Abstract
Objective:
Impulsivity is associated with increased marijuana use and subsequent marijuana-related problems among marijuana users. In addition, single nucleotide polymorphisms (SNPs) in the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes have been associated with cannabis-related phenotypes. This exploratory study tested whether the association between different aspects of impulsivity and the number of marijuana-related problems among users is explicated by variation in these putative cannabinoid-related genes.
Method:
A total of 151 young adult regular marijuana users (used on M = 41.4% of the prior 60 days, SD = 24.3%) provided DNA and completed measures of trait (Barratt Impulsiveness Scale) and behavioral impulsivity (Stop Signal Task and Delay Discounting Questionnaire), as well as a self-report of marijuana-related problems. Three CNR1 and five FAAH SNPs were genotyped, tested for haplotype blocks, and subsequently examined for association with phenotypes described above.
Results:
CNR1 variation significantly moderated the association between trait-level, but not behavioral, impulsivity and marijuana-related problems, such that the combination of higher trait impulsivity and CNR1 variation was associated with a greater number of marijuana-related problems. In contrast, there were no significant FAAH by impulsivity interactions; however, there was a main effect of FAAH on marijuana-related problems.
Conclusions:
These findings support an association with CNR1 and FAAH genes and marijuana-related problems among regular marijuana users. CNR1 variation emerged as a moderator of the relationship between trait impulsivity and marijuana problems, thus suggesting that marijuana users with CNR1 risk variants and a higher trait impulsivity are at greater risk for developing marijuana-related problems and supporting a role for CNR1 in a broader impulsivity phenotype.
Marijuana is the most commonly used illicit drug for individuals ages 12 and older (Substance Abuse and Mental Health Services Administration, 2009). Although marijuana use has been associated with behavioral problems, such as motor vehicle accidents (Drummer et al., 2004; Ramaekers et al., 2000) and risky sexual behaviors (Costa et al., 1996; Fernández et al., 2004), only some marijuana users go on to develop use-related problems and dependence. However, much remains unknown about individual differences that characterize persons most vulnerable to the development of problem cannabis use.
Impulsivity and marijuana
There is strong evidence that impulsivity predicts increased consumption and subsequent use-related negative consequences across a variety of substances and has thus been proposed as a putative mechanism in the development of problematic use (de Wit, 2009; Reynolds, 2006). Impulsivity has been assessed using a variety of measures, including both personality questionnaires and behavioral tasks, reflecting separable components of impulsivity (Reynolds et al., 2006). For example, self-report measures of impulsivity-related personality traits do not correlate highly with behavioral measures of impulsivity, thus showing a distinct trait-level impulsivity construct (Mitchell, 1999; Reynolds et al., 2004, 2006).
Further, factor analyses support separation among behavioral tasks, supporting at least two components of behavioral impulsivity (Reynolds et al., 2006): (a) “impulsive disinhibition” or inhibitory control (e.g., the ability to inhibit a prepotent response as measured by the Stop Task [Logan et al., 1997]) and (b) “impulsive decision-making” (e.g., the valuation of delayed rewards as measured by the Delay Discounting Questionnaire [DDQ; Richards et al., 1999]). Numerous studies demonstrate higher levels of impulsivity in marijuana users on a variety of trait measures (Hayaki et al., 2011), behavioral tasks measuring impulsive disinhibition (e.g., Ramaekers et al., 2006), and impulsive decision making (e.g., Reynolds, 2006; Whitlow et al., 2004). Further, higher levels of trait impulsivity have been specifically associated with increased marijuana misuse and related problems among young adult marijuana users (Day et al., 2013; Hayaki et al., 2011). However, in both studies, impulsive personality traits explained less than 5% of the variance in marijuana problems, suggesting that other factors contribute to the development of problematic use.
CNR1 genetic variation and marijuana-related phenotypes
Genetic variation in the cannabinoid receptor 1 (CNR1) gene has been associated with cannabis dependence (Agrawal et al., 2008; Comings et al., 1997; Hopfer et al., 2006) and related phenotypes (Filbey et al., 2010; Haughey et al., 2008), as well as with substance use phenotypes more generally (e.g., Benyamina et al., 2011; Comings et al., 1997; Hopfer et al., 2006; Hutchison et al., 2008; Zhang et al., 2004; Zuo et al., 2007). Although no studies have directly examined the role of CNR1 in the relationship between impulsivity and substance-related outcomes, one study reported significant associations with individual CNR1 single nucleotide polymorphisms (SNPs; rs1535255, rs2023239, rs1049353, and rs806368) and impulsive personality traits in a population of southwest California Indians (Ehlers et al., 2007). Thus, there is preliminary support for the idea that CNR1 may also be related to impulsivity phenotypes (Lopez-Moreno et al., 2012), which are closely linked with problem substance use (de Wit, 2009; Reynolds, 2006).
Taken together, these findings suggest that associations among impulsivity and marijuana-related outcomes may depend on CNR1 genetic variation. Thus, one potential pathway to marijuana-related problems may be via an interaction with individual differences in impulsivity and CNR1 genetic variation. However, this association has not been directly tested. Further, a critical step is to examine CNR1 variation in relation to distinctive components of impulsivity that putatively play a role in the development of substance use-related problems (e.g., trait level, behavior disinhibition, and impulsive decision-making).
FAAH genetic variation and marijuana-related phenotypes
Although substantially more research efforts have been devoted to examining CNR1 associations with marijuana-related phenotypes, another candidate is the fatty acid amide hydrolase (FAAH) gene. FAAH encodes for fatty acid amide hydrolase, the main enzyme responsible for degradation of the major endocannabinoid anandamide (Ben-Shabat et al., 1998; Muccioli, 2010), and variation in this gene affects local endocannabinoid levels (Basavarajappa and Hungund, 2002; Cravatt et al., 2001). Despite its biological relevance, only a handful of studies have examined FAAH in relation to cannabis phenotypes. The C allele of the rs324420 SNP has been associated with the increased likelihood of progression to cannabis dependence (Tyndale et al., 2007) (according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-IV; American Psychiatric Association, 1994), increased craving after marijuana abstinence (Haughey et al., 2008), and increased severity of withdrawal symptoms after 24-hour abstinence (Schacht et al., 2009).
Thus, the existing literature provides strong, but limited, evidence for the relevance of FAAH to marijuana dependence-related phenotypes. In the current study, we sought to examine FAAH in relation to marijuana-related problems, a phenotype that captures problem use and may precede dependence. Although studies have yet to examine FAAH in relation to impulsivity, given its prior association with cannabis dependence-related phenotypes, we also explored the role of FAAH variation in the associations among impulsivity measures and marijuana-related problems.
