Abstract
The genotoxic effects of high energy ionizing radiation have been largely attributed to the ionization of H2O leading to hydroxyl radicals (OH) and the ionization of DNA leading mostly to damage through base radical cations. However, the contribution of low energy electrons (LEEs; ≤ 10 eV), which involves sub-ionization events, has been considered to be less important than that of hydroxyl radicals and base radical cations. Here, we compare the ability of LEEs and high energy X-ray photons to induce DNA damage using dried thin films of TpTpT trinucleotide as a simple and representative model for DNA damage. The main radiation-induced damage of TpTpT as measured by HPLC-UV and LC-MS/MS analyses included thymine release (-Thy), strand breaks (pT, Tp, pTpT, TpTp and TpT), and the formation of base modifications (5,6-dihydrothymine (5,6-dhT), 5-hydroxymethyluracil (5-hmU) and 5-formyluracil (5-fU)). The global profile of products was very similar for both types of radiation indicating converging pathways of formation. The percent damage of thymine release, fragmentation and base modification was 20, 19 and 61 for high energy X-rays, respectively, compared to 35, 13 and 51 for LEEs (10 eV). Base release was significantly lower for X-rays. In both cases, phosphodiester bond cleavage gave mononucleotides (pT and Tp) and dinucleotides (pTpT and TpTp) containing a terminal phosphate as the major fragments. For base modifications, the ratio of reductive (5,6-dhT) to oxidative products (5-hmU plus 5-fU) was 0.9 for high energy X-rays compared to 1.7 for LEEs. These results indicate that LEEs give a similar profile of products compared to ionizing radiation.
Keywords: Ionizing radiation, DNA damage, X-ray photon, low energy electrons, dissociative electron attachment
INTRODUCTION
It is well known that high energy ionizing radiation, e.g., X-ray and γ-ray, induce DNA damage, including strand breaks and a multitude of base modifications, by the reaction of hydroxyl radicals (•OH) with DNA or via one-electron ionization of DNA components.1 In particular, damage that occurs in close proximity to each other, referred to as clustered damage, is responsible in large part for the deleterious effects of high energy ionizing radiation, including cell death and the induction of mutations and genetic abnormalities that lead to cancer.2 This damage is also important to help eradicate many types of cancer, particularly if they are confined to a specific site in the patient with a well-defined margin from healthy normal tissue.3 To further increase the potential curative effects of ionizing radiation, it is necessary to understand the exact mechanism of radiation-induced DNA damage at the physical chemical and chemical level.3b,c As mentioned above, high energy radiation can generate secondary species along the radiation track, and thus, it is important to differentiate the effect of ionization from the effect of secondary electrons. These studies will hopefully provide additional information needed to treat cancer and design strategies combining both radiation and chemotherapeutic drugs.
During the last two decades, numerous theoretical and experimental studies have shown that low energy electrons (LEEs) can induce DNA damage, including single-strand breaks (SSB), double-strand breaks (DSB), base release and structural modifications.4 LEE-mediated DNA damage may also contribute to the synergistic effects of radiation in combination with certain chemotherapeutic agents, such as cis-platin.5 In general, secondary electrons, positive and negative ions, and radicals are generated along high energy radiation tracks.6 Among these species, secondary electrons with a most probable energy of about 10 eV are the most abundant species (~ 4 × 104 /MeV).6b These species do not readily ionize DNA components but they undergo resonance processes, such as dissociative electron attachment (DEA).6b, 7 After much experimental and theoretical work, it is now well established that LEEs can induce DNA damage by the formation of transient anion states predominantly localized on the nucleobase moieties of DNA.8 Transient anions usually have a short lifetime (10−14 s); either the electron autodetaches or the anion dissociates (e− + AB → AB*− → A + B−) by a process known as dissociative electron attachment (DEA). This can lead to immediate phosphodiester (C-O bond) cleavage and N-glycosidic bond (C-N) cleavage.9
The overall goal of our work is to investigate the mechanism of LEE-induced DNA damage through the identification of intermediates and stable products; in this study, we compare the profile of damage for LEE radiation and high energy radiation. For LEE radiation, thin films of TpTpT were irradiated under ultrahigh vacuum (UHV). For X-ray photon radiation, DNA containers were irradiated at room temperature with a hydration level (Γ) of 2.5 mole H2O / mole nucleotide. To identify differences in the two types of radiation, DNA damage thereby produced was analyzed by high performance liquid chromatography with UV detection (HPLC-UV) and HPLC coupled to tandem mass spectrometry (LC-MS/MS).
EXPERIMENTAL METHODS
Sample preparation and irradiation
Trinucleotide TpTpT was purchased from UCDNA Services (Calgary, AB) and purified by HPLC before use. HPLC grade methanol and acetonitrile were purchased from Fisher Scientific and ammonium acetate (CH3COONH4) from Sigma-Aldrich. The irradiation of TpTpT samples with LEEs was carried out at the University of Sherbrooke while identically prepared samples were sent to the University of Rochester for X-ray irradiation. The experimental setup and procedure for LEE irradiation of TpTpT samples has been described in detail elsewhere.10 In brief, the experimental setup consists of a spin-coating device to deposit oligomer films onto the inside wall (3.2 cm length × 2.6 cm inner diameter) of tantalum (Ta) cylinders and a LEE irradiation system that generates monoenergetic LEEs and irradiates the DNA target under UHV. Such a system allows one to obtain the relatively large quantities of LEE-irradiation products needed for chemical analysis.
For spin-coating, 91 nmol of purified TpTpT (77 μg) was dissolved in 4.9 mL of nanopure grade H2O (Milli-Q water system, 18 M Ω·cm, 25°C) and the solution was evaporated inside seven cylinders. The average thickness of films inside the cylinder was estimated to be 2.5 nm ± 0.1 nm (4 to 5 monolayers (ML)), assuming that the molecules are uniformly distributed onto the inner surface of the cylinder and that the average density of DNA is 1.7 g cm−3.11 After spin-coating, the cylinders were transferred to the LEE irradiation chamber, which was subsequently evacuated for ~24 h to reach a vacuum of 10−8 torr. The electron gun with an energy resolution of 0.5 eV full width at half-maximum produced a monoenergetic 10 eV electron beam by adjusting the potential between the filament and the cylinder. Other parameters that can be adjusted include the time of irradiation, beam current, incident electron energy and the magnitude of the collimating magnetic field.10 The current and irradiation time of LEE exposure were selected to give an exposure within the linear regime of a dose response curve. In this regime, the film does not charge appreciably (i.e., does not accumulate much excess charges). The films were individually irradiated with monoenergetic 10 eV LEEs. The total number of electrons that reach the film (i.e., the fluence) was approximately 1016 with a beam flux of 1.28 × 1012 electrons/s cm2. All procedures were performed at room temperature (RT).
In the case of X-ray irradiation, purified and lyophilized TpTpT was dissolved in ultrapure distilled water (Gibco, Invitrogen). The concentration of this solution was determined by UV absorbance at 260 nm and was found to be approximately 5.7 mM. Aliquots of the TpTpT solution were then pipetted into open-ended silylated suprasil quartz tubes and dried in sealed chambers against P2O5. Under these conditions, DNA contains ~ 2.5 mole H2O/ mole nucleotide of duplex DNA (Γ = 2.5).12 The film weights were measured periodically with a Mettler Toledo XP2U Microbalance with an accuracy of ± 0.1 μg, which in terms of percent error of the film weight is less than 0.2%. The level of hydration of the film was calculated from the difference in the weight of the pre- and post-equilibrated films. The open ends of the suprasil tubes containing solid DNA at a specific level of hydration (Γ = 2.5) were X-ray irradiated at RT in a glove box. The X-ray source was a Varian/Eimac OEG-76H tungsten-target tube operated at 70 keV and 20 mA, and the X-ray beam was filtered by a 25 μm thick aluminum foil. The dosimetry is described elsewhere.13 The dose rate at RT inside the suprasil quartz capillary, after taking into account the attenuation by the quartz, was 1.1 kGy/min. The dose regime extended from 0 to a maximum of 75 kGy for the TpTpT films. After irradiation, the films were dissolved in ultrapure distilled water (Gibco, Invitrogen) to 0.5–1 fold weight to volume and then stored at −20°C before analysis by HPLC-UV.
