Abstract
Si-Wu-Tang (SWT) is a Traditional Chinese Medicine (TCM) formula widely used for the treatments of gynecological diseases. To explore the pharmacological mechanism of SWT, we incorporated microarray data of SWT with our herbal target database TCMID to analyze the potential activity mechanism of SWT's herbal ingredients and targets. We detected 2,405 differentially expressed genes in the microarray data, 20 of 102 proteins targeted by SWT were encoded by these DEGs and can be targeted by 2 FDA-approved drugs and 39 experimental drugs. The results of pathway enrichment analysis of the 20 predicted targets were consistent with that of 2,405 differentially expressed genes, elaborating the potential pharmacological mechanisms of SWT. Further study from a perspective of protein-protein interaction (PPI) network showed that the predicted targets of SWT function cooperatively to perform their multi-target effects. We also constructed a network to combine herbs, ingredients, targets and drugs together which bridges the gap between SWT and conventional medicine, and used it to infer the potential mechanisms of herbal ingredients. Moreover, based on the hypothesis that the same or similar effects between different TCM formulae may result from targeting the same proteins, we analyzed 27 other TCM formulae which can also treat the gynecological diseases, the subsequent result provides additional insight to understand the potential mechanisms of SWT in treating amenorrhea. Our bioinformatics approach to detect the pharmacology of SWT may shed light on drug discovery for gynecological diseases and could be utilized to investigate other TCM formulae as well.
Introduction
Traditional Chinese Medicine (TCM) is an ancient system used in disease treatments for several thousand years [1], [2]. Currently, TCM is not only popular in Asia, but also used in United States and Europe as complementary or alternative medicine [3], [4]. Up to now, nearly 100,000 TCM formulae have been recovered [5], [6], each of which normally contains several herbs. Generally, a TCM formula exerts its therapeutic effects through interactions between herbal ingredients and dysfunctional proteins related to the diseases. These ingredients target many molecules in the cell and function cooperatively to increase the therapeutic efficacy and reduce adverse effects of the TCM [6], [7]. Although great efforts have been made to unveil the mechanisms of TCM formulae, the mechanisms of most formulae are still unknown [6], [8].
Because a TCM formula contains many non-effective and needless ingredients, a new approach which combines only active ingredients in one formula has been suggested for new formula discovery [7]. This method is useful for the modernization of TCM because if a formula is simplified to only contain active ingredients, the production of this new formula will rely less on cultivations of herbs and can be manufactured based on methodology of highly-developed chemical synthesis. However, few formulae were simplified by this way as active ingredients of most formulae were still unclear.
Si-Wu-Tang (SWT), composed of four herbs, Radix Rehmanniae Praeparata, Radix Angelicae Sinensis, Rhizoma Ligustici Chuanxiong and Radix Paeoniae Alba [9], is a popular TCM formula widely used for the treatment of gynecological disease in Asia for a long time [10]. It has been reported in the treatment of menstrual discomfort, climacteric syndrome, peri- or postmenopausal syndrome and other estrogen-related diseases [11]–[15]. Besides, this formula also provides the pharmacological effects of anti-inflammatory, vasodilatation and hematopoiesis [16], [17].
Microarray experiment has been conducted to analyze the mechanism of SWT treatment at gene level, suggesting that SWT has a phytoestrogenic effect and act as an Nrf2 activator [6]. However, the results gained from microarray experiment are not convictive enough because the up/down-regulation of mRNA may not lead to a consistent alteration of protein expression [6], [18], [19]. To further investigate the potential mechanism of SWT on disease treatment, we integrated the microarray expression data with the herbal targets obtained from our TCMID database [20]. TCMID is an integrative database that contains data of herbal ingredients, herbal targets, disease-related gene or proteins, drugs and their targets, many of which were collected through text mining. These data can be effectively applied to complement the results of high throughput experiments.
In particular, we want to check whether genes differentially expressed in cells treated with SWT finally lead to therapeutic effects on protein level. Thus we conducted this study by firstly identifying intersections between symbols of previously known targets of the four herbs in SWT in TCMID database and the differentially expressed genes (DEGs), resulting in 20 predicted targets of SWT. Then pathway enrichment analysis and protein-protein interaction network were utilized to explore SWT's pharmacological effects on gynecological diseases. We further identified compounds (herb ingredients and drugs) that may have potential to become new drugs or drugs that may have new therapeutic effects. Subsequently, a novel herb-ingredient-target-drug network was constructed to visually show the relationships between herbs, ingredients, targets and drugs. We also collected other TCM formulae which have therapeutic effects on gynecological diseases and investigated whether these formulae target most of these 20 predicted targets of SWT as well.
Materials and Methods
2.1. Materials
Microarray data of human breast cancer MCF-7 cells treated with SWT were downloaded from Gene Expression Omnibus (GEO: GSE23610), which consist of 54,675 probe sets. Protein targets of each herb constituting SWT (Radix Rehmanniae Praeparata, Radix Angelicae Sinensis, Rhizoma Ligustici Chuanxiong and Radix Paeoniae Alba) identified experimentally from previously published literature as well as TCM formulae with same or similar effects as SWT were obtained from TCMID by searching herbal names and names of all the gynecological diseases respectively [20]. The target names of four herbs in SWT were also retrieved from TCMID. As for other TCM formulae used for treating gynecological diseases, we obtained 27 formulae which mainly treat menstrual discomfort and climacteric syndromes from our TCMID database (Table S1).