Polymorphism selection and haplotypes
Numerous polymorphisms exist in the CNR1 gene, and a haplotype of markers associated with reduced transcription has been identified within this gene (Zhang et al., 2004). Many reports have focused on a particular SNP (rs2023239) (e.g., Haughey et al., 2008; Zhang et al., 2004), which has been associated with marijuana expectancies (Metrik et al., 2005). However, results for this SNP have not been uniformly consistent (e.g., Hartman et al., 2009) and, thus, the literature has not converged on a single putative CNR1 risk allele. To capitalize on knowledge gained from the existing literature and also in an attempt to more fully characterize the CNR1 gene, three specific polymorphisms were selected in the CNR1 gene—rs2023239 (intronic), rs1049353 (exonic and synonymous), and rs806368 (3′ untranslated region)—based on prior evidence for association with marijuana-related phenotypes (e.g., Hartman et al., 2009; Haughey et al., 2008; Hopfer et al., 2007; Zhang et al., 2004).
A similar strategy was used (in combination with Hap-Map [using an r2 of at least .80 and a minor allele frequency of at least .20] because of the limited FAAH literature) to select five SNPs in the FAAH gene: rs4141964 (intronic), rs324420 (exonic and missense), rs1 1576941 (intronic), rs6703669 (intronic), and rs6429600 (intronic) (Doehring et al., 2007; Haughey et al., 2008; Tyndale et al., 2007). We sought to move beyond a single or multiple SNP approach and characterize each gene for the presence of haplotype blocks (i.e., a set of SNPs that are statistically related), thus allowing for a more statistically powerful and fuller characterization of the variation within the particular polymorphic region (Clark, 2004).
Current study
We conducted an exploratory study focused on phenotypes that contribute to the development and maintenance of cannabis dependence, namely impulsivity and marijuana problem severity. Such psychometrically valid self-report and laboratory behavioral measures offer objective performance-based assessments that go beyond binary diagnostic or symptom count measures to provide better characterization of the spectrum of problem use and related characteristics. Hypotheses were based on converging lines of literature suggesting the following: (a) that impulsivity is a critical factor in risk for problem substance use, and, more specifically, that trait impulsivity increases risk for more severe problems among marijuana users (Day et al., 2013; Hayaki et al., 2011); (b) that CNR1 and FAAH variations are both consistently associated with marijuana dependence and related phenotypes along the dependence pathway (e.g., Hart-man et al., 2009; Haughey et al., 2008; Hopfer et al., 2007; Schacht et al., 2012; Tyndale et al., 2007); and (c) that CNR1 variation may be directly associated with varying levels of trait impulsivity (Ehlers et al., 2007). Taken together, these associations provide putative evidence for the potential of these genes to moderate the relationship between impulsive traits and risk for marijuana-related problems in users.
Thus, our primary hypothesis was that CNR1 genetic variation would interact with trait-level impulsivity, as measured by the Barratt Impulsiveness Scale (BIS; Patton et al., 1995) to influence marijuana-related problems among experienced weekly marijuana users. As a secondary aim, in an effort to test the different components of impulsivity more comprehensively (de Wit, 2009; Reynolds et al., 2008), we also examined the interactions among cannabinoid-related genetic variation and two measures of behavioral impulsivity: (a) the capacity to inhibit already initiated responses as measured by the Stop Signal Task (Logan et al., 1997) and (b) impulsive decision-making as measured by the DDQ (Richards et al., 1999). We genotyped three SNPs in the CNR1 gene and five SNPs in the FAAH gene and subsequently tested each for the presence of haplotypes. CNR1 and FAAH variations were tested as moderators of the relationship between measures of impulsivity and marijuana-related problems.
Method
Sample description
Demographic, marijuana use, and impulsivity variables are presented in Table 1. This study uses data obtained from participants (N = 151) who completed the baseline assessments of an experimental study of marijuana’s acute effects on impulsivity (Metrik et al., 2012). As previously described (see Metrik et al., 2012, for more details), this Brown University Institutional Review Board-approved study of marijuana users comprised participants who met several inclusion criteria: were native English speakers, were 18-30 years of age, used marijuana at least once a week in the past month and at least 10 times in the past 6 months, and had a self-reported ability to abstain from marijuana for 24 hours without withdrawal. Exclusion criteria were history of substance use treatment and intent to quit or receive treatment for cannabis use; past-month affective disorder or history of panic attacks, psychotic state, or suicidal state; DSM-IV alcohol dependence; and smoking 20 or more tobacco cigarettes per day.
Table 1.
Sample demographics, substance use, and impulsivity characteristics (N = 151)
| Variable | n | % |
| Gender | ||
| Male | 96 | 64 |
| Female | 55 | 36 |
| Ethnicity | ||
| White | 108 | 71 |
| Black | 10 | 7 |
| Asian | 7 | 5 |
| American Indian/Alaskan Native | 1 | 1 |
| Mixed ethnic origin | 17 | 11 |
| Other | 8 | 5 |
| Age at first marijuana use, in years | ||
| 13 | 25 | 16 |
| 14-15 | 45 | 30 |
| ≥16 | 81 | 54 |
| Marijuana ounces used per week | ||
| <1/16th | 36 | 24 |
| 1/16th | 35 | 23 |
| 1/8th | 24 | 16 |
| 1/4 | 23 | 15 |
| >1/4 | 33 | 22 |
| M | SD | |
| Age, in years | 21.5 | 3.2 |
| % marijuana use days | 41.4 | 24.3 |
| Marijuana problems scale | 3.1 | 2.6 |
| Times used marijuana on average day | 1.8 | 1.0 |
| Number of alcohol drinks/week | 7.5 | 8.6 |
| % heavy drinking days | 10.6 | 13.1 |
| % smoking tobacco days (for n = 76 tobacco smokers) | 56.3 | 40.1 |
| Impulsivity measures | ||
| Barratt Impulsiveness Scale | 58.7 | 8.5 |
| Stop Signal Reaction Time | 536.3 | 168.6 |
| Delay Discounting Questionnaire: | ||
| Area under the curve | 0.46 | 0.3 |
Note: All marijuana, alcohol, and cigarette use measures reflect use over the past 60 days.
Behavioral measures
The calendar-assisted, clinician-administered Timeline Followback interview (Dennis et al., 2004) is a reliable and valid instrument that was used to assess past-60-day use of marijuana, alcohol, and cigarettes.
