Abstract
Purpose
To investigate the efficiencies of platinum chemotherapeutic drugs (Pt-drugs) in the sensitization of DNA to the direct effects of ionizing radiation and to determine the role of low-energy electrons (LEEs) in this process.
Methods and Materials
Complexes of supercoiled plasmid DNA covalently bound to either cisplatin, carboplatin or oxaliplatin were prepared in different molar ratios. Solid films of DNA and DNA modified by Pt-drugs were irradiated with either 10-KeV or 10-eV electrons. DNA damages were quantified by gel electrophoresis, and the yields for damage formation were obtained from exposure-response curves.
Results
The presence of an average of two Pt-adducts in 3199-bp plasmid DNA increases the probability of a double-strand break by factors of 3.1, 2.5 and 2.4 for carboplatin, cisplatin and oxaliplatin, respectively. Electrons with energies of 10-eV and 10-KeV interact with Pt-adducts to preferentially enhance the formation of cluster lesions. The maximum increase in radiosensitivity per Pt-adduct is found at ratios up to 3.1 × 10−4 Pt-adducts per nucleotide which is equivalent to an average of two adducts per plasmid. Carboplatin and oxaliplatin show higher efficiencies than cisplatin in the radiosensitization of DNA. Since carboplatin and cisplatin give rise to identical reactive species which attach to DNA, carboplatin must be considered as a better radiosensitizers for equal number of Pt-adducts.
Conclusion
Pt-drugs preferentially enhance the formation of cluster damage to DNA induced by the direct effect of ionizing radiation and LEEs are the main species responsible for such an enhancement via the formation of electron resonances.
INTRODUCTION
Concomitant chemoradiation therapy (CRT) is a frequent treatment modality applied to several types of solid tumors and has improved cancer treatment. The primary clinical rationale supporting these types of treatment is the role of chemotherapeutic drugs as radiosensitizers. Platinum chemotherapeutic drugs (Pt-drugs) including cisplatin, carboplatin and oxaliplatin are frequently administered in CRT for the treatment of upper aerodigestive tract, genitourinary and colon malignancies (1). Although it has been shown that the combination of Pt-drugs and radiation improve treatment outcome, the optimum parameters for CRT with Pt-drugs and the underlying mechanisms of their synergistic action remain the subject of active investigation.
Cisplatin has significant activity against several forms of neoplasm, including ovarian, cervical, head and neck, and non-small-cell lung cancer. However, side effects and tumour resistance to the drug have limited its applications (2). Carboplatin has less systemic toxicity than cisplatin. The types of cancer that can be treated by carboplatin are similar to those of cisplatin and it has often replaced cisplatin. Oxaliplatin has a different pattern of sensitivity, a safer toxicity profile and activity against cisplatin-resistant cancer. It is a standard chemotherapeutic drug for treatment of colorectal cancer. After entry of the Pt-drug molecules into the cell, they subsequently convert to chemically reactive forms due to hydrolysis and react with DNA via ligand exchange at the platinum atom to form Pt-drug-DNA adducts (Pt-adducts) including intra and interstrand cross-links (CL) and monofunctional binding to guanine (3). Formation of these CLs leads to the distortion of DNA conformation by unwinding, bending and destabilization of the double helix (4). In addition to affecting transcription and replication processes, the Pt-adducts are believed to specifically inhibit DNA repair of radiation-induced lesions and enhance radiation damage to DNA (5).
The biological impact of ionizing radiation results from the induction of a variety of lesions, predominantly via energy deposition into the DNA itself (direct effect) and its surrounding molecular environment, consisting mostly of water molecules (indirect effect). The energy deposition generates intermediate species including ions, radicals, excited molecules and free electrons in nanoscale volumes that subsequently interact with cellular DNA. The most numerous of these species are non-thermal secondary electrons. Most of the latter have energies below 30-eV and a most probable energy of about 10-eV (6). These LEEs, which carry most of the primary radiation energy, have a mean free path of a few angstroms. Thus they can deposit all of their energies inside DNA and induce damage including single- and double-strand breaks (SSB and DSB), base release and modification via resonance scattering mechanisms (7).