2.2. Methods
2.2.1. Identification of differentially expressed genes and their intersections with herbal targets
In our study, only gene expression profiles of MCF-7 cells treated with SWT in high concentration was used for the identification of differentially expressed genes, because SWT with high concentration has the similar expression profile to Estradiol treatment on MCF-7 cells and is considered as the effective formula for disease treatment in clinical practices [6]. We compared the SWT group in high concentration with control group and obtained differentially expressed genes by setting p-value <0.05 and fold change >1.5 which is consistent with previous study [6].
To identify the potential targets of each herb in SWT, we used each herb's name to query TCMID, and then retrieved the targets for each ingredient in the herb. In total, we obtained 102 non-redundant targets for all of the identified ingredients of SWT (Table S2). For the 102 targets of SWT in TCMID, we firstly obtained their symbols in HUGO Gene Nomenclature Committee (HGNC) by searching the names of targets [21], and then computed intersections between symbols of these targets and names of the differentially expressed genes to get predicted targets of SWT whose encoding genes are differentially expressed.
2.2.2. Pathway enrichment analysis
We carried out pathway enrichment analysis for these differentially expressed genes using ClueGO (a plugin of Cytoscape) [22] and obtained the pathways enriched with these differentially expressed genes (p<0.05 as the threshold). Pathway enrichment analysis for the predicted protein targets of SWT was also identified in a similar way.
2.2.3. Network construction
Based on the data of protein-protein interactions in HPRD [23] and STRING [24], we constructed a PPI network for predicted targets of SWT with Cytoscape [25] using all the data of protein-protein interactions in HPRD and STRING. Then we identified those interactions directly between the predicted proteins or bridged by only one intermediate protein.
To construct a herb-ingredient-target-drug network, we first selected those ingredients, each of which targets at least one of the predicted targets of SWT. Next we downloaded all the drug names and their target names from DrugBank [26], followed by selecting drugs which also target at least one of the predicted targets of SWT. Finally we constructed the herb-ingredient-target-drug network based on the interactions between ingredients (or drugs) and targets using Cytoscape.
2.2.4. Analysis of other TCM formulae with similar effects as SWT
To analyze the 27 other TCM formulae which can also treat gynecological diseases, we firstly obtained the targets of these formulae in TCMID by searching the names of the herbs in each formula. Subsequently we removed the redundant targets in each formula and retained about 513 unique targets (Table S6) for all 27 formulae. Then we calculated the numbers of occurrence in the 27 TCM formulae for all the 513 targets. To identify the common therapeutic effects among these formulae, we counted the numbers of occurrence for the predicted targets of SWT. To explore the connection between gynecological diseases and the predicted targets, we detected the co-occurrence between each target name and each name of gynecological diseases (such as menstrual discomfort and climacteric syndrome) with google scholar. Through text mining, we found that considerable amount of literatures describe the relationships between the predicted targets and the related gynecological diseases.
Results
3.1. Pathway enrichment analysis for differentially expressed genes
To validate the results from a previous study [6], and explore the potential mechanisms of SWT, we looked for differentially expressed genes in downloaded microarray data, followed by pathway enrichment analysis. In all, we obtained 2,405 differentially expressed genes, corresponding to 3,950 probe sets in the microarray. Pathway enrichment analysis of 2,405 differentially expressed genes showed that these genes were enriched in 7 pathways with p-values less than 0.05 ( Table 1 ).
Table 1. Seven pathways enriched based on differentially expressed genes with p-values less than 0.05.
| No. | Pathway name | P-value |
| 1 | Pathways in cancer | 1.61E-05 |
| 2 | Ribosome biogenesis in eukaryotes | 8.91E-04 |
| 3 | p53 signaling pathway | 0.006777907 |
| 4 | Endocytosis | 0.011224262 |
| 5 | Neuroactive ligand-receptor interaction | 0.011869812 |
| 6 | TGF-beta signaling pathway | 0.030380161 |
| 7 | Oxidative Stress Induced Gene Expression Via Nrf2 | 0.041972061 |
Consistent with the previous results [6], we found that Nrf2 was significantly impacted by SWT. Furthermore, our research showed that TGF-β signaling pathway was enriched by 24 differentially expressed genes (Table S3). Besides, TGF-β, which plays an important role in this pathway, has been proved to be involved in a variety of physiological processes and many diseases. It was reported that TGF-β had close relationships with osteoporosis and coronary heart disease in postmenopausal women suffering climacteric syndromes [27]–[29]. Since SWT can treat climacteric syndrome and it significantly down-regulates TGF-β coding gene (one of the 2,045 differentially expressed genes, Table S3), we inferred that one of the therapeutic effects of SWT on osteoporosis and coronary heart disease could attribute to the down-regulation of TGF-β by SWT [30]. In other word, pathway enrichment analysis of differentially expressed genes obtained from microarray experiments suggests the potential mechanisms of SWT on treatment of climacteric syndrome.
