent pathologic phenotype. Even a reduction of frataxin to 30% of normal, matching the estimated protein levels in humans with FRDA, had no effect on the well-being of the animals. Deletion of murine fxn and introduction of homozygous human GAA-expanded FXN caused vacuolation of DRG neurons and iron-reactive inclusions in the heart (57). Puccio et al (58) generated a dramatic cardiac phenotype by deleting exon 4 of the murine fxn gene and targeting the deletion to striated muscle through the muscle creatine kinase promoter. This model is being used in the study of FRDA-like cardiomyopathy (50, 59), but a comparable approach to the nervous system by involving the neuron-specific enolase promoter spared DRG. Peculiarly, the cerebral cortex and peripheral sensory nerve were affected (58). To date, none of the models have generated lesions of DRG or DN that are fully comparable to that of the human disease.
Current State of FRDA Therapy
Friedreich’s Ataxia Research Alliance maintains a list of ongoing phase I, II, and III trials, dividing goals into decreasing oxidative stress, lowering iron toxicity, increasing iron-sulfur clusters, raising frataxin levels or frataxin gene expression, applying gene therapy, replacing frataxin, and search for drug candidates by high-throughput drug screening (60). The anti-oxidant idebenone reportedly benefits FRDA cardiomyopathy, but well-controlled studies did not yield a positive effect on the neurologic phenotype (51, 61). A few case reports of cardiac transplantation in FRDA have appeared (62). Although causative therapy of FRDA is not yet available, patients clearly benefit from drug treatment of their heart disease and diabetes mellitus, physical therapy, and surgical control of progressive scoliosis. Clinicians now have access to a firm genetic diagnosis and may gain insight into the prognosis of FRDA by considering the length of the GAA trinucleotide repeat expansion.
ACKNOWLEDGMENTS
The work leading to this review was performed in the laboratories of the Veterans Affairs Medical Center, Albany, NY, and Albany Medical College. R. Liane Ramirez and Sarah T. Bjork provided expert technical assistance.
This work was supported by the National Institutes of Health, Bethesda, MD (R01 NS069454); Friedreich’s Ataxia Research Alliance, Downingtown, PA; National Ataxia Foundation, Minneapolis, MN; and Neurochemical Research, Inc., Glenmont, NY.
REFERENCES
- 1.Friedreich N. Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen. Nachtrag. Virchows Arch Pathol Anat Physiol Klin Med. 1877;70:140–52. [Google Scholar]
- 2.Koeppen AH, Morral JA, Davis AN, et al. The dorsal root ganglion in Friedreich’s ataxia. Acta Neuropathol. 2009;118:763–76. doi: 10.1007/s00401-009-0589-x. [DOI] [PubMed] [Google Scholar]
- 3.Morral JA, Davis AN, Qian J, et al. Pathology and pathogenesis of sensory neuropathy in Friedreich’s ataxia. Acta Neuropathol (Berl) 2010;120:97–108. doi: 10.1007/s00401-010-0675-0. [DOI] [PubMed] [Google Scholar]
- 4.Mott FW. Case of Friedreich’s disease, with autopsy and systematic microscopical examination of the nervous system. Arch Neurol Psychiat (Lond) 1907;3:180–200. [Google Scholar]
- 5.Spiller WG. Friedreich’s ataxia. J Nerv Ment Dis. 1910;37:411–35. [Google Scholar]
- 6.Urich H, Norman RM, Lloyd OC. Suprasegmental lesions in Friedreich’s ataxia. Confin Neurol. 1957;17:360–71. doi: 10.1159/000105212. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer DR. Brain lesions in Friedreich’s ataxia. Can J Neurol Sci. 1979;6:173–76. doi: 10.1017/s0317167100119596. [DOI] [PubMed] [Google Scholar]