Pt-drugs can enhance radiation damage to DNA by increasing the number of the secondary reactive species generated by primary radiation and/or by sensitizing DNA towards these species. In the indirect effect of radiation, cisplatin sensitizes DNA to hydroxyl radicals and hydrated electrons resulting in the enhanced formation of SSB and DSB (8). Theoretical and experimental studies have also reported that both LEEs and prehydrated electrons can interact with cisplatin and release the chlorine atom from its molecule via dissociative electron attachment (DEA) (9,10,11). Such an indirect effect of radiation produces reactive radicals of cisplatin, which can damage cellular components, including DNA (11,12).
In our laboratory, Zheng et. al. studied direct interaction of LEEs with dry solid films composed of plasmid DNA modified by cisplatin and observed an enhancement in the formation of SSB and DSB (13). Later, Rezaee et. al. showed that the conditions of reaction of Pt-drugs with DNA have substantial effects on the chemical stability of DNA, making it sensitive to the manipulations required for sample preparation (14). Minimizing the adverse effects of platination on DNA integrity, we here investigate the relative efficiency of the Pt-drugs cisplatin, carboplatin and oxaliplatin in DNA sensitization to the direct effects of ionizing radiation, with electrons of 10-KeV and 10-eV. These two electron energies can represent the direct effect of high-energy ionizing radiation and the secondary LEEs on DNA in radiotherapy. Comparing the results obtained with 10-KeV electrons with those of 10-eV, which constitute a major product of ionization, allows us to determine the role of LEEs in the DNA radiosensitization. The optimal DNA radiosensitivity respecting the quantity of Pt-adducts and possible mechanisms responsible for radiosensitisation are determined by measuring the yield of different types of damages and calculating enhancement factors (EFs) as a means of comparing radiosensitization efficiencies. Assuming that the main target in radiotherapy is nuclear DNA, the present results provide guidance for improving CRT.
MATERIALS AND METHODS
Sample Preparation
Plasmid DNA (pGEM-3Zf-) was extracted from E-Coli and mixed with the solutions of Pt-drugs including cisplatin, carboplatin and oxaliplatin. According to the kinetics of binding Pt-drugs to DNA (Fig. 1e, 2e, and3e) measured by inductively coupled plasma-mass spectroscopy (ICP-MS), samples of Pt-drug-DNA complexes were prepared at various concentration ratios between 1:1 and 64:1 and deposited onto tantalum substrate (S1 supplement).
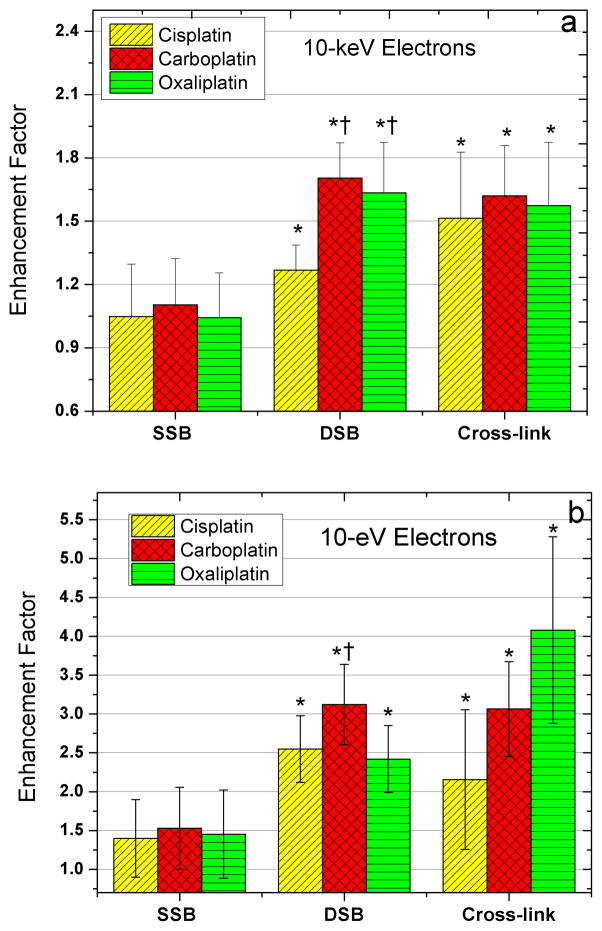
Fig. 1.
Enhancement factors in the yields of SSB, DSB and interduplex CL induced by 10-KeV (a) and 10-eV (b) electrons in the presence of cisplatin, carboplatin and oxaliplatin.