3.2. Pathway enrichment analysis of the20 predicted targets
By focusing on herbal targets of SWT whose encoding genes are differentially expressed, we detected 20 intersections between previously known protein targets of the four herbs of SWT in TCMID and differentially expressed genes, which are 20 predicted targets of SWT ( Table 2 ) and used for further study.
Table 2. 20 predicted targets of SWT.
| No. | Target | Symbol |
| 1 | Vascular endothelial growth factor A | VEGFA |
| 2 | Telomerase protein component 1 | TEP1 |
| 3 | Mitogen-activated protein kinase 1 | MAPK1 |
| 4 | Interleukin-8 | IL8 |
| 5 | Interleukin-6 | IL6 |
| 6 | Intercellular adhesion molecule 1 | ICAM1 |
| 7 | Proto-oncogene c-Fos | FOS |
| 8 | Eukaryotic translation initiation factor 6 | EIF6 |
| 9 | Cytochrome P450 1A1 | CYP1A1 |
| 10 | Cyclin-dependent kinase inhibitor 1 | CDKN1A |
| 11 | Cyclin-A2 | CCNA2 |
| 12 | Caspase-3 | CASP3 |
| 13 | Transcription factor AP-1 | JUN |
| 14 | Activator of 90 kDa heat shock protein ATPase homolog 1 | AHSA1 |
| 15 | Serine/threonine-protein kinase Sgk3 | SGK3 |
| 16 | Heparan sulfate glucosamine 3-O-sulfotransferase 3A1 | HS3ST3A1 |
| 17 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | UCHL1 |
| 18 | Solute carrier family 22 member 5 | SLC22A5 |
| 19 | Choline-phosphate cytidylyltransferase A | PCYT1A |
| 20 | Protein CBFA2T1 | RUNX1T1 |
Pathway enrichment analysis of the 20 predicted targets showed that predicted targets of SWT enriched in 40 pathways with p-values less than 0.05. We ranked these pathways according to the p-value of each pathway in an ascending order. The top 20 pathways are shown in Table 3 . It is interesting to note that several pathways can further illustrate the possibly pharmacological mechanisms of SWT.
Table 3. The top 20 pathways enriched with 20 predicted targets with p-values less than 0.05.
| No. | Pathway name | p-value |
| 1 | Pertussis | 7.21E-07 |
| 2 | Rheumatoid arthritis | 2.89E-06 |
| 3 | TSP-1 Induced Apoptosis in Microvascular Endothelial Cell | 1.92E-05 |
| 4 | Salmonella infection | 7.20E-05 |
| 5 | Bladder cancer | 1.24E-04 |
| 6 | Toll-like receptor signaling pathway | 1.68E-04 |
| 7 | Chagas disease (American trypanosomiasis) | 1.85E-04 |
| 8 | Cadmium induces DNA synthesis and proliferation in macrophages | 1.97E-04 |
| 9 | Cells and Molecules involved in local acute inflammatory response | 3.03E-04 |
| 10 | Oxidative Stress Induced Gene Expression Via Nrf2 | 4.39E-04 |
| 11 | IL 6 signaling pathway | 5.21E-04 |
| 12 | Colorectal cancer | 6.00E-04 |
| 13 | Fc Epsilon Receptor I Signaling in Mast Cells | 0.002144985 |
| 14 | Repression of Pain Sensation by the Transcriptional Regulator DREAM | 0.008579493 |
| 15 | Malaria | 0.010027851 |
| 16 | Legionellosis | 0.013315141 |
| 17 | NOD-like receptor signaling pathway | 0.014802808 |
| 18 | B Cell Survival Pathway | 0.018475697 |
| 19 | D4-GDI Signaling Pathway | 0.018475697 |
| 20 | Pertussis toxin-insensitive CCR5 Signaling in Macrophage | 0.021522095 |
The pathway of “oxidative stress induced gene expression via Nrf2” (ranked 10), which was also enriched by differentially expressed genes, plays an important role in radio-resistance [31]. It was reported that γ-irradiation-induced formation of protein carbonyls was significantly higher in Nrf2-depleted lung cancer cells, and the increased lethality of ionizing radiation in the absence of Nrf2, suggesting Nrf2 has a constitutive activation to protect against ionizing radiation toxicity and confer radio-resistance [31]. Besides, Nrf2 was suggested to be used as a target of chemopreventive agent. Our pathway enrichment analysis showed that three targets of SWT were enriched in this pathway, including proto-oncogene c-fos (FOS), transcription factor AP-1 (JUN), and mitogen-activated protein kinase 1 (MAPK1). As SWT was reported to have a significant effect on radio-resistance [32]–[35], we inferred that the potential effect of radio-resistance was provided by ingredients in the four herbs of SWT targeting these proteins.