Chemical analyses
In the case of LEE irradiation, the samples were recovered from the surface of each Ta cylinder by dissolution with 14 mL of nanopure grade H2O followed by lyophilization until the sample was completely dry (overnight). Usually, six TpTpT films were irradiated with LEEs and one cylinder was used as a control without irradiation. The samples were lyophilized and dissolved in 200 uL for HPLC-UV analysis. Half of the irradiated samples was analyzed directly by HPLC-UV while the other half was treated with alkaline phosphatase (AP, Roche Applied Science) for 60 min at 37°C before analysis to determine whether the fragments contained a terminal phosphate group. TpTpT samples exposed to X-rays were also analyzed by HPLC-UV using the same procedure as that for LEE-irradiated samples. The mixture of products was separated using an Alliance 2695 instrument (Waters) equipped with an YMC-Pack Pro C18 column (5 μm particle size, 250 mm length × 6.0 mm I.D., YMC) using a linear gradient of mobile phase from 0% to 15% acetonitrile combined with 20 mM ammonium acetate buffer for 90 min (flow rate: 1.0 mL/min). The HPLC chromatograms of products were recorded by UV absorption at 210 nm and 260 nm. LC-MS/MS analysis was performed with a Shimadzu HPLC system consisting of an autosampler (SIL-HTc), binary pumps (LC-10ADvp), degasser (DGU-14A), column oven (CTO-10ASvp), UV/Vis detector (SPD-20A) and an API 3000 tandem mass spectrometer with a Turbulon Ionspray source (MDS Sciex, Applied Biosystems). Parameters for the detection of products were optimized prior to analysis using a syringe infusion pump (Harvard Model 22) set at a flow rate of 20 uL/min. For optimal detection of TpTpT, the cone voltage was set to 4500 volts and the collision energy set to 45 volts. The G-value for X-irradiated samples was calculated within the linear region of dose-response curves (0 to 40 kGy) and was based on the estimated target mass consisting of DNA and solvation shell, i.e., DNA alone plus 2.5 H2O per nucleotide.
RESULTS
The irradiation of TpTpT (see structure in Fig. 1) was carried out by exposing a thin film of TpTpT to a monoenergetic beam of LEEs (10 eV) under UHV, whereas in the case of X-rays, solid and dry samples of TpTpT were irradiated in sealed tubes under ambient conditions. The level of hydration of TpTpT was similar for both samples (Γ ≤ 2.5 mole H2O / mole nucleotide) assuming that UHV conditions of LEE irradiation reduces the level of hydration to a minimum, i.e., less than 2.5 mole H2O / mole nucleotide. Thus, one can assume that the formation of hydroxyl radicals is negligible for both LEE and X-ray irradiations. In addition, one can rule out an effect of O2 because both samples were evacuated of O2 before irradiation. It can be expected that both types of radiation generate radicals and ions in the solid state. Depending on the reactivity of these species, they will either transform further in the solid state or ultimately they will transform into stable products when the target is exposed to ambient conditions and dissolved in aqueous solution. The same procedures were used to recovery the products for analysis after irradiation.
Figure 1.
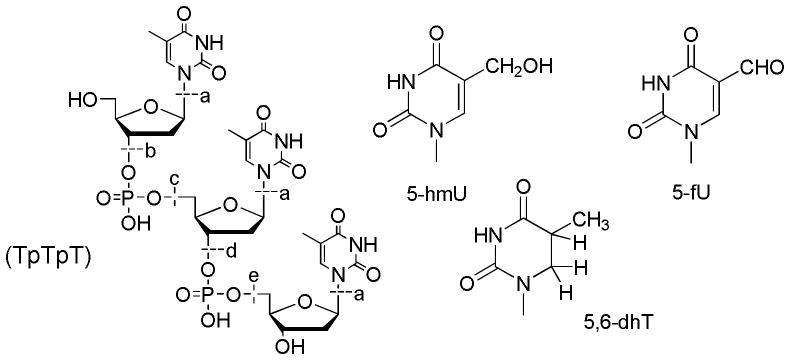
Structure of TpTpT and its modifications. Base release and fragments of TpTpT: Cleavage at (a) gives thymine (Thy) while cleavage at (b to e) gives fragments with a terminal phosphate group (b): pTpT; (c): Tp; (d): pT; and (e): TpTp, together with an unknown fragment. Base modifications of TpTpT: 5-hydroxymethyuracil (5-hmU), 5-formyluracil (5-fU) and 5,6-dihydrothymine (5,6-dhT); note that the bases remain attached to the TpTpT backbone, for example, TpXpT contains a modified base X at the center position of the trinucleotide.
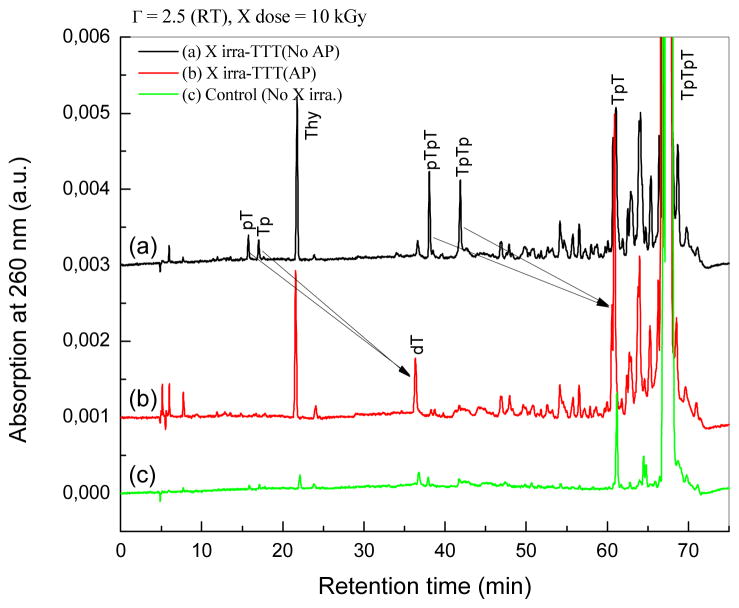
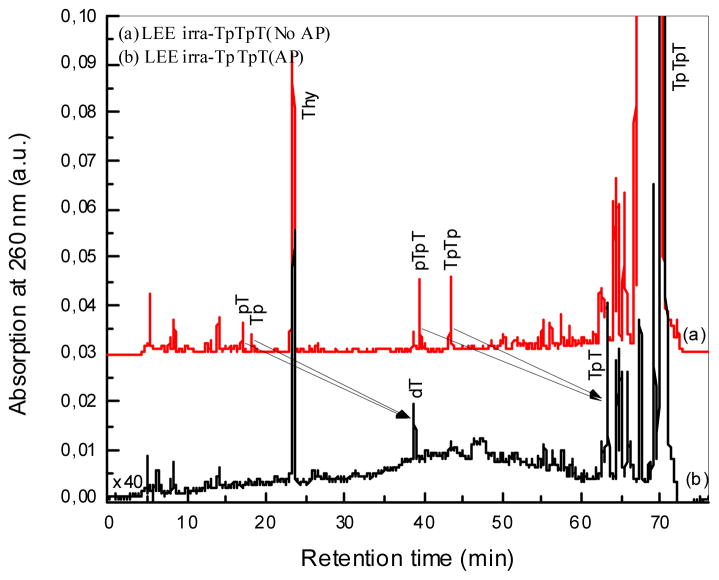
The profile of products for X-ray and LEE irradiated samples was very similar (Figs. 2 and 3). In the case of LEEs, TpTpT gave five characteristic fragments (see Fig. 1 for structures), which includ non-modified thymine base (Thy), two mononucleotides (pT and Tp), and two dinucleotides (pTpT and TpTp). The fragments were identified by co-elution on HPLC-UV with authentic compounds and LC-MS/MS analysis. When the sample was treated with alkaline phosphatase enzyme, which removes the terminal phosphate of nucleotides, we observed the conversion of mononucleotide, e.g., pT and Tp, and dinucleotide fragments, e.g., pTpT and TpTp to thymidine (dT) and TpT, respectively. This confirms the identity of the fragments and indicates that they contain a terminal phosphate. In addition, the chromatograms show that fragments with a terminal phosphate on the 5′ or 3′ terminus are formed in approximately equal yields. This pattern of fragmentation was previously reported for TpTpT as well as several other derivatives exposed to LEEs using the same system.8
Figure 2.