The Marijuana Problems Scale (MPS; Stephens et al., 1994, 2000) is a self-report measure of 22 marijuana-related problems during the past 90 days assessed on a 3-point scale ranging from 0 (no problem) to 2 (serious problem). The dependent variable was a sum count of problems.
The BIS-11 (Patton et al., 1995) includes 30 questions comprising subscales on motor, attentional, and future planning impulsivity, scored on Likert scales from 1 (rarely/ never) to 4 (almost always). The dependent variable was the total score summing all of the subscales.
The Stop Signal Task (Logan et al., 1997) measures inhibition of a prepotent response with two concurrent tasks: (a) the go task is a choice reaction-time task that requires participants to rapidly discriminate two symbols (maximum presentation 1,250 ms), and (b) the stop task involves presentation of a tone (75 ms, 1,000 Hz) that signals one to inhibit the response to the go task. The tone was presented following the visual stimuli at different delays (initial delay at 200 ms) on a quarter of the trials (32 practice trials followed by three blocks of 64 trials). The delay time to the stop signal was adjusted until the participant inhibited responses on approximately 50% of trials. The time in milliseconds required for the participant to stop the go response (stop signal reaction time, SSRT) was the dependent variable, calculated by subtracting the mean stop signal delay from the mean Go Reaction Time. Data from 16 participants were excluded because they almost always (90%-100% of trials) failed to inhibit the stop signal, possibly because of poor comprehension of the instructions (Schachar et al., 2000; Verbruggen and Logan, 2008).
The DDQ (Richards et al., 1999) uses a computerized adjusting-amount procedure to measure discounting of delayed monetary reinforcers. In a series of choice trials, participants were offered the hypothetical choice between $10 available after a delay (0, 2, 30, 180, and 365 days) or a smaller amount available immediately. The dependent variable was area under the curve connecting indifference points and the x-axis, from 0.0 (steepest discounting) to 1.0 (no discounting) (Myerson et al., 2001).
Marker information and haplotype derivation
This study used three markers across the CNR1 gene and five markers in the FAAH gene. Markers that failed the Hardy-Weinberg Equilibrium (HWE) test (p value cutoff = .001) were excluded from all analyses (Barrett et al., 2005; Purcell et al., 2007). Given the low rates of some genotypes (e.g., CNR1 rs806368 - CC [5], CT/TC [52], and TT [91]), we were unable to test the additive effect of an allele with sufficient confidence. Consequently, analyses binned minor allele homozygotes with heterozygotes (i.e., using a carrier code) to maximize statistical power.
To maximize the amount of information provided by the multiple markers and circumvent loss of power because of multiple testing, we used all of the available SNP data to identify haplotype blocks (i.e., the combinations of SNP markers that are statistically associated). Haploview was used to visualize haplotype blocks (Barrett, 2009; Barrett et al., 2005). Haplotypes for both chromosomes were then confirmed and extracted using PHASE Version 2.1 (Stephens and Donnelly, 2003; Stephens et al., 2001), requiring that the probability of a haplotype be greater than or equal to 0.80. PHASE haplotypes were used to construct diplotypes (i.e., combination of haplotypes across the pair of homologous chromosomes) that were used in the regression analyses. Because of the limited and inconsistent literature indicating a putative risk allele in each of our genes of interest, haplotype, and thus diplotype, scores were created using a model based on haplotype dosage for each gene (Lu et al., 2006; Pajewski et al., 2011). We assumed an additive effect for each of the identified haplotypes; consequently, an individual may possess 0, 1, or 2 copies of each haplotype observed. This scoring scheme was used for every haplotype that was more frequent than .20 in the study sample.
Statistical analyses and analysis plan
Descriptive statistics and regression analyses were executed in SPSS (SPSS Statistics Version 17.0, SPSS, Inc., Chicago, IL). The MPS and impulsivity data were initially examined for outliers (using standard scores, criterion Z = 3.29) and for distribution normality (Tabachnick and Fidell, 2001). As described in Tabachnick and Fidell (2001), one outlier for MPS was retained and recoded as one unit greater than the highest nonoutlier value. Initial bivariate correlations examined the relationships among the three measures of impulsivity. Linear regressions were used to test the main and interaction effects of the three measures of impulsivity (BIS, SSRT, and DDQ), and CNR1/FAAH genetic variations on MPS and models were constructed as recommended by Judd et al. (2009). FAAH models were constructed to include the main effect of impulsivity, the main effect of the haplotype, and the Haplotype × Impulsivity interaction effect. For CNR1, in addition to the resulting haplotype block, one putatively associated SNP (rs2023239) from multiple prior studies did not meet our haplotype block inclusion criterion and was also included in the regression model (following Pickard et al., 2009).
This approach maximizes characterization of genetic variation within a particular gene while limiting the number of parameters tested in statistical models. Thus, the CNR1 models were constructed to include the main effect of impulsivity; the main effect of the haplotype; the main effect of the SNP; all possible two-way interactions of the haplotype, SNP, and impulsivity effects; and the three-way interaction of the haplotype, SNP, and impulsivity effect. Separate models were run for each impulsivity measure. In addition, because there is not an agreed-upon putative “risk” haplotype for either CNR1 or FAAH, separate regression models were run for each possible haplotype. We included self-reported race (dichotomously coded as White/non-Hispanic vs. all others) as a covariate in all models to help control for the possible effects of population stratification on genetic associations (Sinha et al., 2006).
Results
Correlations among measures of impulsivity
As expected, the three impulsivity measures were not significantly correlated; SSRT and DDQ were negatively correlated at trend level in the expected direction (BIS - SSRT: r = -.06, p = .50; BIS-DDQ: r = -.05, p = .55; and SSRT-DDQ: r = -.14, p = .08).