With respect to the pathway of “repression of pain sensation by the transcriptional regulator DREAM” (ranked 14), in general, the opioid receptors modulate pain signaling in response to endogenous peptide ligands and opiate drugs such as morphine [36]. Specifically the kappa opioid receptor plays a key role in the profound analgesia of opiates and is activated by the endogenous peptide ligand dynorphin, encoded by the prodynorphin gene. Production of prodynorphin is transcriptionally regulated by a downstream regulatory element (DRE) in the prodynorphin gene. A transcription factor called DREAM (DRE antagonistic modulator) binds to the DRE and represses prodynorphin transcription [36], [37]. The regulation of prodynorphin expression by DREAM leads to the hypothesis that DREAM is involved in pain signaling. Our research showed that differentially expressed genes which encode two targets of SWT, FOS and JUN, enriched in this pathway. The two protein targets interact with DREAM in this pathway to regulate the expression of preprodynorphin [38] and function as third messengers in the signal transduction mechanisms of pain processes [39]. As SWT was also reported to have a significant therapeutic effect on dysmenorrhea [40]–[43], we proposed that SWT may play its therapeutic role on dysmenorrhea by targeting FOS and JUN to regulate the signaling pathway of pain.
In addition, differentially expressed genes which encode targets of SWT were also enriched in three pathways relating to hematopoiesis, erythropoiesis and leukopoiesis, which are “regulation of hematopoiesis by cytokines” (ranked 25), “EPO signaling pathway” (ranked 32) and “TPO signaling pathway” (ranked 35). This finding provides a meaningful explanation for the significant effect of SWT on treating “blood deficiency”, since SWT can prevent the symptoms of blood deficiency through these pathways, such as decreasing erythrocyte and leukocyte [9], [32], [33].
3.3. Protein-protein interaction network analysis
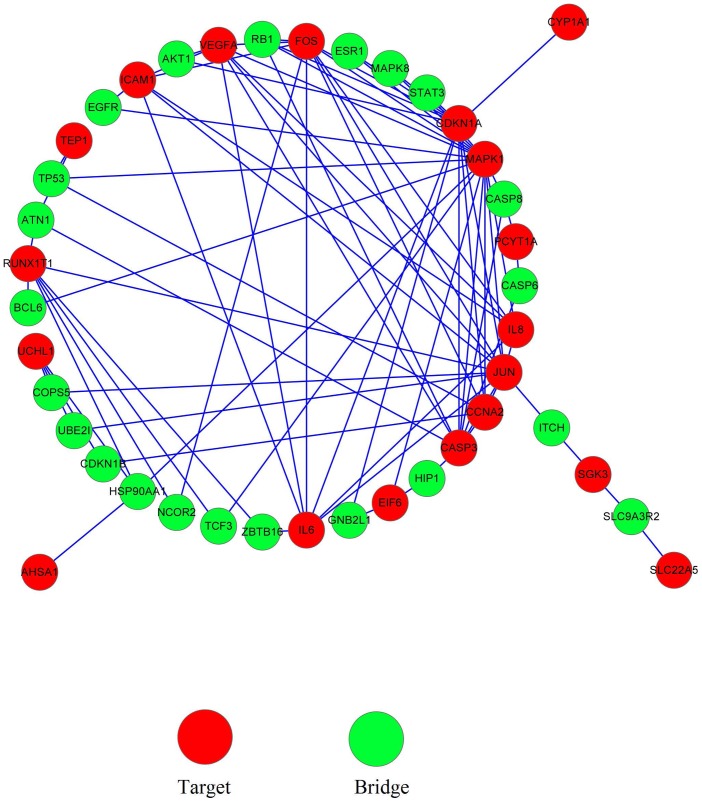
As shown in Figure 1 , the 20 predicted protein targets of SWT in the PPI network connected with each other through direct interaction or bridged by an intermediate protein. 19 out of 20 predicted targets were involved in this network. Five proteins, MAPK1, JUN, CDKN1A, CASP3 and FOS were supposed to be hub proteins because each of them connects with at least 10 predicted targets of SWT or intermediate proteins. Particularly, JUN and FOS participated in 28 and 25 of pathways enriched by differentially expressed genes encoding 20 predicted targets of SWT respectively, suggesting that JUN and FOS function as the main targets of SWT and are the potential targets of new drugs.
Figure 1. PPI network of 20 predicted herbal targets of SWT.
3.4. Herbal ingredients targeting 20 predicted targets of SWT
Since the 102 targets of SWT are targeted by ingredients in the four herbs of SWT, we further selected the ingredients, each of which targets at least one of 20 predicted targets of SWT. For each ingredient with defined targets, it could be a candidate for new drug [44]. The herbs and ingredients which interact with predicted targets of SWT are shown in Table S4.
As shown in Table S4, SWT targets predicted proteins via 16 non-overlapping active ingredients, implying that these 16 ingredients may provide main pharmacological effects of SWT. Accordingly, the four herbs of SWT could potentially be simplified to these 16 ingredients and mass-produced by chemical synthesis, which still need further validation.