* indicates a P-value < 0.05, when the different Pt-drug-DNA complexes are compared to unmodified DNA.
† denotes a P-value < 0.05, when the different Pt-drug-DNA complexes are compared to each other.
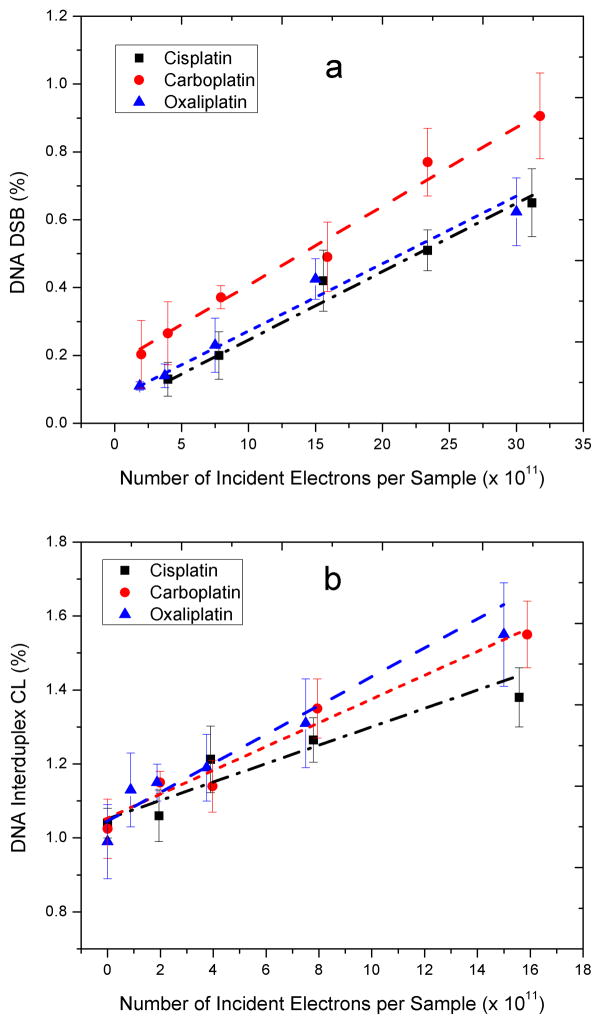
Fig. 2.
Exposure-response curve for the formation of DSB (a, b) and interduplex CL (c, d) by either 10-eV or 10-KeV electrons in DNA modified by cisplatin, carboplatin and oxaliplatin. Data are means ± standard deviation from five measurements. They have been fitted by employing a least-squares regression analysis.
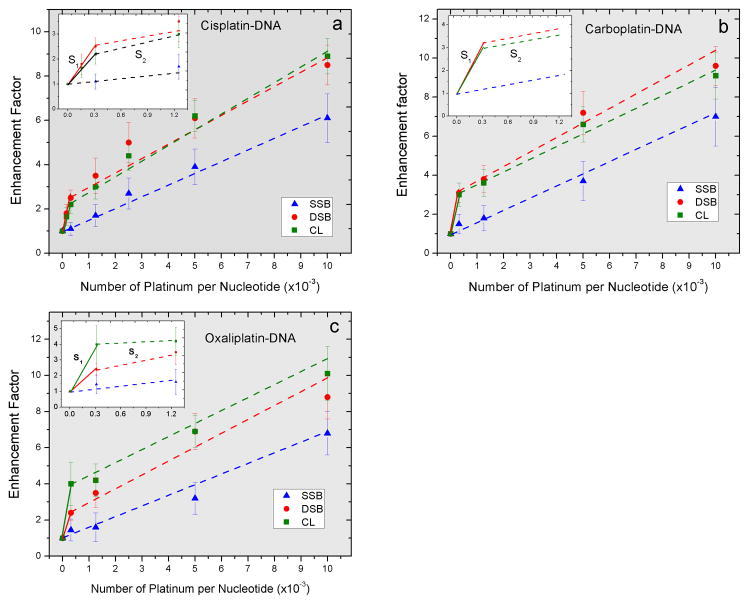
Fig. 3.
Enhancement factors in the yields of SSB, DSB and interduplex CL as a function of the number of Pt-adducts per nucleotide for DNA modified by cisplatin (a), carboplatin (b) and oxaliplatin (c) irradiated with 10-eV electrons. The fitted lines are based on a least-square regression analysis.
