Abstract
MicroRNAs (miRNAs), a group of small non-coding RNAs, have emerged as significant modulators in the establishment and generation of pluripotency, a developmental process that consists of complex cell-fate arrangements. The finding of embryonic stem cell (ESC) cycle-specific miRNAs reveals an important regulation scheme of pluripotency. Subsequent studies showed the ESC-enriched or ESC-depleted miRNAs can regulate induced pluripotent stem cells(iPSC). Moreover, miRNA profiling of iPSC and ESC may distinguish them from one another and facilitate the complex of regulatory network. The accumulative effects of miRNA action enable using miRNA alone to generate iPSCs. Despite the robustness of iPSC studies, further investigations are needed since miRNA may have more impact on induced pluripotency, and the roles of miRNAs in somatic cell nuclear transfer (SCNT), another approach toward cellular reprogramming, remains unclear. This point-of-view article will discuss miRNAs and their impact on the normal and induced pluripotency, as well as bring new insights on somatic cell reprogramming.
Keywords: MicroRNAs, embryonic stem cells, induced pluripotent stem cells, pluripotency regulation, regenerative medicine, somatic cell nuclear transfer, somatic cell reprogramming
Introduction
To date, microRNAs (miRNAs), a group of small and non-coding RNAs, have been recognized as a critical role in the transcriptional and post-transcriptional regulation of human biology and diseases.1 Since the first discovery of miRNA in Caenorhabditis elegans, an increasing number of miRNAs have been reported.2,3 Via base-pairing with imperfect complementary sequences of mRNAs, miRNAs result in repression of target mRNA degradation and/or translational silencing.4,5 Morever, miRNAs are found to target amino acid coding sequence,6 promoters,7 and even long non-coding RNAs.8 It is well-believed that miRNAs hold the ability to influence most developmental processes, including stem-cell fate decision.
Insights from the successful generation of ESC and the technology of somatic cell reprogramming bring prospects for obtaining patient and disease-specific pluripotent stem cell lines for clinical usage.9 However, low efficiency and variability of iPSCs, along with ethical concerns on SCNT, provide barriers for the clinical application of somatic cell reprogramming.9-13 Recent studies have begun to indicate that miRNA plays an active role in shaping and defining pluripotency and reprogramming.14,15 And to pave the way for regenerative medicine, it is important to understand the molecular mechanism underlying ESC pluripotency and cellular reprogramming. In this point-of-view article, we discuss the roles of miRNAs as an emerging class of regulators during the induction and maintenance of the pluripotent state.
miRNAs Control ESC Pluripotency
The initial characterization of miRNAs for ESCs was demonstrated in Dicer- and Dgcr8-deficient mouse ESCs.16-19 Dicer or Dgcr8 deficiency resulted in the failure of the canonical miRNA biogenesis pathway, hence causing global depletion of a large family of miRNAs. This indicated that miRNAs define pluripotent hallmarks of ESC. Studies also showed that Dicer- and Dgcr8-knockout ESCs fail to obtain chimera formation after blastocyst injection, which demonstrated the critical role of miRNA synthesis during early embryonic development.20
Interestingly, studies showed that only a limited number of miRNAs are transcribed in ESC and they are exclusively expressed in the pluripotent state while immediately silenced once receiving differentiation signals.21 Most of these ESC-specific miRNAs constitute two clusters. The two affluent miRNA families are the miR-290 cluster (miR-291-3p, miR-294, and miR-295) in mice, and their human homologs the miR-371-373 family (miR-371, miR-372, and miR-373); and the miR-302-367 cluster (miR-302a, miR-302b, miR-302c, miR-302d, and miR-367) in both mice and humans. These miRNAs have been termed as ESC-specific cell cycle (ESCC) miRNAs since they are predominantly found in the cell cycle. Furthermore, they share a specific seed region (AAGUGCU or AAAGUGC) (Table 1).