3.5. Drugs targeting 20 predicted targets of SWT
Generally, if drugs and TCM formulae target the same proteins, they may have same or similar therapeutic effects, which offer an effective way to connect TCM and conventional medicine. Here, to check whether the predicted targets of SWT are targeted by drugs as well, we further searched drugs which target at least one of the 20 predicted targets in DrugBank. As a result, we found 2 FDA-approved small molecule drugs and 39 experimental drugs. These 41 drugs (Table S5) have the potential for treat gynecological diseases, since each of them interacts with at least one of the targets of SWT, and thereby may have some potential for treating similar diseases as SWT does. But this analysis may have low predictive potential because SWT's therapeutic effects are likely the result of its multiple components targeting multiple protein targets, i.e. not a single component targeting a single target.
3.6. Herb-ingredient-target-drug network analysis
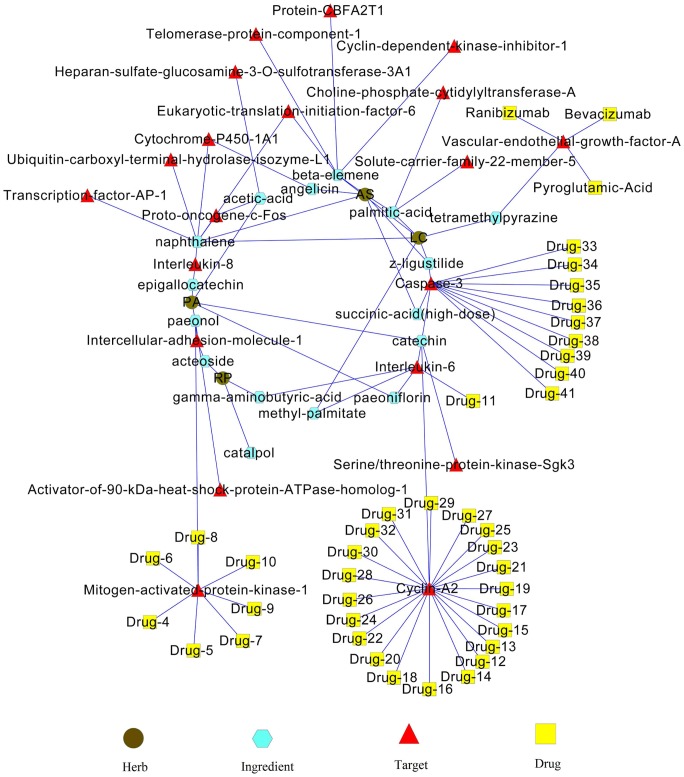
Although TCM and conventional medicine are based on different theories, they both provide their therapeutic effects through chemical molecules (herbal ingredients or drugs) targeting proteins (e.g. enzymes) related to the pathological processes of diseases. Thus targets of both herbal ingredients and drugs can bridge the gap between TCM and conventional medicine. Based on this assumption, a network integrating herbs, ingredients, targets and drugs was constructed ( Figure 2 ). This network visually shows the relationships between herbs, ingredients, targets and drugs, and is a meaningful attempt in bridging TCM and conventional medicine.
Figure 2. The herb-ingredient-target-drug network for SWT.
“PA”, “AS”, “RP”, “LC” represent “Radix Paeoniae Alba”, “Radix Angelicae Sinensis”, “Radix Rehmanniae Praeparata” and “Rhizoma Ligustici Chuanxiong” respectively. “Drug-4”, “Drug-5”, “Drug-6”,..., “Drug-41” represent drugs from “No. 4” to “No. 41” in Table S5 respectively.
In this network, it is easy to find drugs and ingredients connected by the same targets. For example, vascular endothelial growth factor A (VEGFA), Mitogen-activated protein kinase 1, Cyclin-A2 and Caspase-3 are targeted by many drugs and are acting as hubs to connect SWT and conventional medicine. Specially, VEGFA was simultaneously targeted by three drugs, bevacizumab, ranibizumab and pyroglutamic acid. Besides, VEGFA was also targeted by tetramethylpyrazine, an important ingredient in “Rhizoma Ligustici Chuanxiong”. This connection implies that tetramethylpyrazine could potentially have similar therapeutic effects as these drugs, and therefore has some potential to be considered as a new candidate for therapy. For the other three hubs, the related drugs are still under experimental testing. Thus the potential connections between these experimental drugs and SWT remain to be further verified.
3.7. Analysis of TCM formulae with similar effects to SWT
The same or similar therapeutic effects of different TCM formula may be caused by targeting the same targets. Based on this assumption, we checked whether the 27 other formulae treating gynecological diseases retrieved from TCMID also target the 20 predicted targets of SWT (Materials and Methods). The numbers of these formulae containing the predicted targets of SWT are summarized in Table 4 .