Sample Irradiation
The nanoscale films were irradiated with electrons in a home-made laboratory apparatus (Fig. e4). The DNA films were individually irradiated with electrons of either 10- or 10,000-eV for periods between 5 s and 16 min.
Qualification of DNA Damages
After irradiation, the films were immediately retrieved from the apparatus and dissolved in TE buffer from their substrate with 95–98% efficiency. The relative percentage of the different structural forms including supercoiled, nicked circular (SSB), linear (DSB) and interduplex CL in each DNA and Pt-drug-DNA sample was obtained by agarose gel electrophoresis. The amount of each structural form of the DNA was then analyzed by ImageQuant (Molecular Dynamics) software (14).
Calculation of the yields of DNA damages
The yield for electrons induced SSB, DSB and interduplex CL (i.e., dimers of two circular forms of plasmid) were derived from the initial linear slopes of the respective exposure–response curves (S2 Supplement). In addition, ratios of the yields from irradiated the Pt-adduct-DNA molecules to those of unmodified DNA were determined as EFs. These factors represent the radiosensitivity index of Pt-drugs in the induction of SSB, DSB and interduplex CL by ionizing radiation and LEEs.
RESULTS AND DISCUSSION
Relative radiosensitization efficiency of Pt-drugs
Table 1 presents the yields of SSB, DSB and interduplex CL in DNA induced by 10-KeV and 10-eV electrons in unmodified DNA and DNA containing on average of two Pt-adducts per plasmid (i.e., one Pt-drug per 1600 bp). Assuming a Poisson distribution for the binding of Pt-drugs to the plasmids, we expect about 13% of plasmids with no adducts, 74% with 1–3 adducts, and 14% with more than three adducts. Fig. 1 compares the EFs for cisplatin, carboplatin and oxaliplatin. The presence of Pt-adducts substantially enhances the formation of DSB and interduplex CL by electrons, particularly for 10-eV, whereas for SSB formation this enhancement is at the most 50%.
Table 1.
Yields of DNA damage (×10−8 damage/Gy/bp) for 10-KeV (a) and 10-eV (b) electron irradiation of DNA with and without modification by Pt-drugs.
| Irradiation | DNA Damage* | Pure DNA | Cisplatin-DNA | Carboplatin-DNA | Oxaliplatin-DNA |
|---|---|---|---|---|---|
| 10 KeV | SSB | 145.3 ± 23.6 | 152.2 ± 26.5 | 160.4 ± 18.6 | 151.4 ± 18.9 |
| DSB | 0.7 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | |
| CL | 4.5 ± 0.6 | 6.8 ± 1.1 | 7.3 ± 0.3 | 7.1 ± 0.9 | |
|
| |||||
| 10 eV | SSB | 78.7 ± 16.2 | 110.1 ± 15.8 | 120.4 ± 21.7 | 114.3 ± 26.3 |
| DSB | 1.2 ± 0.2 | 3.0 ± 0.2 | 3.7 ± 0.2 | 2.9 ± 0.2 | |
| CL | 3.3 ± 0.9 | 7.2 ± 2.1 | 10.2 ± 1.6 | 13.5 ± 4.6 | |
SSB: single strand break; DSB: double strand break; CL: interduplex cross-link
Photons and electrons of 0.2–20MeV are standard beams in radiotherapy. The interaction of such photons with biomolecules via Compton and pair production produces electrons with a wide energy distribution. Energy deposition by the high-energy electrons into biological matter can be calculated using the Born approximation, in which the interaction between electron and matter leads to individual localized-molecular collisions, with negligible momentum transfer, separated by mean free paths much larger than atomic dimensions. Accordingly, electrons with the energies of a few KeV to a few MeV interact with matter essentially via the same fundamental process. Thus, 10-KeV electrons can generally be considered to represent the direct effects of high-energy ionizing radiation on DNA in radiotherapy. The relative radiosensitization efficiency of Pt-drugs can therefore be discussed in terms of the results of 10-KeV irradiation.