Distribution of alleles and haplotypes
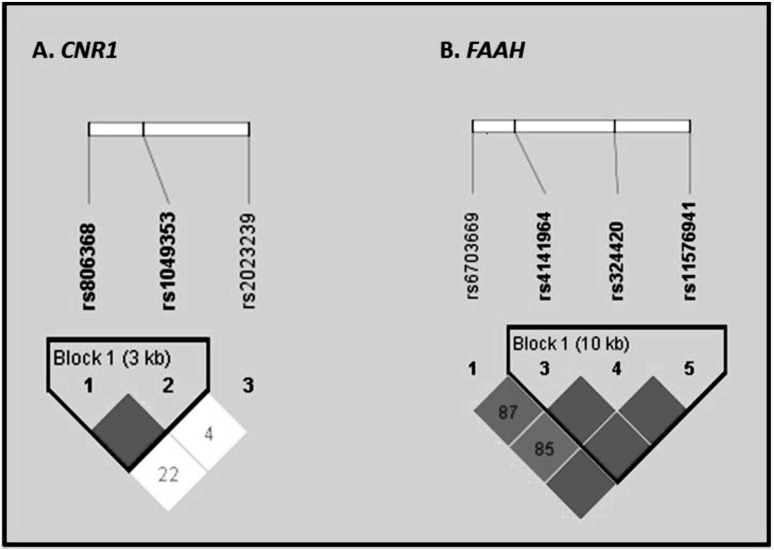
Table 2 describes the prevalence of genotypes and alleles (including the number of missing genotypes) and HWE test p values for each marker. For CNR1, all markers met the criteria for the HWE test (i.e., p > .0010). CNR1 markers rs1049353 and rs806368 formed a single haplotype block (Figure 1a); rs2023239 was not part of a haplotype block. Although it did not meet the criteria for inclusion in the haplotype block, prior evidence from multiple studies supporting the role of rs2023239 in marijuana-related phenotypes (e.g., Haughey et al., 2008; Zhang et al., 2004) led to its inclusion as a separate predictor in the regression models along with the haplotype (following Pickard et al., 2009).
Table 2.
Genotype and minor allele frequencies of CNR1 and FAAH at baseline
| Polymorphism | Genotypes n (%) | Allele frequency | HWE test p | ||||
| CNR1 | |||||||
| rs806368 | CC | CT/TC | TT | ?? | C | T | |
| Frequency | 5 (3.31) | 51 (33.77) | 91 (60.26) | 4 (2.64) | 0.21 | 0.79 | .73 |
| rs1049353 | CC | CT | TT | ?? | C | T | |
| Frequency | 96 (63.58) | 48(31.79) | 7 (4.63) | 0 (0.00) | 0.80 | 0.20 | .78 |
| rs2023239 | CC | TC | TT | ?? | C | T | |
| Frequency | 6(3.97) | 29(19.21) | 110(72.85) | 6 (3.97) | 0.14 | 0.86 | .08 |
| FAAH | |||||||
| rs6703669 | CC | CT | TT | ?? | C | T | |
| Frequency | 75 (49.67) | 55 (36.42) | 2(1.33) | 19(12.58) | 0.78 | 0.22 | .03 |
| rs6429600a | GG | GA | AA | ?? | G | A | |
| Frequency | 43 (28.48) | 20(13.25) | 80 (52.98) | 8 (5.29) | 0.37 | 0.63 | 2.09E-17 |
| rs4141964 | CC | TC | TT | ?? | T | C | |
| Frequency | 55 (36.42) | 63 (42.72) | 26 (17.22) | 7 (4.64) | 0.40 | 0.60 | .35 |
| rs324420 | CC | AC | AA | ?? | C | A | |
| Frequency | 91 (60.27) | 45 (29.80) | 9 (5.96) | 6 (3.97) | 0.78 | 0.22 | .40 |
| rs 11576941 | GG | TG | TT | ?? | T | G | |
| Frequency | 59 (39.07) | 70 (46.36) | 16(10.60) | 6 (3.97) | 0.35 | 0.65 | .69 |
Notes: Table shows the genotypes and frequencies for each marker (in order of chromosomal location).
Denotes that this marker was not included in analyses because it failed the Hardy-Weinberg Equilibrium (HWE) test (criteria for the HWE test = p > .001); ?? indicates individuals who received a missing value for this marker because of failed genotyping calls. CNR1 = cannabinoid receptor 1; FAAH = fatty acid amide hydrolase.
Figures 1a and 1b.
Marker-to-marker D′ values for the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) polymorphisms. D′ varies between 0 and 1 and describes the extent of linkage disequilibrium, a measure of interdependency between genetic loci. A value of 0 for D′ suggests that the examined single nucleotide polymorphisms (SNPs) are independent of one another, whereas a value of 1 suggests that the single nucleotide polymorphisms provide redundant information. An empty box with no numerical value represents D′ of 1. In the color version of the figure available online, bright red indicates a D′of 1, and shades of pink/red indicate D′ < 1. Marker-to-marker r2 values are as follows: (CNR1) rs806368 - rs1049353 = 0.07, rs1049353 - rs2023239 = 0.00, rs806368 - rs2023239 = 0.00. (A) Figure 1a indicates the D′ for genetic variation across CNR1. Marker-to-marker r2 values for CNR1 are as follows: rs806368 - rs1049353 = 0.06; rs1049353 - rs2023239 = 0.00; rs806368 - rs2023239 = 0.00. (B) Figure 1b indicates the D′ for genetic variation across FAAH. Marker-to-marker r2 values for FAAH are as follows: rs6703669 - rs4141964 = 0.16; rs6703669 - rs324420 = 0.06; rs6703669 - rs1 1576941 = 0.15; rs4141964 - rs324420 = 0.42; and rs4141964 - rs1 1576941 = 0.35; rs324420 - rs1 1576941 =0.15.
For FAAH, analyses indicated that the measured genotype frequencies for the FAAH marker (rs6429600) were significantly different from the expectations of the HWE test (p = 1.48E-17); thus, it was not included in the analyses. We observed a single haplotype block comprising FAAH markers rs4141964, rs324420, and rs1 1576941; rs6703669 was not part of a block (Figure 1b). Because the rs6703669 SNP has not emerged as a putatively associated SNP in prior studies of marijuana-related phenotypes, it was not included in the models. Table 3 describes the frequencies of measured haplotypes of CNR1 and FAAH as determined by PHASE (Stephens et al., 2001). Because of missing genotypes at some markers (Tables 2 and 3), the analyses with CNR1 and FAAH included 148 and 145 individuals, respectively. The low prevalence of individuals with two or more copies of a given haplotype led to the use of a haplotype measure coded as carriers (score of 1) versus noncarriers (score of 0).
Table 3.
Haplotypes and frequencies of CNR1 and FAAH
| Proportion n (%) of carriers of haplotype |
|||||
| Variable | Population frequency (SE) | Noncarrier | Carrier | ||
| CNR1 polymorphism | |||||
| rs806368 | rs1049353 | ||||
| T | C | 0.59 (3.74E-3) | 23(15.13) | 125 (82.24) | |
| T | T | 0.20(1.38E-3) | 95 (62.50) | 53 (34.87) | |
| C | C | 0.21 (3.74E-3) | 91 (59.87) | 57 (37.50) | |
| FAAH polymorphism | |||||
| rs4141964 | rs324420 | rs1 1576941 | |||
| T | C | G | 0.18(4.90E-3) | 98 (64.47) | 47 (30.92) |
| T | A | G | 0.22(5.19E-3) | 91 (59.87) | 54 (35.53) |
| C | C | T | 0.35(6.14E-3) | 59 (38.82) | 86 (56.58) |
| C | C | G | 0.25 (5.35E-3) | 79(51.97) | 66 (43.42) |
Notes: Table shows haplotypes extracted from participants using PHASE. Proportions do not sum to 100 to allow for the accurate depiction of missingness in the data. CNR1 = cannabinoid receptor 1; FAAH = fatty acid amide hydrolase
Multivariate regression of impulsivity and endocannabinoid system genes on marijuana-related problems
Barratt Impulsiveness Scale.