Table 1. miRNAs that regulate pluripotency in ESC.
| MiRNA family/cluster |
Roles in pluripotency |
Levels in ESC |
Constituents | Species | References |
|---|---|---|---|---|---|
| miR-302-367 cluster |
promoters |
high |
miR-302a,miR-302b,miR-302c,miR-302d,miR-367 |
M,H |
28,29,30,31,32 |
| miR-290 cluster |
promoters |
high |
miR-291–3p,miR-294,and miR-295 |
M |
21,22,23,24,25,26,27 |
| miR-371 cluster |
unknown |
unknown |
miR-371, miR-372, and miR-373 |
H |
27,49 |
| let-7 family | suppressors | low | let-7 | M,H | 19,33,34,35 |
M, mouse; H, human.
The miR-290 cluster comprises the most highly expressed miRNA cluster in mouse ESCs.21,22 Mouse mutant with a homozygous deletion of the miR-290 cluster are embryonic lethal, which indicates its importance during development.23 This cluster directly target the cell cycle inhibitors p21 and LAST2 and repress the G1-S phase transition. This attributes to the unusually short G1 cell cycle in ESC and, hence, maintains its proliferation ability of pluripotency.24 The transcriptional regulatory circuit of pluripotency consists of Oct-4, Sox2, and Nanog. They have been detected to relate to physical promoters for miR-290 cluster that are predominantly expressed in ESCs.25 Studies have showed that the miR-290 cluster downregulate Oct-4 by targeting epigenetic repressor DNA methyltransferases such as retinoblastoma-like protein 2.26 This miRNA cluster depresses the Oct4 inhibitor, which, in return, favors the expression of itself by promoter binding.27-29 The miR-371,372,373 family, often viewed as human counterpart of miR-290 cluster, is very diverse in human. Though they are predicted to hold the same features as their mouse counterparts, still, further investigations need to be performed.
Another ES cell-specific miRNA cluster is the miR-302-367 cluster. This miRNA cluster is highly and exclusively expressed in undifferentiated ESCs.30,31 The promoter power of miR-302-367 was identified within its transcriptional activity and the ESC-specific transcription factors, Oct-4, Nanog, and Sox-2, were demonstrated to be the upstream regulators of the miR-302-307 promoter.30,31 In addition, miR-302-367 cluster holds the ability of regulating cyclin D1 and cdk4 and maintaining the pluritotency by cell cycle progression.32
In contrast to the miR-290 cluster, the let-7 miRNA cluster remains at very low levels in embryonic stem cells. The accumulation of let-7 mature miRNAs is a specific inducer of cell differentiation.33,34 Studies have demonstrated that let-7 is regulated by LIN28, a RNA-binding protein highly expressed in ESC. In pluripotent stem cells, LIN 28 inhibit the maturation of let-7 by targeting the Dicer-mediated processing of premature let-7.35 Further studies about ESC cell cycle have demonstrated that miR-290 and let-7 miRNAs have quite opposing effects in ESC characterization of self-renewal and pluripotency. The mutually exclusive expression profile suggests they act as a very intersecting pathway just like a leverage or seesaw. In addition, an increasing number of ES-enriched genes have shown to be targets of let-7, such as those coding sequences for the N-myc, c-myc, Sall4, and Lin28.33-35 It is predicted that the two opposite miRNA families form a feedback regulatory circuit which offers a rapid switching mechanism between self-renewal and differentiation.