For 10-KeV electron-irradiated DNA, there is a 1.7-fold increase in the yield of DSB in the presence of carboplatin and oxaliplatin adducts. This factor is reduced to 1.3 for DNA modified by cisplatin. Additionally, the enhancements in DSB yields for DNA modified by either carboplatin or oxaliplatin are significantly larger than that observed in the presence of cisplatin (P-values: 0.0017 and 0.0365, respectively). Similarly, all Pt-drugs sensitize DNA to the interduplex CL formation by 10-KeV electrons. Both carboplatin and oxaliplatin enhance the radiation-induced interduplex CL by a factor of 1.6, compared to 1.4 in the case of cisplatin. In contrast to the DSB yield, there is no substantial difference in the yields of interduplex CL between the three types of modified DNA. Since DSB results from two separate lesions in DNA, these findings suggest that the Pt-drugs preferentially enhance the formation of cluster damage to DNA by ionizing radiation. The enhancement is larger in the presence of carboplatin and oxaliplatin relative to cisplatin. The presence of cluster damages together with Pt-adducts is expected to be extremely toxic for cells owing to the difficulty in performing error-free repair of such locally multiply damaged sites.
Pt-drugs bind to DNA to form mainly intrastrand and interstrand CLs with the purine bases, particularly guanine (4). Despite the similarity in the types of DNA adducts, each Pt-drug produces different proportions of the specific adducts. The major carboplatin adduct, for instance, is reported to be 1,3-d(GpNpG) intrastrand CL, whereas cisplatin and oxaliplatin mostly form 1,2-d(GpG) intrastrand CL (15). The presence of the diaminocyclohexane ligand in oxaliplatin has been shown to lead to several conformational differences between DNA adducts formed by oxaliplatin and cisplatin, predominantly due to the interaction between diaminocyclohexane ligand and DNA constituents (15). Although subtle, such differences distinctively change the conformation of DNA: the carboplatin adduct unwinds DNA to a higher degree than the cisplatin adduct (23° versus 13°, respectively), and oxaliplatin bends DNA less than cisplatin (15). These different alterations in the DNA conformation suggest that each Pt-drug may affect the chemical and physical properties of modified DNA in a distinctive manner, and hence the interaction of LEEs with DNA, as observed in our results. Therefore, it is suggested that the type of Pt-adduct plays a vital role in the DNA sensitization towards ionizing radiation.
In the clinic, carboplatin is an appropriate alternative to cisplatin, owing to its lower side effects and a spectrum of activity similar to cisplatin. Since carboplatin has a more stable leaving group (cyclobutane di-carboxylato) than chloride and oxalate ligands in cisplatin and oxaliplatin, respectively, it shows a lower reactivity to both hydrolysis and sulphur-containing molecules such as glutathione and metallotheonines, thus permitting the administration of larger doses and greater accumulation of carboplatin inside the cell nucleus relative to cisplatin and oxaliplatin (2). For equal number of Pt-adducts, our results indicate that carboplatin, when administered concurrently with radiation, would be expected to be superior to cisplatin due to its greater efficiency in the induction of cluster damages to DNA, predominantly DSB. Hence, the radiosensitization effects of carboplatin are expected be greater than those of cisplatin in the clinic. Oxaliplatin has a different spectrum of activity relative to cisplatin and carboplatin, and at the same concentration, it has a lower reactivity than the two other Pt-drugs to DNA, leading to lower levels of adducts, although its cytotoxicity is similar to cisplatin (16). Our results indicate that oxaliplatin further enhances the formation of DSB compared to cisplatin by a factor of 1.3 for 10-KeV electron radiation. The superiority of oxaliplatin to cisplatin for radiosensitization, on a ‘per adduct’ basis, suggests that one should obtain a similar radiosensitization of tumour cells with lower levels of oxaliplatin adducts.
The role of LEEs in the radiosensitization of modified DNA
Electrons damage DNA through both resonant and non-resonant processes. The latter are single- or multiple-event processes including ionization and dissociative electronic excitation, whereas the former is a single-event process, which induces DNA damages by the formation of a local transient anion and its decay into the dissociative electronic excitation or dissociative electron attachment (DEA) channels. These electron resonances constitute the dominant interaction of electrons with energies lower than 15 eV (LEEs) with DNA. To determine the contribution of resonant processes towards the observed enhancements of DNA damage by high-energy radiation in the presence of Pt-drugs, solid films composed of either unmodified or modified DNA were also irradiated with 10-eV electrons.