Table 4 provides the results for the models testing the moderating role of CNR1 and FAAH genetic variations in the BIS and MPS relationship. The following main effects and Impulsivity × Genotype interactions were found.
Table 4.
Multivariate association of endocannabinoid system genes and Barratt Impulsiveness Scale (BIS) with marijuana-related problems: Main effects and interactions
| Marijuana-related problems |
|||||
| Variable | Estimate | (SE) | β | P | |
| BIS × CNR1 | |||||
| CNR1 TT haplotype | |||||
| Overall model | F = 4.1, adj. R2 = .15, p < .001 | ||||
| Intercept | 0.33 | (0.34) | |||
| BIS | 0.15 | (0.10) | .17 | .14 | |
| SNP | -0.07 | (0.21) | -.00 | .75 | |
| TT haplotype | -0.09 | (0.17) | -.05 | .61 | |
| BIS × SNP | 0.54 | (0.20) | .31 | .007 | |
| BIS × TT haplotype | -0.23 | (0.18) | .14 | .22 | |
| SNP × TT haplotype | -0.43 | (0.37) | -.13 | .25 | |
| BIS × SNP × TT haplotype | -0.77 | (0.36) | -.26 | .03 | |
| CNR1 TC haplotype | |||||
| Overall model | F =4.2, adj. R2 = .15, p < .001 | ||||
| Intercept | 0.52 | (0.37) | |||
| BIS | 0.34 | (0.11) | .37 | .002 | |
| SNP | -0.25 | (0.21) | -.12 | .23 | |
| TC haplotype | -0.23 | (0.18) | -.12 | .20 | |
| BIS × SNP | 0.24 | (0.20) | .14 | .22 | |
| BIS × TC haplotype | -0.36 | (0.18) | -.23 | .05 | |
| SNP × TC haplotype | 0.25 | (0.38) | .07 | .52 | |
| BIS × SNP × TC haplotype | 0.27 | (0.36) | .09 | .46 | |
| CNR1 CC haplotype | |||||
| Overall model | F = 4.1, adj. R2 = .15, p < .001 | ||||
| Intercept | 0.22 | (0.35) | |||
| BIS | 0.16 | (0.11) | .17 | .15 | |
| SNP | -0.28 | (0.23) | -.13 | .23 | |
| CC haplotype | 0.11 | (0.17) | .06 | .53 | |
| BIS × SNP | 0.14 | (0.22) | .08 | .52 | |
| BIS × CC haplotype | 0.16 | (0.18) | .11 | .36 | |
| SNP × CC haplotype | 0.09 | (0.36) | .03 | .81 | |
| BIS × SNP × CC haplotype | 0.43 | (0.35) | .15 | .22 | |
| BIS × FAAH | |||||
| FAAH CCG haplotype | |||||
| Overall model | F = 5.2, adj. R2 = .11, p < .001 | ||||
| Intercept | 0.20 | (0.36) | |||
| BIS | 0.38 | (0.11) | .40 | .001 | |
| CCG haplotype | -0.03 | (0.15) | -.01 | .86 | |
| BIS × CCG haplotype | -0.07 | (0.15) | -.05 | .67 | |
| FAAH CCT haplotype | |||||
| Overall model | F = 5.8, adj. R2 = .12, p < .001 | ||||
| Intercept | 0.29 | (0.36) | |||
| BIS | 0.45 | (0.12) | .47 | <.001 | |
| CCT haplotype | -0.19 | (0.16) | -.10 | .23 | |
| BIS × CCT haplotype | -0.15 | (0.16) | -.12 | .35 | |
| FAAH TAG haplotype | |||||
| Overall model | F = 7.1, adj.R2 = .15, p < .0001 | ||||
| Intercept | -0.03 | (0.36) | |||
| BIS | 0.28 | (0.09) | .29 | .001 | |
| TAG haplotype | 0.32 | (0.15) | .16 | .03 | |
| BIS × TAG haplotype | 0.26 | (0.17) | .14 | .13 | |
| FAAH TCG haplotype | |||||
| Overall model | F = 5.3, adj. R2 = .11, p < .001 | ||||
| Intercept | 0.15 | (0.38) | |||
| BIS | 0.34 | (0.09) | .35 | <.001 | |
| TCG haplotype | 0.08 | (0.17) | .04 | .63 | |
| BIS × TCG haplotype | 0.04 | (0.16) | .02 | .80 | |
Notes: Self-reported race included as a covariate in all models. CNR1 = cannabinoid receptor 1 gene; SNP = single nucleotide polymorphism; CNR1 SNP = rs2023239 and was coded as 0 or 1 vs. 2 T; CNR1 haplotype included rs806368 and rs1049353; FAAH = fatty acid amide hydrolase gene; FAAH haplotype included rs4141964, rs324420, rs1 1576941. Adj. = adjusted.
CNR1.
There were no main effects of BIS or CNR1 genetic variation on MPS. The hypothesized BIS × CNR1 SNP (rs2023239) interaction was observed to be significant (B = 0.54, SE = 0.20, p =.007). This suggests that the effect of BIS on MPS depended on the rs2023239 genotype. Therefore, possessing at least one copy of the C allele and having low BIS scores (low-level trait-level impulsivity) were associated with lower MPS scores. Thus, having a TT genotype and higher BIS scores (greater trait-level impulsivity) was associated with increased MPS scores. In addition, a significant BIS × CNR1 T rs2023239 SNP × CNR1 TT haplotype interaction was observed, suggesting that the presence or absence of the TT haplotype moderated the BIS × rs2023239 SNP Genotype interaction.