HPLC-UV analysis of TpTpT irradiated with X-rays (10 kGy). TpTpT was irradiated at room temperature in the solid state with minimum hydration (2.5 mole H2O/mole nucleotide). Samples were dissolved in H2O and injected without further treatment (a), and after treatment with AP (b), to convert products with a terminal phosphate to a terminal hydroxyl group (black arrows). A control sample without radiation is shown in the bottom chromatogram (c).
Figure 3.
HPLC-UV analysis of TpTpT irradiated with LEEs (10 eV). TpTpT was irradiated as a thin film at room temperature under UHV. Films were recovered from the irradiation cylinders, lyophilized and dissolved in H2O for analysis. Samples were injected without further treatment (a) and after treatment with alkaline phosphatase (b) similar to Fig. 2.
In the case of X-rays, the same fragments were observed as major products (Thy, pT, Tp, pTpT and TpTp; see Fig. 1 for structures). Similarly, mononucleotides converted to dT and dinucleotides to TpT upon treatment with alkaline phosphatase, and the yield of fragments with 5′ and 3′ phosphate termini was approximately equal. Thus, both LEE and X-rays irradiation of TpTpT lead to cleavage of the N-glycosidic bond giving Thy and cleavage of the phosphodiester bond giving fragments with a terminal phosphate group. The yield of fragments without a terminal phosphate, e.g., dT and TpT, was minor compared to those with a terminal phosphate. In addition, there were a number of other peaks in the chromatogram. The region of the chromatogram at short elution times (<15 min) points to the formation of small fragments of TpTpT consisting of a modified nucleobase or mononucleotide; however, the identification of these products was not possible because of the low yield. The region of the chromatogram between 40 and 60 min indicates the formation of as yet unknown di- or tri-nucleotides, which contain a base modification that shifts the polarity either to longer retention times for dinucleotides (more non-polar) or shorter retention times for trinucleotides (more polar). The profile of peaks in this region did not change upon treatment with alkaline phosphatase, suggesting that they do not contain a terminal phosphate. Together, unknown peaks in the chromatogram constituted about 20% of the total UV absorption at 260 nm. We did not attempt to identify these products because of their number and low yields but focused on the region of the chromatogram after 60 min which appears to contain the majority of base modifications.
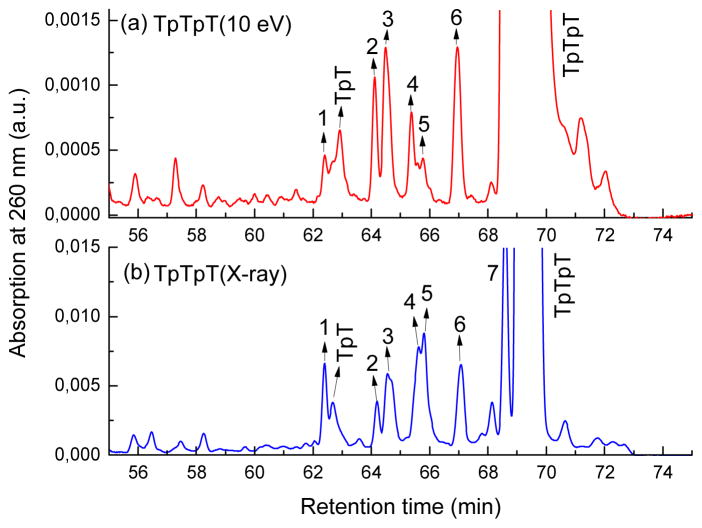
Base modifications of TpTpT eluted in the region of the chromatogram close to or between TpT and TpTpT (60–70 min). Initially, we compared the profile of base modifications on a one-to-one basis for TpTpT exposed to either LEE or X-ray (Fig. 4). On the basis of co-elution with a mixture of LEE and X-ray products, we labeled a total of seven major and common peaks in the chromatograms. The identity of peaks 1–7 was confirmed by LC-MS/MS (Table 1).
Figure 4.
Comparison of base damage by X-rays and LEEs. Panel (a) shows the chromatogram for X-ray induced products and panel (b) shows that for LEE-induced products. Products 1–7 denote TpTpT containing 5-hmU (peaks 1 and 5), 5,6-dhT (peaks 2, 3, 4 and 6), and 5-fU (peak 7). The modification can occur at different positions within TpTpT. Peak 7 did not separate well from the parent peak in the mixture of products from LEEs. The peaks were identified by LC-MS/MS analysis and co-elution with authentic standards (TpTpT containing 5,6-dhT).
Table 1.
Confirmation of products by LC-MS/MS.
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | |
|---|---|---|---|---|---|---|---|
| TpTpT(10 eV) | + | + | + | + | + | + | − |
| TpTpT(X-ray) | + | + | + | + | + | + | + |
| Mass(amu)a | 867 | 853 | 853 | 853 | 867 | 853 | 865 |
| Modified form | 5-hmU | 5,6-dhT | 5,6-dhT | 5,6-dhT | 5-hmU | 5,6-dhT | 5-fU |
Mass indicates (M+H)+ using mass analysis in positive mode. Three base modifications were observed: 5-hydroxymethyluracil (5-hmU), 5,6-dihydrothymine (5,6-dhT), and 5-formylmethyluracil (5-fU). (+: detected, −: non detected).