miRNAs in the Regulation of iPSC
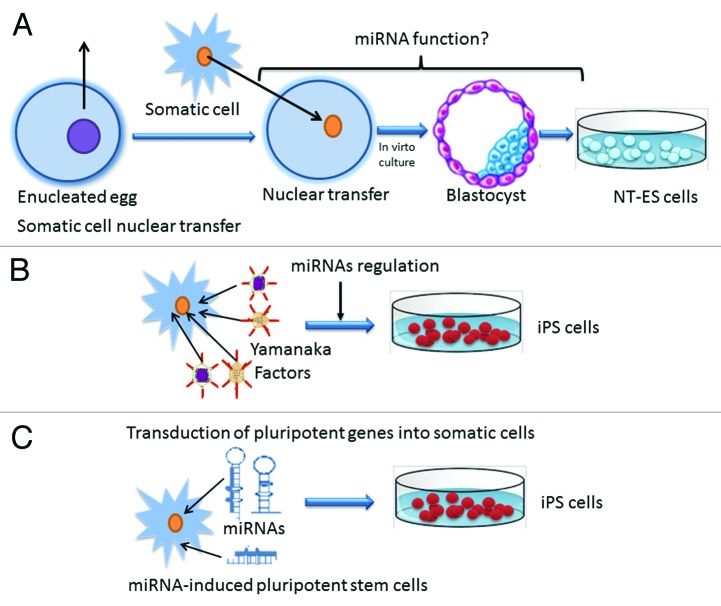
As shown in Figure 1, the routes toward somatic cell reprogramming consist of SCNT and the generation of iPSC-induced pluripotent stem cells (iPSC). The SCNT technology, known as “cloning,” has been achieved successfully in many species, including the first mammal cloning of the sheep, Dolly.36-40 By transferring a somatic cell nucleus into an enuleated egg, this method can enable adult cells to regain pluripotency. Since isogenic embryonic stem cell lines, known as NT-ESCs, can be derived from nuclear-transferred embryos, this technology offers great hope for regenerative medicine.40 However, due to technical restraints and ethical concerns, it is very hard to obtain patient-specific NT-ESC lines,41 and until lately, only one research group has successfully derived hESC lines.42 In 2006, reprogramming of mouse somatic cells into pluripotent stem cells was achieved by ectopically expressing four ESC-enriched transcription factors, namely Oct4, Sox2, Klf4, and C-myc.43 Subsequent studies showed human-induced pluripotent stem cells can be obtained via virtually the same methodology.19,44,45 Although many improved procedures for iPSC generation are available at present, the derivation efficiency and efficacy remains quite low by the method of transcription factor-mediated reprogramming.46-48 Meanwhile, the introduction of certain proto-oncogene can result in oncogenesis, which hurdles the path to clinical application.10-13 These studies suggest that certain road blocks exist during somatic cell reprogramming. Given the features of miRNAs in ESC regulation, in order to address these issues, it is highly important to determine miRNAs’ roles associated with induced somatic cell reprogramming.
Figure 1. Routes to somatic cell reprogramming. (A) In conventional somatic nuclear transfer, the nucleus of an egg is removed and then the nucleus of a somatic adult cell is transplanted into the enucleated egg. The reconstructed embryo is culture into the blastocyst stage and nuclear transferred embryonic stem cells (NT-ESC) can be derived from the inner cell mass(ICM) of the blastocyst. The miRNA function during SCNT needs further investigations. (B) Transduction of pluripotent factors, namely Oct4, Nanog, Myc, and Klf, known as Yamanaka factors, into somatic adult cells can generate iPSCs. Successful iPSC generation and maintenance require the regulation of miRNAs. (C) Using miRNA only can generate iPSC successfully. miRNAs can induce somatic cell reprogramming alone in the absence of exogenous pluripotent factors.