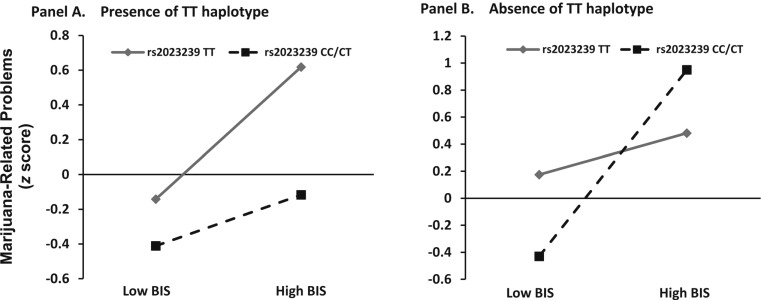
Simple slopes for the association between MPS, BIS, and CNR1 rs2023239 genotype were tested when the CNR1 TT haplotype was present (one or two copies) or absent (Aiken and West, 1991). Each of the simple slope tests revealed a significant positive association between MPS and BIS score, but the effect was strongest for individuals who were C allele carriers at rs2023239 and did not possess the TT haplotype, t(137) = 2.3, p = .02. This three-way interaction suggests that those with higher BIS scores who are rs2023239 C allele carriers and lack the putatively protective TT haplotype manifest higher MPS. Figure 2 plots the simple slopes for the three-way interaction.
Figure 2.
Marijuana Problems Scale as a function of high and low Barratt Impulsiveness Scale (BIS) score, cannabinoid receptor 1 (CNR1) rs2023239 single nucleotide polymorphism (SNP) variation, and CNR1 haplotype variation. Marijuana Problems Scale (standardized z scores) as a function of low BIS score (left side of each panel) and high BIS score (right side of each panel), CNR1 SNP (rs2023239) TT (diamond) versus CC/CT (square) genotype, and CNR1 haplotype variation. (A) Panel A represents the simple slopes of the BIS × SNP interaction in the presence of the TT haplotype. (B) Panel B represents the simple slopes of the BIS × SNP interaction in the absence of the TT haplotype (1 or 2 copies).
There were no significant main effects or interactions in the model testing the CNR1 CC haplotype.
There was a significant main effect of higher BIS (B = 0.34, SE = 0.11, p =.002) on MPS in the model testing the CNR1 TC haplotype. However, there were no significant genetic main effects or any significant Impulsivity × CNR1 TC haplotype interactions.
FAAH.
There was a significant effect of BIS in predicting MPS in all four models (CCG: B = 0.38, SE = 0.11, p = .001; CCT: B = 0.45, SE = 0.12, p < .001; TAG: B = 0.28, SE = 0.09, p = .001; and TCG: B = 0.34, SE = 0.09, p < .001). This suggests that higher trait impulsivity is associated with more marijuana-related problems after FAAH genetic variation was controlled for. Only the model testing the FAAH TAG haplotype indicated a significant genetic main effect (B = 0.32, SE = 0.15, p = .03) in predicting higher MPS. Significant BIS × FAAH interactions were not observed in any of the models.
Stop signal reaction time.
There were no significant main effects or interactions of the models testing CNR1 or FAAH and SSRT in predicting MPS.
Delay Discounting Questionnaire.
There were no significant main effects or interactions of the models testing CNR1 or FAAH and DDQ in predicting MPS.
Sensitivity analysis.
Given the sample size and number of statistical tests, we conducted a post hoc sensitivity analysis of the robustness of our modeling results to test the possibility that the observed significant findings were attributable to Type 1 error. The sensitivity analysis procedure involves first identifying any potentially influential observations, via Cook’s distance (D), and then testing the regression model without those data points (Welsch, 1980). A comparison of the overall model results and individual coefficients is then conducted to determine if these influential values drove significant findings. Regarding the primary hypothesis, sensitivity analysis was conducted for the model resulting in a significant interaction of BIS and CNR1 (SNP and TT haplotype) in predicting marijuana problems. Using a conservative threshold for Cook’s D (e.g., D > 4/n, where n is the number of observations; in this case: D > 4/148 or D > .270) (Bollen and Jackman, 1990), nine observations were deemed potentially influential and the original regression model was rerun without these nine observations.
Results from the modified model were consistent with those of the original model. As before, there were no main effects of BIS or CNR1 genetic variation on MPS, and the hypothesized BIS × CNR1 SNP (rs2023239) interaction was observed to be significant (B = 0.73, SE = 0.28, p = .01), such that having a TT genotype and higher BIS scores (greater trait-level impulsivity) was associated with increased MPS scores. The previously significant BIS × CNR1 T rs2023239 SNP × CNR1 TT haplotype interaction was also observed (B = -0.09, SE = 0.44, p = .04). Again, consistent with our original model, simple slopes analysis revealed that the highest MPS scores were in individuals with high BIS scores who were C allele carriers and who did not possess the TT haplotype, t(137) = 2.4, p = .02. We also conducted sensitivity analysis for the FAAH models in which results had indicated a significant effect of BIS and TAG haplotype; none of the observations in any of these models met the threshold (D > 4/n) for being potentially influential. Together, these findings suggest that high leverage values did not drive our significant results and support the robustness of our findings for both CNR1 and FAAH.
Discussion
In a sample of young adult marijuana users, the present study assessed relationships among endocannabinoid system genetic variation and key intermediate phenotypes that contribute to the development and maintenance of cannabis dependence: namely, impulsivity (trait-level and behavioral) and marijuana-related problems. CNR1 variation moderated the relationship between trait impulsivity and marijuana-related problems, such that higher scores on the BIS combined with CNR1 rs2023239 SNP and haplotype variation predicted MPS scores. Specifically, individuals who report lower levels of trait impulsivity on the BIS and are rs2023239 SNP C allele carriers experience lower levels of marijuana-related problems, whereas individuals who report high levels of trait impulsivity and TT homozygotes experience higher levels of problems. In addition, the effect of rs2023239 SNP genotype was moderated by the presence of the TT haplotype, such that, in the absence of the TT haplotype, individuals who have higher BIS scores and are C allele carriers (rs2023239) have higher MPS scores. However, in the presence of the TT haplotype, it is those with higher BIS scores and the TT genotype at rs2023239 who manifest higher marijuana-related problems. In addition, the FAAH TAG haplotype significantly predicted increased marijuana-related problems, over and above the BIS; however, FAAH did not interact with any of the impulsivity measures to predict marijuana-related problems. All significant associations were robust to sensitivity analysis.