A large number of samples was pooled and carefully purified from each radiation mixture in order to obtain sufficient amounts for product analysis by LC-MS/MS. A major molecular ion was observed in each of the purified peaks. Thereby, peaks 2, 3, 4 and 6 were identified as TpTpT containing a single 5,6-dihydrothymine (5,6-dhT) on the basis of their molecular ion (m/z 853), fragment ions (m/z 629, 531, 225 and 127) and identification of the corresponding modified nucleoside after enzymatic digestion of the trinucleotide. These results confirm the formation of 5,6-dhT in TpTpT exposed to LEEs as reported in a previous study.8h Further analysis of the purified peaks revealed two addition molecular ions (m/z 867 for peaks 1 and 5 and m/z 865 for peak 7). The modification within TpTpT was subsequently identified from the analysis of peaks 1 and 5 as 5-hydroxymethyluracil (5-hmU), whereas that in peak 7 was identified as 5-formyluracil (5-fU), on the basis of enzymatic digestion of the trinucleotide and comparison of the mixture of nucleosides with authentic standards by LC-MS/MS analysis. The chromatograms depicted nonmodified dT together with modified nucleosides of 5-hmU or 5-fU with the same molecular ion, fragmentation spectra, and retention time on HPLC as authentic standards.14
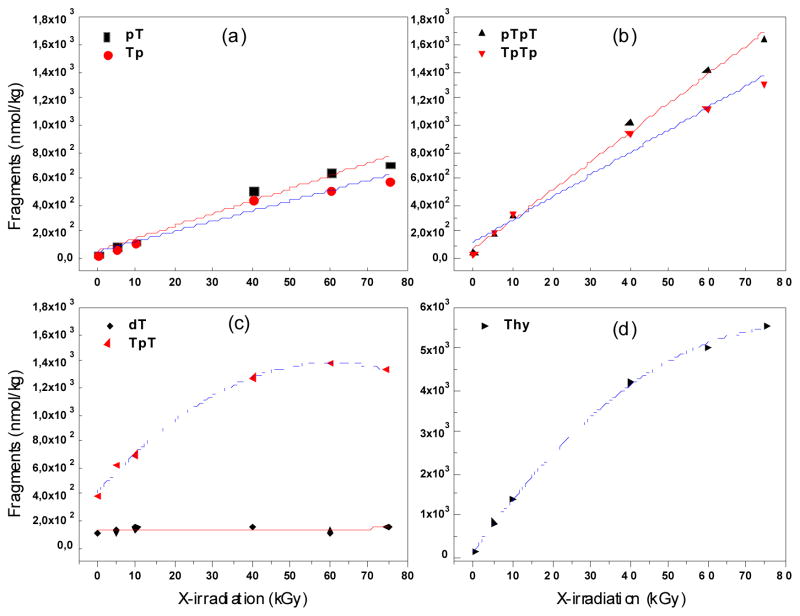
The relative yield of products by X-rays was estimated from the formation of products as a function of dose taking the initial and linear part of the function (Fig. 5; Table 2). The majority of products increased linearly as a function of dose through the entire dose range (0–75 kGy) with the exception of dT and Thy, which appeared to reach a plateau at doses above 10 kGy (Fig. 5). Thus, we used 10 kGy X-irradiated TpTpT in order to compare the yield of each group of products under single hit conditions. In the case of LEEs, the formation of products was linear as a function of time of irradiation up to 5 min, at which point, the formation of products decreased and reached a plateau at about 10 min. No reaction occurred at longer times (>10 min) due to charging of the film by trapped electrons. Thus, the yield of products for LEE experiments was taken in the linear region of the dose response curve. From these analyses, the relative yields of each group of products were compared for LEE and X-ray radiation (Table 2). For LEEs, the yield of base release and base modification dominated over fragmentation with percentages of 35% and 51% of the total damage, respectively. The ratio of base modifications to base release plus fragmentation was 1.06; thus, half of the incident LEEs induced DNA base modifications while the other half induced fragmentation upon interaction with DNA subunits. For X-rays, the percent of base release, fragmentation and base modifications was 20, 19, and 61, respectively. The dominant process was base modification, and the ratio of base modification base release plus fragmentation was 1.6. The main difference between LEE and X-ray radiation was the greater relative formation of base release (-Thy) in the case of LEEs. The yield of base modifications was also determine by enzymatic digestion of irradiated TpTpT followed by LC-MS/MS in multiple reaction monitoring (MRM) mode of the instrument. These results indicate that the ratio of reductive (5,6-dhT) to oxidative (5-hmU and 5-fU) was 0.9 and 1.7 for X-rays and LEEs, respectively (Table 3).
Figure 5.
Base release and strand break formation as a function of X-ray dose. The formation of several products is shown: pT and Tp (a), pTpT and TpTp (b), dT and TpT (c) and Thy (d) (see Fig. 1 for structures). The points were fit to either a linear or polynomial function.
Table 2.
Comparison of damage by LEE and X-ray radiationa
| Target TpTpT | Total Damageb | Base release | Fragments | Modification | ratiod | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Thy | %c | Sum | %c | Sum | %c | |||
| LEE (10 eV) | 16.7 | 5.9 | 35 | 2.2 | 13 | 8.6 | 51 | 1.06 |
| X-ray(10 kGy) | 12.2 | 2.4 | 20 | 2.3 | 19 | 7.5 | 61 | 1.60 |
See structure of TpTpT and products in Fig. 1. Yields are expressed as the number of products per 1000 parent molecules.
Total damage includes base release, fragments and modified forms of initial targeted molecules based on HPLC-UV analysis.
Percentage of total damage.
Ratio of modification to fragmentation.
Table 3.
Yield of identified base modifications induced by LEE and X-ray radiationa
| LEE (10 eV) | X-ray (10 kGy) | |
|---|---|---|
|
| ||
| 5-hmU | 3.02 ± 0.53 | 2.99 ± 0.42 |
| 5-fU | 0.22 ± 0.05 | 0.98 ± 0.17 |
| 5,6-dhT | 5.36 ± 0.66 | 3.53 ± 0.52 |
| Ratiob | 1.7 | 0.9 |
Yields were obtained by enzymatic digestion of irradiated TpTpT and LC-MS/MS analysis of the modified nucleotides14 expressed as the number of products per 1000 parent molecules.
Ratio of reduction products (5,6-dhT) to oxidation products (5-hmU and 5-fU).
The G-value for total damage induced by X-irradiated samples was estimated to be 363 ± 14 nmol/J from the formation of product as a function of dose (Fig. 5). It was not possible to estimate the G-values for LEE-induced damage using this irradiation system because the number of electrons actually absorbed by the film is not known with certainty. As shown by Alizadeh and Sanche, determination of G values for LEEs requires a study of the thickness dependence on the total degradation yields; such measurements were not possible with the present irradiator owing to large local variations in film thickness. The G value for strand breaks obtained from a study of the film thickness dependence of LEE-induced damage to plasmid DNA has been estimated to be 260 ± 50 nmol/J.15 Thus, assuming that strand breaks in plasmid DNA is equal to base release and fragmentation in our study with TpTpT, the G values for damage by LEE are comparable to those by X-rays.
DISCUSSION
A major finding of the present study is that exposure of TpTpT to either X-rays or LEEs lead to the same products. The reaction of both X-rays and LEEs with TpTpT involved the release of the nonmodified nucleobase, Thy, the formation of specific fragments (i.e., pT, Tp, pTpT and TpTp), and the formation of modified bases. The major base modification included a reductive product (5,6-dhT) and two oxidative products (5-hmU and 5-fU). The above fragments and base damage accounted for approximately 80% of the total UV absorbing products in the HPLC chromatogram upon irradiation of TpTpT. These results indicate that X-rays and LEEs induce similar radical intermediates and pathways of decomposition. Indeed, the formation of all of the products can be rationalized by pathways that involve either one-electron ionization, in the case of X-rays (Scheme 1), or sub-ionization processes, i.e., dissociative electron attachment (DEA), in the case of LEEs (Scheme 2).
Scheme 1.
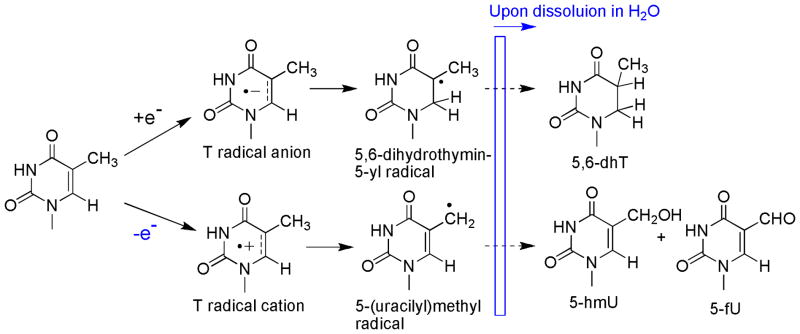
Proposed mechanism of base damage by one-electron ionization (X-rays)
Scheme 2.