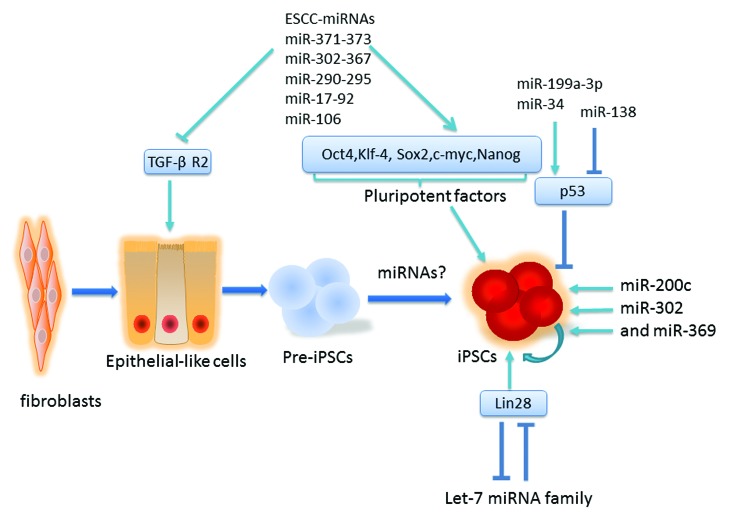
The ESC-specific cell cycle (ESCC) miRNAs comprise a number of cluster families sharing a common seed sequence as mentioned above. The transduction of the four classic reprogramming factors, Oct3/4, Sox2, Klf4, and C-myc, can directly affect the expression of these miRNA clusters, varying from miR-290-295 (and its human homolog miR-371-373),49,50 miR-302a-367,50 to miR-17-92 (and its human homolog miR-106b-25 and miR-106a-363).51 (Fig. 2) They can suppress G1-S transition during cell cycle to maintain self-renewal and pluritotency at the early stage of somatic reprogramming.24 Furthermore, these miRNAs can influence epigenetic status with significant impacts on pluripotent gene expression. For instance, studies show that the ESCC miRNAs can enhance the re-activation of stem cell core pluripotency factors, such as OCT3/4, by repressing NR2F2, a transcriptional repressor of OCT3/4.52 Global demethylation is required for establishing pluritotency, which can be triggered by miR-302 miRNA clusters as they suppress epigenetic regulators AOF2, AOF1, MECP1-p66, and MECP2.53 Also, a recent investigation suggests overexpression of miR-302 enhances the pluripotent marker, Nanog, hence increases the generation of iPSC by suppressing methyl-DNA-binding domain protein 2 (MBD2).54 On the other hand, the inhibition of the let-7 miRNA family, with a negative role in the maintenance of ESC pluripotency, can influence the function of ESCC miRNAs and promote induced pluripotency from somatic cells.55 Since let-7 miRNAs constitute a major barrier for de-differentiation of somatic cells by repressing stem cell proliferation genes and Myc family of proteins, Lin 28, a negative regulator of let-7, can serve as one of the reprogramming factors.19
Figure 2. Roles of miRNA in the regulation of iPSC. By suppressing TGF-β receptor-2, ESCC miRNAs can promote the early stage of somatic cell reprogramming, known as MET. miRNa involvement and the later stage of reprogramming remains unclear. ESCC miRNAs also target pluripotent genes and enhance induced reprogramming. On the other hand, Let-7 provides a barrier for somatic cell reprogramming and the inhibition of let-7 can result in pluripotency. By targeting p53, miR-34 family inhibits pluripotent genes; namely Nanog, Sox2, and N-Myc, and suppress adult cell reprogramming. Using miRNA (miR-200c, miR-302, miR-369) only can also induce reprogramming.
Other than regulating pluripotent cell cycle and epigenetic factors, ESC-enriched miRNAs are found to promote the generation of iPSCs by regulating TGF-β/Activin signaling pathway, a well-believed pluripotency regulation pathway.50,56,57 Directly targeting the TGF-β type II receptor (TGF-β R2), ESCC miRNAs repress TGF-β signaling to enhance the mesenchymal-to-epithelial transition (MET), an early stage of induced reprogramming.37,41,50,56 Interestingly, a profiling study has indicated that the levels of miR-24-1, miR-23b, and miR-21 are high in human fibroblasts, and by regulating TGF-β signaling pathway, their expression has remarkably silenced in iPSCs derived from the same colonies of fibroblasts.58 This finding suggests suppressing TGF-β signaling-specific miRNAs promote the generation and establishment of iPSCs.