- 8.Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 2011;122:323–30. doi: 10.1007/s00401-011-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campuzano V, Montermini L, Moltò MD, et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–27. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 10.Harding AE. Friedreich’s ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589–620. doi: 10.1093/brain/104.3.589. [DOI] [PubMed] [Google Scholar]
- 11.Andermann E, Remillard GM, Goyer C, et al. Genetic and family studies in Friedreich’s ataxia. Can J Neurol Sci. 1976;3:287–301. doi: 10.1017/s0317167100025476. [DOI] [PubMed] [Google Scholar]
- 12.Barbeau A. Friedreich’s ataxia 1976—An overview. Can J Neurol Sci. 1976;3:389–97. doi: 10.1017/s0317167100025646. [DOI] [PubMed] [Google Scholar]
- 13.Koeppen AH. Friedreich’s ataxia: Pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cnop M, Igoillo-Esteve M, Rai M, et al. The central role and mechanism of β-cell dysfunction and death in Friedreich ataxia–associated diabetes. Ann Neurol. 2012;72:971–82. doi: 10.1002/ana.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedreich N. Ueber degenerative Atrophie der spinalen Hinterstränge. Virchows Arch Pathol Anat Physiol Klin Med. 1863;26:391–419. [Google Scholar]
- 16.Evans W, Wright G. The electrocardiogram in Friedreich disease. Brit Heart J. 1942;4:91–102. doi: 10.1136/hrt.4.3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell DS. Myocarditis in Friedreich’s ataxia. J Pathol Bacteriol. 1946;63:739–48. doi: 10.1002/path.1700580414. [DOI] [PubMed] [Google Scholar]
- 18.Cossée M, Puccio H, Gansmuller A, et al. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum Mol Genet. 2000;9:1219–26. doi: 10.1093/hmg/9.8.1219. [DOI] [PubMed] [Google Scholar]
- 19.Cossée M, Dürr A, Schmitt M, et al. Friedreich’s ataxia: Point mutations and clinical presentation of compound heterozygotes. Ann Neurol. 1999;45:200–6. doi: 10.1002/1531-8249(199902)45:2<200::aid-ana10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Dürr A, Cossée M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335:1169–75. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 21.Koeppen AH, Morral JA, McComb RD, et al. The neuropathology of late-onset Friedreich’s ataxia. Cerebellum. 2011;10:96–103. doi: 10.1007/s12311-010-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue K, Hirano A, Hasson J. Friedreich’s ataxia selectively involves the large neurons of the dorsal root ganglia. Trans Am Neurol Assoc. 1979;104:75–76. [PubMed] [Google Scholar]
- 23.Nageotte J. Greffe de ganglion rachidiens, survie deséléments nobles et transformation des cellules unipolaires en cellules multipolaires. Compt Rend Hebd Séances Mem Soc Biol; Paris, France: 1907. pp. 62–64. [Google Scholar]
- 24.Hughes JT, Brownell B, Hewer RL. The peripheral sensory pathway in Friedreich’s ataxia. Brain. 1968;91:803–18. doi: 10.1093/brain/91.4.803. [DOI] [PubMed] [Google Scholar]
- 25.Goto S, Hirano A. Immunohistochemical evidence for the selective involvement of dorsal root fibres in Friedreich’s ataxia. Neuropathol Appl Neurobiol. 1990;16:365–70. doi: 10.1111/j.1365-2990.1990.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 26.Morral JA, Davis AN, Qian J, et al. Pathology and pathogenesis of sensory neuropathy in Friedreich’s ataxia. Acta Neuropathol. 2010;120:97–108. doi: 10.1007/s00401-010-0675-0. [DOI] [PubMed] [Google Scholar]
- 27.Jitpimolmard S, Small J, King RHM, et al. The sensory neuropathy of Friedreich’s ataxia: An autopsy study of a case with prolonged survival. Acta Neuropathol. 1993;86:29–35. doi: 10.1007/BF00454895. [DOI] [PubMed] [Google Scholar]