Table 4. Numbers of occurrence in 27 other TCM formulae for each predicted targets of SWT.
| No. | Target | No. of occurrence |
| 1 | Caspase-3 | 27 |
| 2 | Transcription factor AP-1 | 27 |
| 3 | Proto-oncogene c-Fos | 27 |
| 4 | Vascular endothelial growth factor A | 25 |
| 5 | Interleukin-8 | 25 |
| 6 | Interleukin-6 | 25 |
| 7 | Cyclin-dependent kinase inhibitor 1 | 25 |
| 8 | Eukaryotic translation initiation factor 6 | 23 |
| 9 | Cytochrome P450 1A1 | 23 |
| 10 | Telomerase protein component 1 | 23 |
| 11 | Mitogen-activated protein kinase 1 | 22 |
| 12 | Intercellular adhesion molecule 1 | 22 |
| 13 | Solute carrier family 22 member 5 | 22 |
| 14 | Choline-phosphate cytidylyltransferase A | 22 |
| 15 | Protein CBFA2T1 | 22 |
| 16 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 21 |
| 17 | Activator of 90 kDa heat shock protein ATPase homolog 1 | 19 |
| 18 | Heparan sulfate glucosamine 3-O-sulfotransferase 3A1 | 16 |
| 19 | Cyclin-A2 | 14 |
| 20 | Serine/threonine-protein kinase Sgk3 | 14 |
As shown in Table 4 , CAPS3, JUN and FOS were targeted by all the 27 formulae, which probably explain the common mechanism of the 27 formulae having same or similar therapeutic effects as SWT on gynecological diseases. Additionally, VEGFA was targeted by 25 out of 27 TCM formulae. Previous research showed that there was a significant reduction in the expression of VEGFA for women with amenorrhoea [45], whereas our study concerning differentially expressed genes showed that the mRNA of VEGFA was significantly up-regulated under the influence of SWT. Thus it is very likely that SWT can help facilitating the expression of VEGFA. As a result, the symptoms of amenorrhea could be potentially relieved by the increase of VEGFA, which provides the potential pharmacological mechanism for SWT to treat amenorrhea.
Discussion
TCM formulae typically utilize multi-component therapeutics, similarly drug combination provides increased therapeutic effects by synergisms of two or more drugs and decreased side effects by antagonism, playing a more and more important role in clinical practices and attracting great attention of drug companies and biomedical researchers. Our study also provides a new perspective to find drugs that may provide synergetic effects on treatment of diseases through combinational strategies. For example, if MAPK1 and CASP3, the hub proteins in our constructed PPI network, are targeted by herbal ingredients, the disturbance of the two hub proteins will affect many other proteins in this network and thereby provide same or similar effects as SWT. In addition, as shown in Figure 2 , MAPK1 and CASP3 can be targeted by 7 and 9 drugs respectively. Therefore, we suppose that drug combinations, such as “Drug-4” and “Drug-33” in Figure 2 which target MAPK1 and CASP3 respectively, may provide similar therapeutic effects as SWT.
Traditional research methodologies of TCM's pharmacological effects and molecular mechanism are based on the model of single ingredient and single target, which is similar to the rationale applied in modern drug discovery. Thus, methods of natural pharmaceutical chemistry have been widely applied to extract and isolate individual ingredients in herbs. Pharmacological effects of each ingredient are then tested by the methods of modern pharmacology. It is worth noting that individual ingredient may not necessarily have significant therapeutic effects without the coordination with other ingredients, owing to the combinational therapeutic effects for a TCM formula. In other word, the combinational effects of a formula are not necessarily the sum of the individual effect of each ingredient in the formula, which makes it a great challenge for experiments to test the therapeutic effects of TCM [46], [47]. Fortunately, bioinformatic approaches can be applied to this field and to illustrate the potential mechanisms of TCM formulae at the systematic level. In this study, we successfully applied a bioinformatics approach to detect the potential pharmacology of SWT, which may be more reasonable than methods adopted in previous works. For example, the pharmacology of a formula-Qing Luo Yin (QLY) was analysed by Zhang, etc [48]. But they found targets of herbal ingredients based on information of drugs' targets in DrugBank, and this may miss out many targets because only a few herbal ingredients are FDA-approved drugs. In our work, we collected all targets for each herbal ingredients form TCMID and the data was more complete.
According to our results, SWT could be simplified to 16 herbal ingredients. Of course, to verify whether these 16 ingredients can provide similar effects as SWT requires more preclinical experiments. Also, a breast cancer cell line may not be a relevant target cell for observing the effects of drugs on non-cancerous gynecological diseases. Nevertheless, our study demonstrates that the pharmacological effects of TCM formulae can be explored by the integration of multi-level data, such as formulae, herbs, herbal targets, herbal ingredients and drugs as well as PPI network. Our analysis pipeline can also be effectively extended to study mechanisms of other TCM formulae.
Supporting Information
27 TCM formulae used for treating women's diseases.
(DOCX)
102 targets of SWT.
(DOCX)
Symbols of 24 genes enriched in TGF-beta signaling pathway.
(DOCX)
Herbs and ingredients which target 20 predicted targets of SWT.
(DOCX)
41 Drugs interacting with 20 predicted targets of SWT.
(DOCX)
513targets of the 27 formulae.