As shown in Table 1 and Fig. 1, 10-eV yields of DSB increase by factors of 3.1, 2.5, and 2.4, respectively, when carboplatin, cisplatin and oxaliplatin are bound to DNA (P-value < 0.001). Moreover, the enhancement of DSB is greater with carboplatin than with either cisplatin or oxaliplatin (P-value: 0.013, 0.008, respectively). The yields of interduplex CL induced by 10-eV electrons are also enhanced significantly by factors of 2.2, 3.1 and 4.1 in the presence of cisplatin, carboplatin and oxaliplatin, respectively, (P-value < 0.05). Since LEEs constitute a major portion of the secondary species generated by high-energy radiation, these findings suggest that, in the presence of Pt-adducts, LEEs play a major role in the enhancement of DNA damage, particularly cluster damages.
Fig. 2 shows the exposure-response curves for the formation of DSB and interduplex CL in modified DNA irradiated with 10-eV electrons. These curves exhibit a linear behaviour, which suggests that a single-hit process is responsible for the LEEs-induced damages. For interduplex CL, it is reasonable to suggest that a reactive specie formed on one DNA molecule may attack an adjacent molecule, thus one would expect a linear exposure-response curve. However, the interaction of only one LEE with DNA also leads to DSB, which are due to two separate leasions. At 10-eV, the incident electrons essentially break the bonds between DNA constituents via core-excited resonances (7, 17). Dissociative TNI can rupture chemical bonds between DNA constituents via DEA; alternatively they may decay via electron autodetachment resulting in the departure of an electron with lower kinetic energy and the formation of an electronically excited neutral molecule that may itself dissociate into various fragments (18). In the presence of Pt-adducts, we hypothesize that dissociation of one TNI formed at the phosphate group of DNA may lead to the formation of the second lesion via two mechanisms: subsequent formation of a TNI and mechanical stress. The former may occur when the TNI decays via electron autodetachment and leaves the molecular group in an electronically excited dissociative state. In this manner, the phosphate group can dissociate to produce a SSB, and the departing electron can be recaptured by the Pt-adduct on the adjacent strand to form a subsequent TNI, which dissociates into an anion and a radical. The mechanical stress results from the modification of DNA conformation due to the Pt-adducts, which reduces the chemical stability of DNA and weakens certain bonds, particularly at the site of DNA platination. Thus, during the conversion of supercoiled to circular plasmid, which normally leads to a SSB, other weak bonds could rupture resulting in the formation of a second lesion.
Such mechanical stress can also be responsible for the observed differences in the EFs among the Pt-drugs. Structural alterations of the double helix of DNA induced by carboplatin (due to the formation of 1,3-d(GpNpG) intrastrand CL), for example, are more severe than those induced by cisplatin and oxaliplatin (i.e., the formation of 1,2-d(GpG) intrastrand CL) (15). This difference results in a greater perturbation of the chemical bonds between DNA components at the platination site. Such perturbation could enhance the formation of TNI and dissociation of the chemical bonds.
Dependence of DNA radiosensitization on the number of Pt-adducts
Fig. 3 shows the EFs for the formation of DSB, interduplex CL and SSB by 10-eV electrons as a function of the number of Pt-adducts. The curves of these EFs for DSB and interduplex CL formations exhibit a biphasic behaviour, with an initial steep slopes (S1) of about (4.0–9.5)×103 up to the 0.31×10−3 Pt-adducts per nucleotide and a final slopes of (0.5–0.7)×103. Hence, optimum radiosensitization of DNA, in terms of damage per Pt-adduct lies below ratios of 0.31×10−3 Pt-adducts per nucleotide.
Intrastrand CLs are the primary Pt-adducts observed in short synthetic DNA and in cultured cells. However studies on the platination of plasmid DNA show that at a low concentration of Pt-drugs (less than 0.5×10−3 Pt-molecules/nucleotide), interstrand CLs are the most probable adducts in the supercoiled form, while intrastrand CLs are the most probable in the relaxed and linear DNA forms (19). This concentration of Pt-drugs is very close to that of which the EF curve changes slope in the present study (Fig. 3). These findings suggest that the substantial difference observed in the radiosensitivity of modified DNA at the ratios beyond 0.31×10−3 Pt-adducts per nucleotide may depend on the type of Pt-adduct (e.g., interstrand versus intrastrand crosslinks). This ratio however has a value of one or two orders of magnitude higher than those measured from the malignant tissue of the patients typically treated with Pt-drugs in clinical trials. Since Pt-drugs, particularly cisplatin and oxaliplatin have severe side effects such as neurotoxicity, renal and gastrointestinal toxicity, it is impossible to increase the dose of the drugs in clinical applications. In contrast, the ratio of Pt-drugs to DNA in the cancer tissue of the patients treated with liposomal Pt-drugs, i.e., encapsulation of the drug into nanoparticle formulation, is similar to our proposed ratio resulting in the optimal radiosensitization (20).