Our findings support a role for trait impulsivity and endocannabinoid system genes in predicting risk for marijuana-related problems among regular users. The present study replicates previous behavioral findings linking trait impulsivity and the severity of marijuana problems in users (Day et al., 2013; Hayaki et al., 2011). Further, findings of a CNR1 × BIS interaction extend prior work to suggest that CNR1 genetic variation further increases risk for marijuana-related problems in individuals with high levels of trait impulsivity. Our results did not demonstrate a main effect of CNR1 rs2023239 SNP or haplotype variation on marijuana problems. This result is partially in contrast with studies showing direct associations with CNR1 and DSM-III-R (American Psychiatric Association, 1987), DSM-IV cannabis dependence (Comings et al., 1997; Hopfer et al., 2006), and other related phenotypes (Haughey et al., 2008). However, our findings are among the first to test and demonstrate an interaction among CNR1 variation and trait impulsivity in predicting marijuana outcomes. These findings suggest that CNR1’s influence on cannabis dependence may be in part through a risk-associated trait impulsivity phenotype. Although results need to be replicated, the current findings add to the literature supporting a broader role for CNR1 in substance use vulnerability that includes risk for impulsive personality and problem substance use beyond marijuana use (Hutchison et al., 2008; López-Moreno et al., 2012).
Results also support a role for FAAH in risk for marijuana-related outcomes but suggest that FAAH does not interact with measures of impulsivity to increase risk for marijuana-related problems. The finding that variation in FAAH was significantly associated with an increased number of marijuana problems adds to prior studies suggesting a role for FAAH in DSM-IV cannabis dependence (Tyndale et al., 2007), as well as in craving and withdrawal processes (Haughey et al., 2008; Schacht et al., 2009). Taken together, these findings suggest that FAAH variation increases risk for more severe outcomes along the cannabis dependence pathway.
Although no prior studies have examined the role of CNR1 or FAAH in the pathway from impulsivity to increased marijuana problems, our findings are consistent with several laboratory studies that provide compelling evidence that CNR1 and FAAH variations play a role in cannabis dependence-related intermediate phenotypes (Filbey et al., 2010; Haughey et al., 2008; Schacht et al., 2012). Because of the very limited number and nature of these studies, further investigations are critical, and our findings may be considered preliminary. Greater knowledge from similar experimental studies examining endocannabinoid system genes that use a variety of behavioral and laboratory analogs of critical dependence-related mechanisms will continue to advance our understanding of individual differences in the trajectories of cannabis use disorders and problem use.
The finding of both a two-way Trait Impulsivity × CNR1 rs2023239 SNP interaction and a three-way Trait Impulsivity x rs2023239 SNP × CNR1 TT haplotype interaction in predicting marijuana-related problems suggests that there may be at least two functional loci within the CNR1 gene. Thus, the full genetic picture only emerges when the variants are modeled together while accounting for individual differences in BIS scores. These findings require replication in an independent sample to improve confidence in their reliability but suggest that the genetic architecture of the CNR1 gene may be complicated and require complex modeling of multiple variants that may not all fall into haplotype blocks.
There was neither a main nor genetic interaction effect of behavioral impulsivity, measured by SSRT and the DDQ, on marijuana-related problems. These findings are consistent with weaker associations of levels of marijuana use with delay discounting compared with other drugs, such as tobacco, cocaine, opioids, and alcohol (e.g., Johnson et al., 2010; Mitchell et al., 2005). In addition, laboratory findings from our prior study indicate that acute marijuana intoxication increases SSRT, albeit to a small extent (Metrik et al., 2012). Thus, it could be that such measures of behavioral impulsivity are better suited for measurement of acute drug effects, whereas trait-level impulsivity measured by the BIS may be implicated in the development of problematic marijuana use over time and, further, may be a more sensitive measure of an underlying latent vulnerability to behavioral undercontrol. Indeed, research suggests that behavioral undercontrol and vulnerability to substance use may share genetic influences (Young et al., 2009). Our study provides further evidence linking substance use and trait-level impulsivity in a cluster of genetically influenced behaviors.
Limitations and future directions
The primary limitation of our study is that the sample size is relatively small given the number of hypotheses tested, which may have inflated the risk of Type I error. Although we have protected against this possibility with our hierarchical hypothesis structure and further confirmed the robustness of our results via sensitivity analyses, replication is needed to substantiate these findings. In addition, although this was not viable in our study because of an already large number of statistical tests, future studies may wish to examine these interactions from an endocannabinoid system level, including Gene × Gene and Gene × Gene × Trait level interactions. Finally, although we controlled for self-reported race in all models, our results may still be vulnerable to the effects of population stratification.
Conclusions
Despite these limitations, this study is strengthened by going beyond a single or multiple SNP approach to characterize variation in the endocannabinoid system genes. Further, by examining interactions with impulsivity and endocannabinoid system variation on risk for problems related to marijuana use, our study integrates biological and behavioral factors to help explicate the mechanisms by which genetic variability influences risk for marijuana dependence. As has been shown in alcohol dependence (Hutchison, 2010; Ray et al., 2010), the identification of candidate phenotypes that serve as markers for heightened substance use disorder vulnerability has the potential to greatly advance the examination of genetic influences on cannabis dependence. The present study provides an important step toward identifying dispositional and genetic risk factors for marijuana-related outcomes. This indicates that the relationships among impulsive personality traits and marijuana outcomes may be further qualified by genetic risk factors and supporting a role for CNR1 in risk for impulsive personality traits that increase the risk of a variety of substance-related problems. Additional research is needed to identify the neurobiological and genetic mechanisms that underlie these unique associations.
Acknowledgments
The authors gratefully acknowledge Amy Mochel, Suzanne Sales, Timothy Souza, and Adrienne Umali for their contributions to this project.
Footnotes
This research was supported by National Institute on Drug Abuse (NIDA) Grants R01 DA021403 (to Jane Metrik), K23 DA033302 (to L. Cinnamon Bidwell), and R01 DA023134 (to Valerie S. Knopik); by a Research Career Development Award from the Medical Research Service of the Department of Veterans Affairs, 1S10RR023457-01A1, and Shared Equipment Evaluation Program (ShEEP) grants from the Medical Research Service of the Department of Veterans Affairs (to John McGeary); and by National Institute on Alcohol Abuse and Alcoholism Grant K01 AA021113 (to Rohan H. C. Palmer).