Another barrier for induced reprogramming is the activation of p53, a well-known tumor suppressor gene.59 Studies have demonstrated that miR-34,59 miR-199a-3p,60 and miR-138,61 as transcriptional targets of p53 tumor suppressor pathway, play a critical role in reprogramming. miR-34 represses downstream expression of p53 and suppresses the establishment of induced pluripotency. And reprogramming from miR-34a-depleted mouse fibroblasts can promote the efficiency of iPSC generation.59 Similarly, the repression of miR-199a-3p also enhances and facilitates somatic cell reprogramming.60 miR-138, on the other hand, serves as enhancer of p53 by targeting its 3′ untranslated region (UTR).61 It is appreciated that the successful reprogramming involves genetically suppressing the p53 pathway, and p53 pathway is known to ensure genomic integrity and reduce tumor progression.62 Could the repression of microRNA in p53 pathway enhance reprogramming at the cost of genetic instability? It is postulated that p53-mediated miRNA might be dosage-sensitive and only a certain amount of these miRNA expression can establish pluripotency and hold genome integrity at the same time. Therefore, further investigations need to be done on p53-related miRNA expression patterns.
miRNA Profiling Distinguishes iPSC and ESC
Are iPS and ESC exactly the same and hold the same properties? This issue remains controversial ever since the first successful generation of iPSCs in 2006. On a genome-wide scale, Doi et al. found that certain variations of tissue- and cancer-specific CpG methylation differ iPSCs and ESCs.63 iPSCs have been compared with ESC for gene expression, chormation structure, and histone modifications by many groups. Interestingly, the results of these studies seem quite contradictory. Presumably, miRNA expression difference between iPS and ESC, along with other epigenetic and post-transcriptional modifications, can be observed at the molecular level. To date, only few studies were conducted. Studies showed that several miRNA clusters, including miR-371/-372/-373 and many others, as more highly expressed in hESCs than iPSCs, while miR-181a, -199b-3p, and -214 are iPS-specific miRNAs and remain low in ESC types.58,64 These findings indicate that miRNAs are differentially expressed in iPSCs and ESCs and global gene expression patterns may be different between them. However, another recent study demonstrated that no individual miRNA can be found to distinguish ESC and iPSC based on larger profiling statistics.65 Additionally, p53 status can be related to the classification of pluripotent stem cell lines and the expression of p53-targeting miRNA can distinguish different types of iPSCs and ESCs.65
miRNAs Alone Can Induce Reprogramming
Since a multitude of miRNAs can be used to enhance somatic reprogramming efficiency, can single miRNAs alone achieve induced pluripotency? Several exciting discoveries have been made recently. The stable lentiviral delivery of miR-302/367 miRNA clusters alone can generate iPSCs successfully. Without any protein-encoding reprogramming factors, the efficiency using miR-302/367 alone was 100-fold higher than the conventional approach of introducing retroviral infection.66 Another study conducted by Miyoshi et al. showed successful generation of iPSCs by transfection with miR-200c, miR-302, and miR-369 miRNA.67 However, Hu et al. failed to reprogram human adipose stem cells to iPSCs by miR-302, which indicates that miRNA-induced pluripotent stem cells could only be derived from certain types of adult cell types.68 It is worth noting that since miRNA-mediated reprogramming are mostly dependent on endogenous pathways and, hence, maintain a smooth epigenetic modification, it shows certain advantages in producing better, safer, and more ES-like iPSCs over the conventional methods. Therefore, more studies about reprogramming different adult cell types and transduction of other miRNA families need to be performed to tackle this issue.