Proposed mechanism of base damage by LEEs with sub-ionization events
Mechanism of formation of products by one-electron ionization
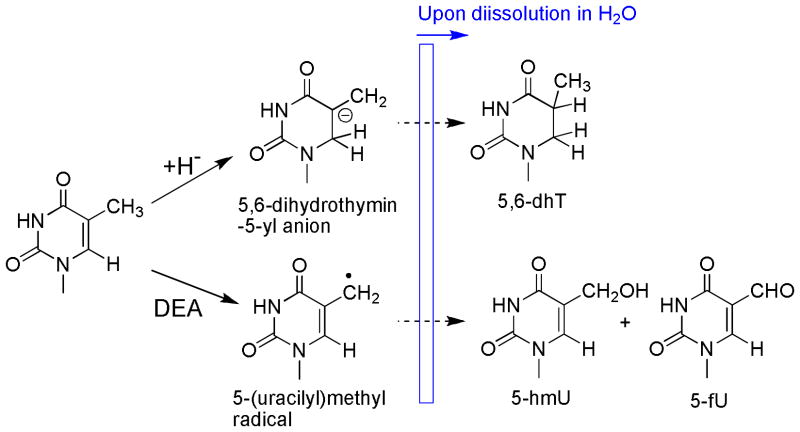
The removal of an electron from TpTpT upon exposure to X-rays can give the corresponding radical cations of the base (Thy), sugar and phosphate moieties (Scheme 1; only radical cations of Thy are shown). The formation of stable products from the nucleobase and nucleoside radical cations of Thy has been studied in detail by the analysis of intermediate species and stable products.1b, 16 Thereby, Thy derivatives undergo deprotonation from the methyl group leading to the corresponding 5-(uracilyl)methyl radical in competition with hydration at C6 of the pyrimidine ring leading to 6-hydroxyl-5,6-dihydrothymin-5-yl radicals. The division of these pathways depends on the experimental conditions such that deprotonation is favored (>90%) in dry solid samples, whereas hydration is favored over deprotonation with a ratio of 7:3 in aqueous solutions.16a Thus, the formation of 5-hmU and 5-fU modifications of TpTpT upon exposure to X-rays under dry conditions can be explained by initial deprotontation of Thy radical cations to 5-(uracilyl)methyl radicals (Scheme 1). It should be noted that 5-(uracilyl)methyl radicals are fairly long-lived in the condensed phase.16d The formation of 5-hmU likely involves the oxidation of 5-(uracilyl)methyl radicals to a 5-(uracilyl)methyl cation, followed by hydration of the cation once the sample is dissolved in H2O. The oxidation of 5-(uracilyl)methyl radicals may be favored in the condensed phase by the presence of nearby oxidizing radicals, such as, 5,6-dihydrothymin-5-yl radicals. Similar redox reactions have been proposed to account for the greater yield of products compared to free radical precursors.16d Electron transfer between a 5-(uracilyl)methyl radical and a 5,6-dihydrothymin-5-yl radical can give the corresponding cation and anion, respectively. This reaction not only explains the formation of 5-hmU, but also another major product in the reaction mixture, 5,6-dhT (described in more detail below). In contrast, the formation of 5-fU likely takes place when the sample is dissolved in aerated aqueous solution. The reaction of 5-(uracilyl)methyl radical and O2 leads to the formation of peroxyl radicals that in turn can give 5-fU by a number of pathways. For example, one-electron oxidation of the nucleoside (dT) in aerated aqueous solution gives a high yield of 5-fU nucleoside due to bimolecular decay of the intermediate peroxyl radicals.17 In contrast, peroxyl radicals attached to the trinucleotide can decompose by other pathways, which in aqueous solution, includes intramolecular reactions with adjacent bases and the sugar moiety.18 These reactions produce a hydroperoxide, i.e., 5-hydroperoxymethyluracil, which in turn can decompose to 5-fU by dehydration. It is also possible that intermediate peroxyl radicals afford 5-fU directly by reactions involving metal ions that are present in trace amounts in aqueous solution. Interestingly, X-ray irradiation favors the formation of 5-hmU over 5-fU (3:1) (Table 3), whereas dT radical cations generated in aqueous give a much lower amount of 5-hmU (1:3).14, 17 This suggests that 5-hmU is the major product of 5-(uracilyl)methyl radicals by reactions in the condensed phase whereas 5-fU is formed predominantly when the sample is dissolved in aerated aqueous solutions.
The exposure of thymine derivatives to X-rays leads to the addition of electrons to the pyrimidine ring leading to an intermediate thymine radical anion that subsequently undergoes protonation at C6 in aqueous solution.16d, 19 5,6-Dihydrothymine modifications (5,6-DhT) are the main reductive product observed upon exposure of thymine, thymine derivatives and DNA to ionizing radiation.16b, 16d, 20 Likewise, this reaction explains the formation of 5,6-dhT upon exposure of solid TpTpT to X-rays. Thy radical anions undergo protonation at temperatures above 140°K to give C5 centered radicals.19a Following protonation of Thy radical anions, the resulting 5,6-dihydrothymin-5-yl radicals can either undergo reductive or oxidative chemistry to give either 5,6-dhT or 5-hydroxy-5,6-dihydrothymine, respectively. Under aerobic conditions, 5,6-dihydrothymin-5-yl radicals should be diverted to the oxidative pathway because of the addition of O2. In the present study, however, we did not detect this product after purification the main peaks and LC-MS/MS analysis. Other studies have also reported a lack of products from this pathway when DNA is irradiated in the solid state.16d, 20b
The mechanism of formation of base release and strand breaks by one-electron ionization likely involves radical cations of the sugar-phosphate backbone. For example, a radical cation on the sugar moiety can undergo deprotonation to give a neutral carbon centered radical with the most likely sites of deprotonation occurring from C5′ and C3′.21 In turn, neutral radicals of the sugar moiety can convert to strand breaks by a number of pathways that have been proposed on the basis of their independent generation under controlled conditions.22 Another pathway of base release involves deprotonation of thymine and cytosine radical cations from C1 of the sugar moiety giving 2-ribonolactone as the final product.16a, 23 In addition, guanine and adenine radical cations transform into neutral sugar radicals at 3′ or 5′ upon electronic excitation of the cation.24 The yield of base release by direct ionization is estimated to be 2.8 lower than that by OH radicals.12 In general, the yield of base release is comparable to the formation of strand breaks although neutral sugar radicals such as those at 1′ give fragments containing the base moiety and certain products of neutral sugar radicals are known to slowly transform into strand breaks, such as 2-ribonolactone.22a, 25 Other interesting findings related to base release from DNA irradiated in the solid state include the lack of stoichiometry between base release and sugar radicals in EPR analyses taken at low temperature, variation of base release according to base type or sequence, effects of DNA hydration and O2.12, 21, 26
Mechanism of formation of products by LEEs
The formation of base release, strand breaks and base modification by way of LEEs (0–10 eV) has been described in detail with monomeric nucleic acids, short oligonucleotides, and plasmid DNA under UHV at room temperature.4d, 8 The initial step of LEE-induced DNA damage involves transient capture of the electron by DNA bases followed by bond cleavage within the base or transfer of the electron to another site, which often leads to scission of the N-glycosidic bond (C-N) or the phosphodiester bond (C-O), respectively. These bonds are prone to cleavage by interaction of transient molecular anion with low lying antibonding orbitals of the molecule. The bonds can rupture by a process known as dissociative electron attachment (DEA) such that the bond breaks giving a neutral radical species and a stable anion. The formation of DEA products is governed by the occupation of an electron in a localized orbitals of the molecule, and thus, the process shows distinctive shape or core-excited resonances below the ionization threshold of the molecule. In the case of nucleic acid components, these resonances are clearly visible in the region between 0–12 eV, with a broad peak around 10 eV, when the yield of DEA products is measured as a function of electron energy.4d, 7, 8c, 8d, 27 Above 12 eV, the yield of products decreases and then slowly rises at higher energies (>20 eV) indicating the onset of non-resonant fragmentation via either electronic excitation of high lying dissociative states or dipolar dissociation (DD).4d, 28 The total ionization cross-sections as a function of energy peak at about 80 eV and appear to be negligible at energies below 10 eV for various DNA and RNA components.29 Thus, it is reasonable to assume that the exposure of DNA to LEEs of 10 eV or lower induces damage nearly entirely through DEA with a minor contribution from ionization.