- 28.Stefansson K, Wollmann RL, Moore RB. Distribution of S-100 protein outside the central nervous system. Brain Res. 1982;234:309–17. doi: 10.1016/0006-8993(82)90871-x. [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ, Lais AC. Evidence for segmental demyelination secondary to axonal degeneration in Friedreich’s ataxia. In: Kakulas BA, editor. Clinical Studies in Mycology; Proceedings of the International Congress on Muscle Diseases; Amsterdam, Netherlands. Excerpta Medica; 1974. pp. 253–63. [Google Scholar]
- 30.Ouvrier RA, McLeod JG, Conchin TE. Friedreich’s ataxia. Early detection and progression of peripheral nerve abnormalities. J Neurol Sci. 1982;55:137–45. doi: 10.1016/0022-510x(82)90095-8. [DOI] [PubMed] [Google Scholar]
- 31.Koeppen AH, Michael SC, Knutson MD, et al. The dentate nucleus in Friedreich’s ataxia: The role of iron-responsive proteins. Acta Neuropathol. 2007;114:163–73. doi: 10.1007/s00401-007-0220-y. [DOI] [PubMed] [Google Scholar]
- 32.Koeppen AH, Ramirez RL, Yu D, et al. Friedreich’s ataxia causes redistribution of iron, copper, and zinc in the dentate nucleus. Cerebellum. 2012;11:845–60. doi: 10.1007/s12311-012-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi F, Gianola S, Corvetti L. The strange case of Purkinje axon regeneration and plasticity. Cerebellum. 2006;5:174–82. doi: 10.1080/14734220600786444. [DOI] [PubMed] [Google Scholar]
- 34.Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55. doi: 10.1007/s12311-009-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeppen AH. Neuropathology of the inherited ataxias. In: Manto U-B, Pandolfo M, editors. The Cerebellum and Its Disorders. Cambridge University Press; Cambridge, UK: 2002. pp. 387–405. [Google Scholar]
- 36.Schmucker S, Argentini M, Carelle-Camels N, et al. The in vivo mitochondrial two-step maturation of human frataxin. Hum Mol Genet. 2008;17:3521–31. doi: 10.1093/hmg/ddn244. [DOI] [PubMed] [Google Scholar]
- 37.Rötig A, De Lonlay P, Chretien D, et al. Aconitase and mitochondrial iron-sulfur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–17. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 38.Mühlenhoff U, Richhardt N, Ristow M, et al. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum Mol Genet. 2002;11:2025–36. doi: 10.1093/hmg/11.17.2025. [DOI] [PubMed] [Google Scholar]
- 39.Vaubel RA, Isaya G. Iron-sulfur cluster synthesis, iron homeostasis and oxidative stress in Friedreich ataxia [published online ahead of print August 11, 2012] Mol Cell Neurosci. doi: 10.1016/j.mcn.2012.08.003. doi: 10.1016/j.mcn.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabczyk E, Usdin K. The GAA-TTC triplet repeat expanded in Friedreich’s ataxia impedes transcription elongation by T7 RNA polymerase in a length and supercoil dependent manner. Nucleic Acids Res. 2000;28:2815–22. doi: 10.1093/nar/28.14.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman D, Jenssen K, Burnett R, et al. Histone deacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat Chem Biol. 2006;2:551–58. doi: 10.1038/nchembio815. [DOI] [PubMed] [Google Scholar]
- 42.Kim E, Napierala M, Dent SY. Hyperexpansion of GAA repeats affect post-initiation steps of FXN transcription in Friedreich’s ataxia. Nucleic Acids Res. 2011;39:8366–77. doi: 10.1093/nar/gkr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mahdawi S, Pinto RM, Ismail O, et al. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet. 2008;17:735–46. doi: 10.1093/hmg/ddm346. [DOI] [PubMed] [Google Scholar]
- 44.Evans-Galea MV, Carrodus N, Rowley SM, et al. FXN methylation predicts expression and clinical outcome in Friedreich ataxia. Ann Neurol. 2012;71:487–97. doi: 10.1002/ana.22671. [DOI] [PubMed] [Google Scholar]