miRNA and Somatic Cell Nuclear Transfer
As mentioned above, SCNT technology is viewed as a promising tool for providing patient-specific stem cell lines for clinical usage. Despite the robustness of iPSC researches, SCNT hold better advantages in maintaining genome integrity and treating mitochondrial diseases compared with iPSCs.9,69,70 The uncertainties and low efficiencies of iPSC may hurdle the clinical applications. During the process of induced pluripotency, genome instability is observed and epigenetic memories of donor cell are still harbored.11,12 Recently, a study showed human patient-specific ESCs can be derived by SCNT with optimized activation and culture methods.42-Although the results need to be reproduced, this technology shows great promise in regenerative medicine. Since mouse ESCs derived by SCNT showed genome and transcriptome stability,71 it is postulated that human SCNT-ESCs might hold better capacity in regenerative therapeutics. Hence, it is worthwhile to compare the epigenetic and post-transcriptional differences, including miRNA profiling, between iPSCs and SCNT-ESC in both mouse and human beings. To date, only very few reports have been made underlying miRNA and somatic cell nuclear transfer. Castro et al. demonstrated the miRNA expression profiling differences in elongated cloned and in vitro-fertilized bovine embryos.72 Another study conducted by Cui et al. showed different expression levels of mir-127 and mir-136 between normal fertilized mouse embryos and SCNT-embryos.73 However, these studies are limited and would need more investigations to provide ongoing data. Recently, Inoue et al. reported that deletion and knockdown Xist, a non-coding RNA that control X-chromosome inactivation in female mammals, can increase SCNT efficiency greatly, and correct epigenetic reprogramming errors.74,75 Although other types of long non-coding RNA instead of miRNA was used to mediate reprogramming in this case, it is possible that miRNA can also be exploited to mediate SCNT. These findings suggest that non-coding RNA plays an important role in mouse reprogramming, and miRNA, along with other epigenetic and post-transcriptional factors, could provide new insights into human somatic cell nuclear transfer.
Outlook
The understanding of miRNA regulation and function in pluripotent stem cells and somatic cell reprogramming is essential for the development of regenerative medicine. Over the past decade, a large multitude of miRNAs has emerged as a crucial part of a complex molecular network of gene expression associated with the pluripotent state of cells. In spite of the above-mentioned importance of miRNAs in regulation and function of pluripotency and somatic cell reprogramming, many further investigations need to be performed.
First, can any new miRNAs be identified? The emerging technologies such as deep sequencing can be used to predict large numbers of new miRNAs in pluripotent stem cells. The new tools can also help us to discover the direct profiling of interactions between miRNAs and mRNAs. Second, what is the proper miRNAs dosage requirement of regulation of pluripotency? It is believed that miRNAs in the function and regulation of both ESC and iPSC are dose-sensitive.76,77 miRNA-mediated regulatory effects on pluripotent factors are fully described in this review. Understanding specific miRNA dosages may provide a powerful mechanism in fine-tuning the final gene products and induced pluripotent outcomes to an optimized level. Third, do mouse miRNAs and human miRNAs hold the same properties? Though mouse and human cell biology share a lot of similarities, mouse and human ESCs show notable differences in the regulation and maintenance of pluripotent state. It is thus important to determine mouse miRNAs and their human counterpart, despite their identical seed, contribute to the functional difference of one another by targeting overlapping or distinctive mRNAs. Furthermore, it is believed that human ESCs are more like epiblast stem cells compared with mouse ESC; and mouse ESC are more “embryonic” than their human counterpart.78,79 Is it possible to derive more “embryonic,” i.e., more mouse ESC-resembling, human ESC lines with the transduction of miRNAs? Last but not least, there are many other classes of non-coding RNAs that may also play a role in regulating pluripotency and differentiation, and miRNA binding mechanism is an imperfect recognition. One miRNA might be regulating many targets. Is there a cumulative effect on the simultaneous miRNA functions? And how do other non-coding RNAs work with miRNA to establish pluripotency? In brief, a better understanding of regulatory and functional networks underlying pluripotency and somatic cell reprogramming is required before these pluripotent stem cells can be put into clinical practice.
Acknowlegements
HYS’s laboratory is supported by The Science and Technology Commission of Shanghai Municipality Project (11JC1401000), National Basic Research Program (2010CB945500, 2012CB966300), and Zhengyi Scholarship (S06-7).