On the basis of this and previous studies8h, 5,6-dhT, 5-hmU and 5-fU are the major types of base damage formed upon exposure of TpTpT to LEEs (10 eV). The formation of 5,6-dhT was proposed to take place by the initial reaction of hydride anions (H-) at C6 of T followed by protonation of the anion upon dissolution of the sample in H2O.8h Hydride anions are major DEA products generated from the reaction of LEEs (5.5–12 eV) with Thy.27, 30 In addition, H atom radicals are also generate by LEEs but at lower energies (< 3 eV).31 Although 5-hmU and 5-fU are generally considered to be produced by Thy radical cations, this work establishes that they are also produced in high yields when TpTpT is bombarded with LEEs. A previous study32, however, reported the formation of the nucleobases of 5-hmU and 5-fU after bombardment of Thy with synchrotron ultrasoft X-rays, which generate low energy auger electrons from the ionization of the nitrogen and oxygen K shells of the molecule. The formation of 5-hmU and 5-fU can be explained by a DEA mediated pathway involving cleavage of the C-H bond of the exocyclic methyl group of Thy leading to 5-(uracilyl)methyl radicals and H anions. The sites of DEA were determined by monitoring the formation of H and D anions from differentially labeled Thy as a function of energy.30 Interestingly, H anions from N1 and N3 of Thy were predominantly observed at low energies (4–6 eV) while D anions from either C6 or the CH3 group of Thy were observed with equal intensity at higher energies (7–12 eV). These results suggest that 5-(uracilyl)methyl radicals and H anions should be the preferential products of DEA when TpTpT is exposed to LEEs at 10 eV. The 5-(uracilyl)methyl radical can subsequently convert into 5-hmU and 5-fU once the samples are dissolved in water (see explanation above for X-rays). In addition to base damage, the mechanism of formation of base release and strand breaks by LEEs likely involves the initial formation of a transient anion followed by transfer to either C-N or C-O bonds leading to their cleavage by DEA, respectively. The most favorable DEA products of N-glycosidic bond cleavage (C-N) are a neutral radical at C1 of the sugar moiety and a Thy anion, whereas those of phosphodiester bond cleavage (C-O), include a neutral radical at C3′ of C5′ of the sugar together with a phosphate anion.4b, 8a, 8b, 9b, 33
Role of LEEs in X-ray induced DNA damage
The percentage of base release to total measured damage was significantly lower for X-rays (20%) compared to that for LEEs (35%). This result indicates that LEEs induce base release more efficiently than X-rays. The pathway to base release by LEEs, which involves DEA mediated cleavage of the C-N bond, implicates fewer steps than a pathway through base or sugar ionization, which invokes initial deprotonation of the sugar radical cation and multistep reactions of subsequent radicals. The higher amount of base release at oligonucleotide termini may be a specific phenomenon of LEE reactions. In tri- and tetra-nucleotides, the yield of base release was several fold higher from the termini than from the center, e.g., the release of Thy was at least 6-fold higher than the release of X from TpXpT, where X = U, C, A, G.8f, 8g The end effect mediated by LEE is rationalized in terms of preferential capture of the electron by terminal bases and/or a lower probability of transfer to the C-O bond by terminal compared to central bases because termini can only transfer to a single CO bond while central bases can transfer to two C-O bonds. It is worth noting that transfer of an electron from the base to the C-O bond leading to C-O bond cleavage is driven by DEA to the phosphate group, and thus, this reaction only takes place next to a phosphodiester bond.9b In contrast, the end effect observed when oligonucleotides are exposed to X-rays is smaller and dependent on the presence of G, which can undergo electron transfer with sugar radicals and change the course of the reaction.26
There are other arguments suggesting that LEEs play a role in the formation of DNA damage induced by high energy X-rays. The inability of anthracycline to inhibit base release when intercalated in DNA indicates that OH radicals, base radicals and possibly certain sugar radicals are not immediate precursors of base release in three crystalline double-stranded oligomers after X-irradiation at 4K.21 Although this effect was attributed to the high reactivity of sugar radicals, one cannot rule out the reaction of LEEs, which occurs on a very short time-scale (10−14s) and releases Thy anion and a sugar radical at C1′. Another example in which LEEs may be implicated in DNA damage reported that the yield of 5,6-dihydropyrimidines decreases concomitantly with an increase in H2 as a function of increasing hydration, both reaching a plateau with maximum hydration.19b This phenomenon was explained by intra-spur reactions involving bulk water, and a source of H2 emanating from reactions involving DNA. However, the matching but inverse association of 5,6-dihydropyrimidine products and H2 may also point to the formation of H anions by the reaction of LEEs.
CONCLUSION
DNA damage induced by exposure of TpTpT to LEEs and X-rays was examined by HPLC-UV and LC-MS/MS. The final products included base release (-Thy), strand breaks (pT, Tp, pTpT, TpTp and TpT), and base modifications (5,6-dhT, 5-hmU and 5-fU). The finding that LEE induces the formation 5-hmU and 5-fU permits us to provide a more complete profile of LEE-induced damage to Thy. We propose that the above products can arise from both initial one-electron ionization (X-rays) and the reaction of LEEs leading to sub-ionization events such as DEA. The profile of products in both cases was very similar, except for the greater yield of base release obtained from LEEs compared to X-rays. We argue that LEE-induced pathways involving DEA may constitute an important component of DNA damage induced by ionizing radiation. However, further experiments will be necessary to estimate the contribution of ionization and LEE-induced reactions in the overall chemical effects of ionizing radiation.
Acknowledgments
We would like to thank the Natural Science and Engineering Council of Canada and the Canadian Institutes of Health Research for financial support.
ABBREVIATIONS
- LEE
low energy electrons
- DEA
dissociative electron attachment
- LC-MS/MS
HPLC tandem mass spectrometry
- 5,6-dhT
5,6-dihydrothymine
- 5-hmU
5-hydroxymethyluracil
- 5-fU
5-formyluracil
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This paper is dedicated to Professor William (Bill) A. Bernhard from the Department of Biochemistry and Biophysics at University of Rochester, Rochester, NY, who initiated this work in collaboration with the other authors but unfortunately passed away before its completion.