CONCLUSION
The presence of Pt-adducts preferencially enhances the formation of DNA cluster damages, including DSB and interduplex CL, induced by the direct effects of ionizing radiation, particularly those produced by LEEs. Despite similarities between Pt-drugs in the enhancement of the DNA lesions, carboplatin and oxaliplatin have a higher efficiency than cisplatin. Since the reactive forms of the Pt-drugs are identical between cisplatin and carboplatin, and similar to oxaliplatin, the type of Pt-adducts must be responsible for the observed different efficienies in DNA radiosensitization by Pt-drugs. The yields and EFs for the formation of cluster lesions by 10-eV electrons are larger than those by 10-KeV electrons, suggesting that LEEs are the main secondary species responsible for the enhancement of DNA damage in the presence of Pt-drugs. In addition, the induction of cluster lesions by 10-eV electrons results from a single-hit process as deduced from the linearity of the exposure-response curves. Moreover, radiosensitization enhancement versus quantity of Pt-adducts is bi-phasic with a change in slope at a ratio of 3.1×10−4 Pt-adducts per nucleotide. It appears that the radiosensitization by Pt-drugs depends considerably on the type of Pt-adducts.
For clinical applications, therefore, it is reasonable to infer that the effectiveness of radiation beams generating more LEEs would be higher in Pt-based CRT. Owing to the greater ionization density, the radiation beams with high linear energy transfer (LET), such as alpha, proton and heavy ion beams, produce a large number of LEEs in their tracks compared to low LET radiation (e.g., photons and electrons), hence they should be more efficient in Pt-based CRT.
Supplementary Material
Table 2.
Enhancement Factors for the induction of SSB, DSB and interduplex CL as a function of the number of Pt-adducts per nucleotide. S1 and S2 denote the slopes of the fitted lines to the Enhancement Factor curves for the DNA damages presented in Fig. 3 at ratios less and more that 3.1 × 10−4 Pt-adducts per nucleotide, respectively. R1,2 is the ratio of S1 to S2.
| DNA Damage* | Pt-drug | S1 (× 103) | S2 (× 103) | R1,2 |
|---|---|---|---|---|
| SSB | Cisplatin | 0.51 ± 0.03 | 0.51 ± 0.03 | 1 ± 0.1 |
| Carboplatin | 0.57 ± 0.04 | 0.57 ± 0.04 | 1 ± 0.1 | |
| Oxaliplatin | 0.56 ± 0.06 | 0.56 ± 0.06 | 1 ± 0.1 | |
|
| ||||
| DSB | Cisplatin | 4.8 ± 0.2 | 0.54 ± 0.07 | 8.9 ± 1.1 |
| Carboplatin | 6.7 ± 0.8 | 0.65 ± 0.1 | 10.3 ± 2.0 | |
| Oxaliplatin | 4.5 ± 0.6 | 0.59 ± 0.1 | 7.6 ± 1.6 | |
|
| ||||
| CL | Cisplatin | 3.84 ± 0.2 | 0.65 ± 0.06 | 5.9 ± 0.8 |
| Carboplatin | 6.4 ± 0.9 | 0.62 ± 0.08 | 10.3 ± 1.9 | |
| Oxaliplatin | 9.5 ± 2.6 | 0.67 ± 0.03 | 14.2 ± 3.9 | |
SSB: single strand break; DSB: double strand break; CL: interduplex cross-link
SUMMARY.
Concurrent administration of platinum chemotherapeutic drugs (Pt-drugs) and radiation improves the treatment of several solid tumours by enhancing local therapy; however, the underlying mechanisms of the synergistic action between Pt-drugs and radiation remain the subject of active investigation. We report that Pt-drugs preferentially enhance the formation of cluster damage to DNA by ionizing radiation and that LEEs play the major role in the induction of such lesions through a quantum process known as electron resonances.