References
- Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, Madden PAF. An autosomal linkage scan for cannabis use disorders in the Nicotine Addiction Genetics Project. Archives of General Psychiatry. 2008;65:713–721. doi: 10.1001/archpsyc.65.6.713. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Newbury Park, CA: Sage; 1991. Multiple regression: Testing and interpreting interactions. [Google Scholar]
- American Psychiatric Association. Washington, DC: 1987. Diagnostic and statistical manual of mental disorders (3rd ed., Revised) Author. [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: 1994. Diagnostic and statistical manual of mental disorders. Author. [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harbor Protocols. 2009 doi: 10.1101/pdb.ip71. doi: 10.1101 /pdb.ip71. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: An overview. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2002;66:287–299. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee M-H, Vogel Z, Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. European Journal of Pharmacology. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- Benyamina A, Kebir O, Blecha L, Reynaud M, Krebs MO. CNR1 gene polymorphisms in addictive disorders: A systematic review and a meta-analysis. Addiction Biology. 2011;16:1–6. doi: 10.1111/j.1369-1600.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Jackman RW. Regression diagnostics: An expository treatment of outliers and influential cases. In: Fox J, Long JS, editors. Modern methods of data analysis. Newbury Park, CA: Sage; 1990. pp. 257–291. [Google Scholar]
- Clark AG. The role of haplotypes in candidate gene studies. Genetic Epidemiology. 2004;27:321–333. doi: 10.1002/gepi.20025. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): Association with i.v. drug use. Molecular Psychiatry. 1997;2:161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Costa FM, Jessor R, Fortenberry JD, Donovan JE. Psychosocial conventionality, health orientation, and contraceptive use in adolescence. Journal of Adolescent Health. 1996;18:404–416. doi: 10.1016/1054-139X(95)00192-U. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Metrik J, Spillane NS, Kahler CW. Working memory and impulsivity predict marijuana-related problems among frequent users. Drug and Alcohol Dependence. 2013;131:171–174. doi: 10.1016/j.drugalcdep.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Funk R, Godley SH, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (GAIN) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction, 99, Supplement. 2004;2:120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Doehring A, Geisslinger G, Lötsch J. Rapid screening for potentially relevant polymorphisms in the human fatty acid amide hydrolase gene using Pyrosequencing. Prostaglandins & Other Lipid Mediators. 2007;84:128–137. doi: 10.1016/j.prostaglandins.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn J, Robertson MD, Swann P. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accident Analysis and Prevention. 2004;36:239–248. doi: 10.1016/s0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the cannabinoid receptor gene (CNR1) and impulsivity in southwest California Indians. Twin Research and Human Genetics. 2007;10:805–811. doi: 10.1375/twin.10.6.805. [DOI] [PubMed] [Google Scholar]
- Fernández MI, Collazo JB, Hernández N, Bowen GS, Varga LM, Vila CK, Perrino T. Predictors of HIV risk among Hispanic farm workers in South Florida: Women are at higher risk than men. AIDS and Behavior. 2004;8:165–174. doi: 10.1023/B:AIBE.0000030247.00140.62. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Hopfer CJ, Haberstick B, Rhee SH, Crowley TJ, Corley RP, Ehringer MA. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug and Alcohol Dependence. 2009;104:11–16. doi: 10.1016/j.drugalcdep.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Herman DS, Hagerty CE, de Dios MA, Anderson BJ, Stein MD. Expectancies and self-efficacy mediate the effects of impulsivity on marijuana use outcomes: An application of the acquired preparedness model. Addictive Behaviors. 2011;36:389–396. doi: 10.1016/j.addbeh.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Lessem JM, Hartman CA, Stallings MC, Cherny SS, Corley RP, Crowley TJ. A genome-wide scan for loci influencing adolescent cannabis dependence symptoms: Evidence for linkage on chromosomes 3 and 9. Drug and Alcohol Dependence. 2007;89:34–41. doi: 10.1016/j.drugalcdep.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, Ehringer MA. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Filbey F. The incentive salience of alcohol: Translating the effects of genetic variant in CNR1. Archives of General Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, McClelland GH, Ryan CS. 2nd ed. New York, NY: Routledge; 2009. Data analysis: A model comparison approach. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- López-Moreno JA, Echeverry-Alzate V, Bühler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. Journal of Psychopharmacology. 2012;26:133–143. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- Lu J, Wei Q, Bondy ML, Li D, Brewster A, Shete S, Wang L-E. Polymorphisms and haplotypes of the NBS1 gene are associated with risk of sporadic breast cancer in non-Hispanic white women ≤55 years. Carcinogenesis. 2006;27:2209–2216. doi: 10.1093/carcin/bgl077. [DOI] [PubMed] [Google Scholar]
- Metrik J, Kahler CW, Reynolds B, McGeary JE, Monti PM, Haney M, Rohsenow DJ. Balanced placebo design with marijuana: Pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology. 2012;223:489–499. doi: 10.1007/s00213-012-2740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, McGeary J, Hutchison K. The Cannabinoid Receptor Gene (CNR1) polymorphism and expectancies in marijuana withdrawal among college drinkers [Abstract 347] Alcoholism: Clinical and Experimental Research, 29, Supplement. 2005;S1:65. [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discovery Today. 2010;15:474–483. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajewski NM, Parker SD, Poland GA, Ovsyannikova IG, Song W, Zhang K, Kaslow RA. The role of HLA-DR-DQ haplotypes in variable antibody responses to Anthrax Vaccine Adsorbed. Genes and Immunity. 2011;12:457–465. doi: 10.1038/gene.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51 doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pickard BS, Christoforou A, Thomson PA, Fawkes A, Evans KL, Morris SW, Muir WJ. Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder. Molecular Psychiatry. 2009;14:874–884. doi: 10.1038/mp.2008.24. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Robbe HW, O’Hanlon JF. Marijuana, alcohol and actual driving performance. Human Psychopharmacology. 2000;15:551–558. doi: 10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use & Misuse. 2010;45:1742–1765. doi: 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioural Pharmacology. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40:305–315. [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyper-activity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology. 2009;203:511–517. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Larkin EK, Elston RC, Redline S. Self-reported race and genetic admixture. The New England Journal of Medicine. 2006;354:421–422. doi: 10.1056/NEJMc052515. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: U.S. Government Printing Office; 2009. Results from the 2008 National Survey on Drug Use and Health: National findings (NSDUH Series H-36, HHS Publication No. SMA 09–4434) [Google Scholar]
- Tabachnick BG, Fidell LS. 4th ed. Boston, MA: Allyn & Bacon; 2001. Using multivariate statistics. [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: Studies of drug use and dependence in Caucasians. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology: General. 2008;137:649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch RE. Regression sensitivity analysis and bounded-influence estimation. In: Kmenta J, Ramsey JB, editors. Evaluation of econometric models. New York, NY: Academic Press; 1980. pp. 153–167. [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P-W, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, Uhl GR. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Molecular Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- Zuo L, Kranzler HR, Luo X, Covault J, Gelernter X. CNR1 variation modulates risk for drug and alcohol dependence. Biological Psychiatry. 2007;62:616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]