References
- 1.(a) von Sonntag C. Free-Radical-Induced DNA Damage and Its Repair. A Chemical Perspective. Springer-Verlag; Berlin-Heidelberg-NewYork: 2006. [Google Scholar]; (b) Cadet J, Douki T, Gasparutto D, Ravanat JL, Wagner JR. Oxidatively Generated Nucleobase Modifications in Isolated and Cellular DNA. John Wiley & Sons Ltd; Chichester, UK: 2012. [Google Scholar]
- 2.Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing Cancer Risks of Low-Dose Radiation. Nat Rev Cancer. 2009;9:596–604. doi: 10.1038/nrc2677. [DOI] [PubMed] [Google Scholar]
- 3.(a) Begg AC, Stewart FA, Vens C. Strategies to Improve Radiotherapy with Targeted Drugs. Nature Reviews Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]; (b) Choy H. Chemoradiation in Cancer Therapy. Humana Press; Totowa, N.Y: 2003. [Google Scholar]; (c) Tippayamontri T, Kotb R, Paquette B, Sanche L. Synergism in Concomitant Chemoradiotherapy of Cisplatin and Oxaliplatin and Their Liposomal Formulation in the Human Colorectal Cancer HCT116 Model. Anticancer Res. 2012;32:4395–4404. [PubMed] [Google Scholar]
- 4.(a) Boudaiffa B, Cloutier P, Hunting D, Huels MA, Sanche L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science. 2000;287:1658–1660. doi: 10.1126/science.287.5458.1658. [DOI] [PubMed] [Google Scholar]; (b) Li XF, Sevilla MD, Sanche L. Density Functional Theory Studies of Electron Interaction with DNA: Can Zero eV Electrons Induce Strand Breaks? J Am Chem Soc. 2003;125:13668–13669. doi: 10.1021/ja036509m. [DOI] [PubMed] [Google Scholar]; (c) Sanche L. Low Energy Electron Damage to DNA. In: Shukla MK, Leszczynski J, editors. Radiation Induced Molecular Phenomena in Nucleic Acid. Springer; Dordrecht, Netherlands: 2008. pp. 531–575. [Google Scholar]; (d) Sanche L. Nanoscale Dynamics of Radiosensitivity: Role of Low Energy Electrons. In: Gomez-Tejedor GG, Fuss MC, editors. Radiation Damage in Biomolecular Systems, Biological and Medical Physics, Biomedical Engineering. Springer; Dordrecht, Netherlands: 2012. pp. 3–43. [Google Scholar]
- 5.Sanche L. Role of Secondary Low Energy Electrons in Radiobiology and Chemoradiation Therapy of Cancer. Chem Phys Lett. 2009;474:1–6. doi: 10.1103/PhysRevLett.100.198101. [DOI] [PubMed] [Google Scholar]
- 6.(a) LaVerne JA, Pimblott SM. Electron Energy Loss Distributions in Solid and Gaseous Hydrocarbons. J Phys Chem. 1995;99:10540–10548. [PubMed] [Google Scholar]; (b) Pimblott SM, LaVerne JA. Production of Low-Energy Electrons by Ionizing Radiation. Radiat Phys Chem. 2007;76:1244–1247. [Google Scholar]
- 7.Sanche L. Low Energy Electron-Driven Damage in Biomolecules. Eur Phys J D. 2005;35:367–390. [Google Scholar]
- 8.(a) Zheng Y, Cloutier P, Hunting DJ, Wagner JR, Sanche L. Glycosidic Bond Cleavage of Thymidine by Low-Energy Electrons. J Am Chem Soc. 2004;126:1002–1003. doi: 10.1021/ja0388562. [DOI] [PubMed] [Google Scholar]; (b) Zheng Y, Cloutier P, Hunting DJ, Sanche L, Wagner JR. Chemical Basis of DNA Sugar-Phosphate Cleavage by Low-Energy Electrons. J Am Chem Soc. 2005;127:16592–16598. doi: 10.1021/ja054129q. [DOI] [PubMed] [Google Scholar]; (c) Zheng Y, Wagner JR, Sanche L. DNA Damage Induced by Low-Energy Electrons: Electron Transfer and Diffraction. Phys Rev Lett. 2006;9620:8101–8101. doi: 10.1103/PhysRevLett.96.208101. [DOI] [PubMed] [Google Scholar]; (d) Zheng Y, Cloutier P, Hunting DJ, Wagner JR, Sanche L. Phosphodiester and N-Glycosidic Bond Cleavage in DNA Induced by 4–15 eV Electrons - Art. No. 064710. J Chem Phys. 2006;124:64710–64710. doi: 10.1063/1.2166364. [DOI] [PubMed] [Google Scholar]; (e) Li ZJ, Zheng Y, Cloutier P, Sanche L, Wagner JR. Low Energy Electron Induced DNA Damage: Effects of Terminal Phosphate and Base Moieties on the Distribution of Damage. J Am Chem Soc. 2008;130:5612–5613. doi: 10.1021/ja077601b. [DOI] [PubMed] [Google Scholar]; (f) Li ZJ, Cloutier P, Sanche L, Wagner JR. Low-Energy Electron-Induced DNA Damage: Effect of Base Sequence in Oligonucleotide Trimers. J Am Chem Soc. 2010;132:5422–5427. doi: 10.1021/ja9099505. [DOI] [PubMed] [Google Scholar]; (g) Li Z, Cloutier P, Sanche L, Wagner JR. Low-Energy Electron-Induced Damage in a Trinucleotide Containing 5-Bromouracil. J Phys Chem B. 2011;115:13668–13673. doi: 10.1021/jp205194g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Park Y, Li ZJ, Cloutier P, Sanche L, Wagner JR. DNA Damage Induced by Low-Energy Electrons: Conversion of Thymine to 5,6-Dihydrothymine in the Oligonucleotide Trimer Tptpt. Radiat Res. 2011;175:240–246. doi: 10.1667/rr2381.1. [DOI] [PubMed] [Google Scholar]; (i) Park Y, Polska K, Rak J, Wagner JR, Sanche L. Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J Phys Chem B. 2012;116:9676–9682. doi: 10.1021/jp304964r. [DOI] [PubMed] [Google Scholar]
- 9.(a) Sanche L. Bond Dissociation and Formation of Transient Anions, Radicals, and Radical Anions. In: Greenberg M, editor. Reactive Intermediates in Chemistry and Biology. John Wiley & Sons, Inc; 2010. pp. 239–293. [Google Scholar]; (b) Simons J. How Do Low-Energy (0.1–2 eV) Electrons Cause DNA-Strand Breaks? Acc Chem Res. 2006;39:772–779. doi: 10.1021/ar0680769. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Cloutier P, Wagner JR, Sanche L. Irradiator to Study Damage Induced to Large Nonvolatile Molecules by Low-Energy Electrons. Rev Sci Instrum. 2004;75:4534–4540. [Google Scholar]
- 11.Fasman GD. Handbook of Biochemistry and Molecular Biology. CRC Press; Boca Raton, FL: 1995. [Google Scholar]
- 12.Swarts SG, Sevilla MD, Becker D, Tokar CJ, Wheeler KT. Radiation-Induced DNA Damage as a Function of Hydration. I. Release of Unaltered Bases. Radiat Res. 1992;129:333–344. [PubMed] [Google Scholar]
- 13.Sharma KKK, Milligan JR, Bernhard WA. Multiplicity of DNA Single-Strand Breaks Produced in Puc18 Exposed to the Direct Effects of Ionizing Radiation. Radiat Res. 2008;170:156–162. doi: 10.1667/RR1277.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samson-Thibault F, Madugundu GS, Gao S, Cadet J, Wagner JR. Analysis of Cytosine Modifications in Oxidized DNA by Enzymatic Digestion and HPLC-MS/MS. Chem Res Toxicol. 2012;25:1902–1911. doi: 10.1021/tx300195f. [DOI] [PubMed] [Google Scholar]
- 15.Alizadeh E, Sanche L. Measurements of G Values for DNA Damage Induced by Low-Energy Electrons. J Phys Chem B. 2011;115:14852–14858. doi: 10.1021/jp207922n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Decarroz C, Wagner JR, van Lier JE, Krishna CM, Riesz P, Cadet J. Sensitized Photooxidation of Thymidine by 2-Methyl-1,4-Naphthoquinone. Characterization of Stable Products. Int J Radiat Biol. 1986;50:491–507. doi: 10.1080/09553008614550901. [DOI] [PubMed] [Google Scholar]; (b) Shaw AA, Voituriez L, Cadet J, Gregoli S, Symons MCR. Identification of the Products Resulting from the Direct Effects of Γ-Radiation Onthymidine. J Chem Soc Perk Trans 2. 1988:1303–1307. [Google Scholar]; (c) Sagstuen E, Hole EO, Nelson WH, Close DM. Radiation-Induced Free-Radical Formation in Thymine Derivatives. Epr/Endor of Anhydrous Thymine Single Crystals X-Irradiated at 10 K. J Phys Chem. 1992;96:1121–1126. [Google Scholar]; (d) Sharma KKK, Swarts SG, Bernhard WA. Mechanisms of Direct Radiation Damage to DNA: The Effect of Base Sequence on Base End Products. J Phys Chem B. 2011;115:4843–4855. doi: 10.1021/jp200902h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sevilla MD, Engelhardt ML. Mechanisms for Radiation Damage in DNA Constituents and DNA: Reactions of the N1-Substituted Thymine Π- Cation Radicals. J Chem Soc, Faraday Discussions. 1977;63:255–263. [Google Scholar]