Acknowledgments
Financial support for this work was provided by Canadian Institutes of Health Research (CIHR). The authors would like to thank Dr. AD. Bass for his helpful comments and P.Cloutier for technical support.
Footnotes
Conflict of interest: none
References
- 1.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm - general principles. Nat Clin Pract Oncol. 2007;4(2):86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 2.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 4.Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107(5):1387–407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 5.Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation. Semin Radiat Oncol. 2006;16(1):2–9. doi: 10.1016/j.semradonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Pimblott SM, LaVerne JA. Production of low-energy electrons by ionizing radiation. Radiat Phys Chem. 2007;76(8–9):1244–7. [Google Scholar]
- 7.Sanche L. Low-energy electron interaction with DNA: Bond dissociation and formation of transient anions, radicals, and radical anions. In: Greenberg Marc M., editor. Radical and radical ion reactivity. New Jersey: John Wiley & Sons; 2009. pp. 239–94. [Google Scholar]
- 8.Rezaee M, Sanche L, Hunting DJ. Cisplatin enhances the formation of DNA single- and double-strand breaks by hydrated electrons and hydroxyl radicals. Radiat Res. 2013;179(3):323–31. doi: 10.1667/RR3185.1. [DOI] [PubMed] [Google Scholar]
- 9.Kopyra J, Koenig-Lehmann C, Bald I, Illenberger E. A single slow electron triggers the loss of both chlorine atoms from the anticancer drug cisplatin: Implications for chemoradiation therapy. Angewandte Chemie-International Edition. 2009;48(42):7904–7. doi: 10.1002/anie.200903874. [DOI] [PubMed] [Google Scholar]
- 10.Kuduk-Jaworska J, Chojnacki H, Jański JJ. Non-empirical quantum chemical studies on electron transfer reactions in trans- and cis-diamminedichloroplatinum(II) complexes. J Mol Modeling. 2011;17(9):2411–21. doi: 10.1007/s00894-011-1060-1. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q-B, Kalantari S, Wang C. Electron transfer reaction mechanism of cisplatin with DNA at the molecular level. Molecular Pharmaceutics. 2007;4(4):624–8. doi: 10.1021/mp070040a. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q-B. Molecular reaction mechanisms of combination treatments of low-dose cisplatin with radiotherapy and photodynamic therapy. J Med Chem. 2007;50(11):2601–4. doi: 10.1021/jm061416b. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Role of secondary low-energy electrons in the concomitant chemoradiation therapy of cancer. Phys Rev Lett. 2008;100(19) doi: 10.1103/PhysRevLett.100.198101. [DOI] [PubMed] [Google Scholar]
- 14.Rezaee M, Alizadeh E, Hunting D, Sanche L. DNA-platinum thin films for use in chemoradiation therapy studies. Bioinorg Chem Appl. 2012;2012:923914. doi: 10.1155/2012/923914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics. 2009;1(4):280–91. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woynarowski JM, Faivre S, Herzig MCS, Arnett B, Chapman WG, Trevino AV, et al. Oxaliplatin-induced damage of cellular DNA. Mol Pharmacol. 2000;58(5):920–7. doi: 10.1124/mol.58.5.920. [DOI] [PubMed] [Google Scholar]
- 17.Boudaïffa B, Cloutier P, Hunting D, Huels MA, Sanche L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science. 2000;287(5458):1658–60. doi: 10.1126/science.287.5458.1658. [DOI] [PubMed] [Google Scholar]
- 18.Sanche L. Role of secondary low energy electrons in radiobiology and chemoradiation therapy of cancer. Chemical Physics Letters. 2009;474(1–3):1–6. [Google Scholar]
- 19.Vrána O, Boudńy V, Brabec V. Superhelical torsion controls DNA interstrand crosslinking by antitumor cis-diamminedichloroplatinum(II) Nucleic Acids Res. 1996;24(20):3918–25. doi: 10.1093/nar/24.20.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulikas T, Stathopoulos GP, Volakakis N, Vougiouka M. Systemic lipoplatin infusion results in preferential tumor uptake in human studies. Anticancer Res. 2005;25(4):3031–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.