Glossary
Abbreviations:
- miRNA
microRNA
- ESC
embryonic stem cell
- iPSC
induced pluripotent stem cell
- SCNT
somatic cell nuclear transfer
Submitted
03/13/2013
Revised
07/16/2013
Accepted
07/22/2013
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/25828
References
- 1.Czech B, Hannon GJ. Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet. 2011;12:19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 4.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 7.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–13. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–69. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–25. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 11.Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–31. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 13.Han Z, Vandevoort CA, Latham KE. Therapeutic cloning: status and prospects. Curr Opin Mol Ther. 2007;9:392–7. [PubMed] [Google Scholar]
- 14.Bao X, Zhu X, Liao B, Benda C, Zhuang Q, Pei D, Qin B, Esteban MA. MicroRNAs in somatic cell reprogramming. Curr Opin Cell Biol. 2013;25:208–14. doi: 10.1016/j.ceb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Parsons XH. MicroRNA profiling reveals distinct mechanisms governing cardiac and neural lineage-specification of pluripotent human embryonic stem cells. J Stem Cell Res Ther. 2012;2:124. doi: 10.4172/2157-7633.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–5. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 21.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351–8. doi: 10.1016/S1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 22.Zovoilis A, Smorag L, Pantazi A, Engel W. Members of the miR-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation. 2009;78:69–78. doi: 10.1016/j.diff.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros LA, Dennis LM, Gill ME, Houbaviy H, Markoulaki S, Fu D, White AC, Kirak O, Sharp PA, Page DC, et al. Mir-290-295 deficiency in mice results in partially penetrant embryonic lethality and germ cell defects. Proc Natl Acad Sci U S A. 2011;108:14163–8. doi: 10.1073/pnas.1111241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–83. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benetti R, Gonzalo S, Jaco I, Muñoz P, Gonzalez S, Schoeftner S, Murchison E, Andl T, Chen T, Klatt P, et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nat Struct Mol Biol. 2008;15:268–79. doi: 10.1038/nsmb.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P, Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–67. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 28.Morin RD, O’Connor MD, Griffith M, Kuchenbauer F, Delaney A, Prabhu AL, Zhao Y, McDonald H, Zeng T, Hirst M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18:610–21. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshmipathy U, Love B, Goff LA, Jörnsten R, Graichen R, Hart RP, Chesnut JD. MicroRNA expression pattern of undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev. 2007;16:1003–16. doi: 10.1089/scd.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barroso-delJesus A, Romero-López C, Lucena-Aguilar G, Melen GJ, Sanchez L, Ligero G, Berzal-Herranz A, Menendez P. Embryonic stem cell-specific miR302-367 cluster: human gene structure and functional characterization of its core promoter. Mol Cell Biol. 2008;28:6609–19. doi: 10.1128/MCB.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28:6426–38. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barroso-del Jesus A, Lucena-Aguilar G, Menendez P. The miR-302-367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2009;8:394–8. doi: 10.4161/cc.8.3.7554. [DOI] [PubMed] [Google Scholar]
- 33.Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhong X, Li N, Liang S, Huang Q, Coukos G, Zhang L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J Biol Chem. 2010;285:41961–71. doi: 10.1074/jbc.M110.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–40. [PubMed] [Google Scholar]