- 17.Wagner JR, van Lier JE, Johnston LJ. Quinone Sensitized Electron Transfer Photooxidation of Nucleic Acids: Chemistry of Thymine and Thymidine Radical Cations in Aqueous Solution. Photochem Photobiol. 1990;52:333–343. doi: 10.1111/j.1751-1097.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 18.In SH, Carter KN, Sato K, Greenberg MM. Characterization and Mechanism of Formation of Tandem Lesions in DNA by a Nucleobase Peroxyl Radical. J Am Chem Soc. 2007;129:4089–4098. doi: 10.1021/ja0692276. [DOI] [PubMed] [Google Scholar]
- 19.(a) Wang W, Sevilla MD. Protonation of Nucleobase Anions in Gamma-Irradiated DNA and Model Systems. Which DNA Base is the Ultimate Sink for the Electron? Radiat Res. 1994;138:9–17. [PubMed] [Google Scholar]; (b) Falcone JM, Becker D, Sevilla MD, Swarts SG. Products of the Reactions of the Dry and Aqueous Electron with Hydrated DNA: Hydrogen and 5,6-Dihydropyrimidines. Radiat Phys Chem. 2005;72:257–264. [Google Scholar]
- 20.(a) Gromova M, Balanzat E, Gervais B, Nardin R, Cadet J. The Direct Effect of Heavy Ions and Electrons on Thymidine in the Solid State. Int J Radiat Biol. 1998;74:81–97. doi: 10.1080/095530098141753. [DOI] [PubMed] [Google Scholar]; (b) Dawidzik JB, Budzinski EE, Patrzyc HB, Cheng HC, Iijima H, Alderfer JL, Tabaczynski WA, Wallace JC, Box HC. Dihydrothymine Lesion in X-Irradiated DNA: Characterization at the Molecular Level and Detection in Cells. Int J Radiat Biol. 2004;80:355–361. doi: 10.1080/09553000410001695877. [DOI] [PubMed] [Google Scholar]
- 21.Razskazovskiy Y, Debije MG, Bernhard WA. Direct Radiation Damage to Crystalline DNA: What Is the Source of Unaltered Base Release? Radiat Res. 2000;153:436–441. doi: 10.1667/0033-7587(2000)153[0436:drdtcd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Dedon PC. The Chemical Toxicology of 2-Deoxyribose Oxidation in DNA. Chem Res Toxicol. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]; (b) Al-Oudat B, Salyer A, Trabbic K, Bryant-Friedrich A. 3′-Modified Oligodeoxyribonucleotides for the Study of 2-Deoxyribose Damage in DNA. Bioorg Med Chem Lett. 2013;23:854–859. doi: 10.1016/j.bmcl.2012.11.050. [DOI] [PubMed] [Google Scholar]; (c) Bryant-Friedrich AC. Fate of DNA Sugar Radicals. In: Fishbein J, editor. Advances in Molecular Toxicology. Vol. 4. Elsevier; New York: 2010. pp. 127–155. [Google Scholar]
- 23.(a) Watanabe T, Tashiro R, Sugiyama H. Photoreaction at 5′-(G/C)Aa(Br)Ut-3′ Sequence in Duplex DNA: Efficent Generation of Uracil-5-yl Radical by Charge Transfer. J Am Chem Soc. 2007;129:8163–8168. doi: 10.1021/ja0692736. [DOI] [PubMed] [Google Scholar]; (b) Roginskaya M, Razskazovskiy Y, Bernhard WA. 2-Deoxyribonolactone Lesions in X-Ray-Irradiated DNA: Quantitative Determination by Catalytic 5-Methylene-2-Furanone Release. Angew Chem. 2005;44:6210–6213. doi: 10.1002/anie.200501956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Adhikary A, Collins S, Khanduri D, Sevilla MD. Sugar Radicals Formed by Photoexcitation of Guanine Cation Radical in Oligonucleotides. J Phys Chem B. 2007;111:7415–7421. doi: 10.1021/jp071107c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Adhikary A, Khanduri D, Kumar A, Sevilla MD. Photoexcitation of Adenine Cation Radical [A.+] in the near Uv-Vis Region Produces Sugar Radicals in Adenosine and in Its Nucleotides. J Phys Chem B. 2008;112:15844–15855. doi: 10.1021/jp808139e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Sheppard TL. Half-Life and DNA Strand Scission Products of 2-Deoxyribonolactone Oxidative DNA Damage Lesions. Chem Res Toxicol. 2004;17:197–207. doi: 10.1021/tx034197v. [DOI] [PubMed] [Google Scholar]
- 26.Sharma KK, Razskazovskiy Y, Purkayastha S, Bernhard WA. Mechanisms of Strand Break Formation in DNA Due to the Direct Effect of Ionizing Radiation: The Dependency of Free Base Release on the Length of Alternating CG Oligodeoxynucleotides. J Phys Chem B. 2009;113:8183–8191. doi: 10.1021/jp900803b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptasinska S, Sanche L. Dissociative Electron Attachment to Abasic DNA. PCCP. 2007;9:1730–1735. doi: 10.1039/b616619a. [DOI] [PubMed] [Google Scholar]
- 28.Huels MA, Boudaiffa B, Cloutier P, Hunting D, Sanche L. Single, Double, and Multiple Double Strand Breaks Induced in DNA by 3–100 eV Electrons. J Am Chem Soc. 2003;125:4467–4477. doi: 10.1021/ja029527x. [DOI] [PubMed] [Google Scholar]
- 29.Vinodkumar M, Limbachiya C, Barot M, Swadia M, Barot A. Electron Impact Total Ionization Cross Sections for All the Components of DNA and RNA Molecule. Int J Mass spectrom. 2013;339–340:16–23. [Google Scholar]
- 30.Ptasinska S, Denifl S, Grill V, Mark TD, Illenberger E, Scheier P. Bond- and Site-Selective Loss of H- from Pyrimidine Bases. Phys Rev Lett. 2005;9509:3201–3201. doi: 10.1103/PhysRevLett.95.093201. [DOI] [PubMed] [Google Scholar]
- 31.(a) Abdoul-Carime H, Gohlke S, Illenberger E. Site-Specific Dissociation of DNA Bases by Slow Electrons at Early Stages of Irradiation. Phys Rev Lett. 2004;9216:8103–8103. doi: 10.1103/PhysRevLett.92.168103. [DOI] [PubMed] [Google Scholar]; (b) Denifl S, Ptasinska S, Probst M, Hrusak J, Scheier P, Mark TD. Electron Attachment to the Gas-Phase DNA Bases Cytosine and Thymine. J Phys Chem A. 2004;108:6562–6569. [Google Scholar]; (c) Denifl S, Ptasinska S, Hanel G, Gstir B, Probst M, Scheier P, Mark TD. Electron Attachment to Gas-Phase Uracil. J Chem Phys. 2004;120:6557–6565. doi: 10.1063/1.1649724. [DOI] [PubMed] [Google Scholar]
- 32.Akamatsu K, Fujii K, Yokoya A. Qualitative and Quantitative Analyses of the Decomposition Products That Arise from the Exposure of Thymine to Monochromatic Ultrasoft X Rays and 60Co Gamma Rays in the Solid State. Radiat Res. 2004;161:442–450. doi: 10.1667/3151. [DOI] [PubMed] [Google Scholar]
- 33.(a) Becker D, Bryant-Friedrich A, Trzasko C, Sevilla MD. Electron Spin Resonance Study of DNA Irradiated with an Argon-Ion Beam: Evidence for Formation of Sugar Phosphate Backbone Radicals. Radiat Res. 2003;160:174–185. doi: 10.1667/rr3037. [DOI] [PubMed] [Google Scholar]; (b) Becker D, Adhikary A, Tetteh ST, Bull AW, Sevilla MD. Kr-86 Ion-Beam Irradiation of Hydrated DNA: Free Radical and Unaltered Base Yields. Radiat Res. 2012;178:524–537. doi: 10.1667/RR3066.3. [DOI] [PMC free article] [PubMed] [Google Scholar]