- 45.De Biase I, Rasmussen A, Endres D, et al. Progressive GAA expansions in dorsal root ganglia of Friedreich’s ataxia patients. Ann Neurol. 2007;61:55–60. doi: 10.1002/ana.21052. [DOI] [PubMed] [Google Scholar]
- 46.Ezzatizadeh V, Pinto RM, Sandi C, et al. The mismatch repair system protects against intergenerational GAA repeat instability in a Friedreich ataxia mouse model. Neurobiol Dis. 2012;46:165–71. doi: 10.1016/j.nbd.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halabi A, Ditch S, Wang J, et al. DNA mismatch repair complex MutSA promotes GAA•TTC repeat expansion in human cells. J Biol Chem. 2012;287:29958–67. doi: 10.1074/jbc.M112.356758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamarche JB, Côté M, Lemieux B. The cardiomyopathy of Friedreich’s ataxia. Morphological observations in 3 cases. Can J Neurol Sci. 1980;7:389–96. doi: 10.1017/s0317167100022927. [DOI] [PubMed] [Google Scholar]
- 49.Michael S, Petrocine SV, Qian J, et al. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich’s ataxia. Cerebellum. 2006;5:257–67. doi: 10.1080/14734220600913246. [DOI] [PubMed] [Google Scholar]
- 50.Huang ML, Becker EM, et al. Elucidation of the mechanism of mitochondrial iron loading in Friedreich’s ataxia by analysis of a mouse mutant. Proc Natl Acad Sci USA. 2009;106:16381–86. doi: 10.1073/pnas.0906784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearny M, Orrell RW, Fahey M, et al. The Cochrane Collaboration. Wiley; Hoboken, NJ: 2012. Antioxidants and Other Pharmacological Treatments for Friedreich Ataxia [Review] pp. 1–27. [DOI] [PubMed] [Google Scholar]
- 52.Bayot AZ, Santos R, Camadro J-M, et al. Friedreich’s ataxia: The vicious circle hypothesis revisited. BMC Med. 2011;9:112. doi: 10.1186/1741-7015-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramirez RL, Qian J, Santambrogio P, et al. The relation of cytosolic iron excess to the cardiomyopathy of Friedreich’s ataxia. Am J Cardiol. 2012;110:1820–7. doi: 10.1016/j.amjcard.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein S, Myerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15:435–45. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 55.Rare and Orphan Disease Center, Jackson Laboratories [Accessed December 17, 2012]; www.jax.org/rare.
- 56.Martelli A, Napierala M, Puccio H. Understanding the genetic and molecular pathogenesis of Friedreich’s ataxia through animal and cellular models. Dis Model Mech. 2012;5:165–76. doi: 10.1242/dmm.008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Mahdawi S, Pinto RM, Varshney D, et al. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal cardiac pathology. Genomics. 2006;88:580–90. doi: 10.1016/j.ygeno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puccio H, Simon D, Cossée M, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet. 2001;27:181–86. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 59.Payne RM, Pride PM, Babbey CM. Cardiomyopathy of Friedreich’s ataxia: Use of mouse models to understand human disease and guide therapeutics. Pediatr Cardiol. 2011;32:366–78. doi: 10.1007/s00246-011-9943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedreich’s Ataxia Research Alliance [Accessed December 17, 2012]; Available at: http://www.curefa.org/pipeline.htlml.
- 61.Perlman SL. A review of Friedreich ataxia clinical trial results. J Child Neurol. 2012;27:1217–22. doi: 10.1177/0883073812453872. [DOI] [PubMed] [Google Scholar]
- 62.Yoon G, Soman T, Wilson J, et al. Cardiac transplantation in Friedreich ataxia. J Child Neurol. 2012;27:1193–96. doi: 10.1177/0883073812448229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. JB Lippincott; Philadelphia, PA: 1954. [Google Scholar]