(DOCX)
Funding Statement
This work was supported by the National 973 Key Basic Research Program (Grant No. 2013CB127000 and 2010CB945401), the National Natural Science Foundation of China (Grant No. 31240038, 31071162, and 31000590), the Science and Technology Commission of Shanghai Municipality (11DZ2260300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhao J, Jiang P, Zhang W (2010) Molecular networks for the study of TCM Pharmacology. Brief Bioinform 11: 417–430. [DOI] [PubMed] [Google Scholar]
- 2. Li S, Zhang ZQ, Wu LJ, Zhang XG, Li YD, et al. (2007) Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst Biol 1: 51–60. [DOI] [PubMed] [Google Scholar]
- 3. Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE (2010) Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 13: 50–65. [PubMed] [Google Scholar]
- 4. Cheung F (2011) TCM: Made in China. Nature 480: S82–S83. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Zhou GB, Liu P, Song JH, Liang Y, et al. (2008) Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. PNAS 105: 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen Z, Wang Z, Wang S, Ravula R, Yang L, et al. (2011) Discovery of Molecular Mechanisms of Traditional Chinese Medicinal Formula Si-Wu-Tang Using Gene Expression Microarray and Connectivity Map. PLoS One 6: e18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan SK, Zhao J, Dou SS, Jiang P, Liu RH, et al. (2009) Methodology of Modernization Research in Traditional Chinese Medicine Based on Systems Biology and Network Biology. Chinese Journal of Natural Medicine 7: 249–259. [Google Scholar]
- 8. Tian P (2011) Convergence: Where West meets East. Nature 480: S84–S86. [DOI] [PubMed] [Google Scholar]
- 9. Gao Y, Ma ZC, Liang QD, Tan HL, Liu M, et al. (2010) Combined Research of Si wu tang and Blood Deficiency. World Science And Technology 12: 211–216. [Google Scholar]
- 10. Wang L, Wang Z, Wo S, Lau CB, Chen X, et al. (2011) A bio-activity guided in vitro pharmacokinetic method to improve the quality control of Chinese medicines, application to Si Wu Tang, Int J Pharm. 406: 99–105. [DOI] [PubMed] [Google Scholar]
- 11. Yeh LL, Liu JY, Lin KS, Liu YS, Chiou JM, et al. (2007) A randomised placebo controlled trial of a traditional Chinese herbal formula in the treatment of primary dysmenorrhoea. PLoS One 2: e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohta H, Ni JW, Matsumoto K, Watanabe H, Shimizu M (1993) Peony and its major constituent, paeoniflorin, improve radial maze performance impaired by scopolamine in rats. Pharmacol Biochem Behav 45: 719–723. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe H (1998) Protective effect of a traditional medicine, shimotsu-to, on brain lesion in rats. J Toxicol Sci 23: 234–236. [DOI] [PubMed] [Google Scholar]
- 14. Wang ZJ, Wo SK, Wang L, Lau CB, Lee VH, et al. (2009) Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal 50: 232–244. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Shen P, Cheng Y (2004) Identification and determination of the major constituents in traditional Chinese medicine Si-Wu-Tang by HPLC coupled with DAD and ESI-MS. J Pharm Biomed Anal 34: 705–713. [DOI] [PubMed] [Google Scholar]
- 16. Dai Y, But PP, Chan YP, Matsuda H, Kubo M (2002) Antipruritic and Antiinflammatory Effects of Aqueous Extract from Si-Wu-Tang. Biol Pharm Bull 25: 1175–1178. [DOI] [PubMed] [Google Scholar]
- 17. Liang QD, Gao Y, Tan HL, Guo P, Li YF, et al. (2006) Effects of four Si-Wu-Tang's constituents and their combination on irradiated mice. Biol Pharm Bull 29: 1378–1382. [DOI] [PubMed] [Google Scholar]
- 18. Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology 4: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between Protein and mRNA Abundance in Yeast. Mol Cell Biol 19: 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue RC, Fang Z, Zhang MX, Yi ZH, Shi TL (2013) TCMID: Traditional Chinese Medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res 41: D1055–D1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seal RL, Gordon SM, Lush MJ, Wright MW, Bruford EA (2011) genenames.org: the GNC resources in 2011. Nucleic Acids Res 39: D514–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, et al. (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, et al. (2009) Human Protein Reference Database–2009 update. Nucleic Acids Res 37: D767–D772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, et al. (2011) The STRING database in 2011: functional interaction networks of roteins, globally integrated and scored. Nucleic Acids Res 39: D561–D568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, et al. (2008) DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res 36: D901–D906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinke V, Seck T, Clanget C, Scheidt-Nave C, Ziegler R, et al. (2001) Association of transforming growth factor-β1 (TGFβ1) T29→C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-β1 in a population-based sample of postmenopausal German women. Calcif. Tissue Int 69: 315–320. [DOI] [PubMed] [Google Scholar]