- 37.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 38.Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–74. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 39.Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- 41.Egli D, Chen AE, Saphier G, Ichida J, Fitzgerald C, Go KJ, Acevedo N, Patel J, Baetscher M, Kearns WG, et al. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat Commun. 2011;2:488. doi: 10.1038/ncomms1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–38. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–74. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–9. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–61. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443–8. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Yang C-S, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–34. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosa A, Brivanlou AH. A regulatory circuitry comprised of miR-302 and the transcription factors OCT4 and NR2F2 regulates human embryonic stem cell differentiation. EMBO J. 2011;30:237–48. doi: 10.1038/emboj.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin SL, Chang DC, Lin CH, Ying SY, Leu D, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–65. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryul Lee M, Prasain N, Chae HD, Kim YJ, Mantel C, Yoder MC, et al. Epigenetic Regulation of Nanog by miR-302 cluster-MBD2 Completes iPS Cell Reprogramming. Stem Cells. 2012;31:666–81. doi: 10.1002/stem.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, Xue Y, Cai J, Guo X, Qin B, et al. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–64. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson KD, Venkatasubrahmanyam S, Jia F, Sun N, Butte AJ, Wu JC. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev. 2009;18:749–58. doi: 10.1089/scd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–60. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, He Q, Han C, Gu H, Jin L, Li Q, Mei Y, Wu M. p53-facilitated miR-199a-3p regulates somatic cell reprogramming. Stem Cells. 2012;30:1405–13. doi: 10.1002/stem.1121. [DOI] [PubMed] [Google Scholar]
- 61.Ye D, Wang G, Liu Y, Huang W, Wu M, Zhu S, Jia W, Deng AM, Liu H, Kang J. MiR-138 promotes induced pluripotent stem cell generation through the regulation of the p53 signaling. Stem Cells. 2012;30:1645–54. doi: 10.1002/stem.1149. [DOI] [PubMed] [Google Scholar]
- 62.Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–53. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R,et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 2009;41:1350–3. [DOI] [PMC free article] [PubMed]
- 64.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neveu P, Kye MJ, Qi S, Buchholz DE, Clegg DO, Sahin M, Park IH, Kim KS, Daley GQ, Kornblum HI, et al. MicroRNA profiling reveals two distinct p53-related human pluripotent stem cell states. Cell Stem Cell. 2010;7:671–81. doi: 10.1016/j.stem.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 66.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Hu S, Wilson KD, Ghosh Z, Han L, Wang Y, Lan F, Ransohoff KJ, Burridge P, Wu JC. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 2013;31:259–68. doi: 10.1002/stem.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurdon J, Murdoch A. Nuclear transfer and iPS may work best together. Cell Stem Cell. 2008;2:135–8. doi: 10.1016/j.stem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, Zimmer M, Kahler DJ, Goland RS, Noggle SA, et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–7. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci U S A. 2006;103:933–8. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castro FO, Sharbati S, Rodríguez-Alvarez LL, Cox JF, Hultschig C, Einspanier R. MicroRNA expression profiling of elongated cloned and in vitro-fertilized bovine embryos. Theriogenology. 2010;73:71–85. doi: 10.1016/j.theriogenology.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Cui XS, Zhang DX, Ko YG, Kim NH. Aberrant epigenetic reprogramming of imprinted microRNA-127 and Rtl1 in cloned mouse embryos. Biochem Biophys Res Commun. 2009;379:390–4. doi: 10.1016/j.bbrc.2008.12.148. [DOI] [PubMed] [Google Scholar]
- 74.Matoba S, Inoue K, Kohda T, Sugimoto M, Mizutani E, Ogonuki N, Nakamura T, Abe K, Nakano T, Ishino F, et al. RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos. Proc Natl Acad Sci U S A. 2011;108:20621–6. doi: 10.1073/pnas.1112664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoue K, Kohda T, Sugimoto M, Sado T, Ogonuki N, Matoba S, Shiura H, Ikeda R, Mochida K, Fujii T, et al. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science. 2010;330:496–9. doi: 10.1126/science.1194174. [DOI] [PubMed] [Google Scholar]
- 76.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–63. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 77.Hanina SA, Mifsud W, Down TA, Hayashi K, O’Carroll D, Lao K, Miska EA, Surani MA. Genome-wide identification of targets and function of individual MicroRNAs in mouse embryonic stem cells. PLoS Genet. 2010;6:e1001163. doi: 10.1371/journal.pgen.1001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 79.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]