- 28. Yamada Y, Miyauchi A, Goto J, Takagi Y, Okuizumi H, et al. (1998) Association of a polymorphism of the transforming growth factor-β1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J Bone Miner Res 13: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 29. Os I, Djurovic S, Seljeflot I, Berg K (2002) Transforming growth factor (TGF)-β1 inversely related to vascular cell adhesion molecule-1 in postmenopausal women with coronary artery disease. A possible mechanism for the putative cardioprotective role of TGF-β1? Journal of Internal Medicine 251: 223–227. [DOI] [PubMed] [Google Scholar]
- 30. Chen ZW, Xu HY, Ma WC, Wang LH (2010) Study of Siwu Tang formula granule on the expression of TGF-B1 and Smads in bone marrow depressed mice. China Occupational Medicine 37: 279–281. [Google Scholar]
- 31. Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S (2010) Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal 13: 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao Y, Ma ZC, Tan HL, Tong Li, Dai WQ, et al. (2003) Effects of Si-Wu-Tang and it s extracts on medullary hematopoiesis in mice of blood deficiency induced by 60Coγ irradiation. Tianjin Journal of Traditional Chinese Medicine 20: 47–51. [Google Scholar]
- 33. Tan HL, Ma ZC, Gao Y, Liu YX, Xiao CR, et al. (2002) Effects of Siwu Tang on CD34 Antigen Expression in one Marrow of Mice with Blood Deficiency. Traditional Chinese Drug Research & Clinical Pharmacology 13: 11–13. [Google Scholar]
- 34. Ma ZC, Gao Y, Tang HL, Wang SQ (2003) The Effects of Si-Wu-Tang on Serum Protein of Blood Deficient Mice Induced by Radiation. China Journal of Chinese Materia Medica 28: 1050–1053. [PubMed] [Google Scholar]
- 35. Zhang T, Tan YY, Pan YS (2000) Effects of Siwu Decotion on RBC Immunoadhesive Function and Stem Cell Multiplication. Journal of Beijing University of TCM 23: 36–38. [Google Scholar]
- 36. Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, et al. (2002) DREAM is a critical transcriptional repressor for pain modulation. Cell 108: 31–43. [DOI] [PubMed] [Google Scholar]
- 37. Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR (1999) DREAM is a Ca2+-regulated transcriptional repressor. Nature 398: 80–84. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Zhang Y, Han JS, Wang Y (2008) Distinct Responses of DREAM to Electroacupuncture Stimulation with Different Frequencies During Physiological and Inflammatory Conditions in Rats. Neurochem Res 33: 2070–2077. [DOI] [PubMed] [Google Scholar]
- 39. Naranjo JR, Mellström B, Achaval M, Sassone-Corsi P (1991) Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron 6: 607–617. [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Duan JA, Liu P, Zhu M, Su SL, et al. (2012) Study on compare Taohong Siwu Decoction,Taoren-Honghua pair and Siwu Decoction. Pharmacology and Clinics of Chinese Materia Medica 28: 2–6. [Google Scholar]
- 41. Liu L, Ma H, Tang Y, Chen W, Lu Y, et al. (2012) Discovery of estrogen receptor α modulators from natural compounds in Si-Wu-Tang series decoctions using estrogen-responsive MCF-7 breast cancer cells. Bioorg Med Chem Lett 22: 154–163. [DOI] [PubMed] [Google Scholar]
- 42. Zhang CB, Lu Y, Duan JA, Su SL, Tang YP, et al. (2007) Study on Siwu Decoction and its serial decoctionsin dysmenorrhea model mice. Pharmaceutical and Clinical Research 15: 459–461. [Google Scholar]
- 43. Hua YQ, Duan JA, Su SL, Lu Yin, Wang QJ, et al. (2008) Bioactivity evaluation of Siwu Decoction and its derived formulaes in the treatment of primary dysmenorrhea (I). Journal of China Pharmaceutical University 39: 72–76. [Google Scholar]
- 44. Debnath B, Al-Mawsawi LQ, Neamati N (2010) Are we living in the end of the blockbuster drug era? Drug News Perspect 23: 670–684. [DOI] [PubMed] [Google Scholar]
- 45. Narvekar N, Critchley HO, Cheng L, Baird DT (2006) Mifepristone-induced amenorrhoea is associated with an increase in microvessel density and glucocorticoid receptor and a decrease in stromal vascular endothelial growth factor. Hum Reprod 21: 2312–2318. [DOI] [PubMed] [Google Scholar]
- 46. Li G, Miao R, Zhao H (2012) Progression and prospects of translational medicine in China. Science China Life Sciences 55: 1022–1025. [DOI] [PubMed] [Google Scholar]
- 47.Leng F (2012) Progression and prospects of translational medicine in China. Science China Life Sciences 55 (: 1022–1025. [DOI] [PubMed]
- 48.Zhang B, Wang X, Li S (2013) An Integrative Platform of TCM Network Pharmacology and Its Application on a Herbal Formula, Qing-Luo-Yin. Evid Based Complement Alternat. doi: 10.1155/2013/456747. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
27 TCM formulae used for treating women's diseases.
(DOCX)
102 targets of SWT.
(DOCX)
Symbols of 24 genes enriched in TGF-beta signaling pathway.
(DOCX)
Herbs and ingredients which target 20 predicted targets of SWT.
(DOCX)
41 Drugs interacting with 20 predicted targets of SWT.
(DOCX)
513targets of the 27 formulae.
(DOCX)