Abstract
An α-glucosidase inhibitor was developed from Aspergillus oryzae N159-1, which was screened from traditional fermented Korean foods. The intracellular concentration of the inhibitor reached its highest level when the fungus was cultured in tryptic soy broth medium at 27℃ for five days. The inhibitor was purified using a series of purification steps involving ultrafiltration, Sephadex G-25 gel permeation chromatography, strong cation exchange solid phase extraction, reverse-phase high performance liquid chromatography, and size exclusion chromatography. The final yield of the purification was 1.9%. Results of the liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis indicated that the purified α-glucosidase inhibitor was a tri-peptide, Pro-Phe-Pro, with the molecular weight of 360.1 Da. The IC50 value of the peptide against α-glucosidase activity was 3.1 mg/mL. Using Lineweaver-Burk plot analysis, the inhibition pattern indicated that the inhibitor acts as a mixed type inhibitor.
Keywords: α-Glucosidase inhibitory peptide, Anti-obesity, Aspergillus oryzae N159-1
Recently, the social and economic cost incurred by the increase in the obese population has risen annually. Annually, approximately 40% of deaths by circulating system disease, including atherosclerosis, cerebro-, cardiovascular complications, hypertension, diabetes, and functional depression of certain organs were caused by obesity [1]. The causes of obesity are complex, with multiple factors, such as interaction among genetic and biological factors (age, gender, ethnicity, hormonal), environmental (diet, exercise, social factors, chemicals, etc.) and behavioral factors acting through the physical activation of energy intake and expenditure [2, 3]. In addition to reducing fat intake for prevention of obesity, reduction of carbohydrate intake is very important. Excessive consumption of carbohydrate is a major factor in development of obesity in humans [4-6]. In general, obese persons tend to have addiction to consumption of carbohydrate rich-foods as meals or snacks [7].
Carbohydrates, which are the major compounds of our daily foods, are hydrolyzed into simple sugars, such as glucoses, and then absorbed through the intestine. Lipogenesis is the energy-storage process by which acetyl-CoA from the Embden-Meyerhof-Parnas pathway of absorbed simple sugars is converted to fat. Lipogenesis occurs as the process of fatty acid synthesis and subsequent triglyceride synthesis to form fats [8]. This process also influences accumulation of fatty acid in adipose tissue [9]. Therefore, carbohydrate is important in control of balance between fat intake and fat oxidation [10].
Some studies on therapeutic approaches to obesity as delay of glucose absorption by carbohydrates [11], and decrease of postprandial hyperglycan, which retards absorption of glucose by inhibition of carbohydrate hydrolyzing enzymes, such as α-amylase or α-glucosidase in digestive organs have been reported [12].
α-Glucosidase (EC3.2.1.20), an enzyme located in the epithelium of the small intestine, plays an important role in control of blood glucose level in the body and it is the key enzyme that catalyzes cleavage of disaccharides and oligosaccharides to glucose [13]. Many papers have reported on commercial α-glucosidase inhibitors such as acarbose [14], and voglibose [15], nojirimycin [16], and 1-deoxynojirimycin [17], and these are currently used in combination with diet or as an antidiabetic [18]. However, side effects of these compounds, such as headaches, insomnia, vomiting, flatulence, and diarrhea, have been reported [19]. Therefore, several studies have been conducted in an effort to search for effective α-glucosidase inhibitors without side effects and for development of a physiologically functional food or lead compound from plant and microorganisms, including Streptomyces sp. [20], Bacillus sp. [21], Nelumbo nucifera [22], Grateloupia elliptica [23], Ganoderma lucidum [24], and Pine bark [25]. However, study of α-glucosidase inhibitors from microorganisms has been limited. Therefore, development of a potential new high efficacy α-glucosidase inhibitor from microorganisms is necessary.
This study was conducted for screening of α-glucosidase inhibitor-producing fungi from traditional fermented Korean foods for development of a new anti-obesity drug candidate. Optimal conditions for production of α-glucosidase inhibitor from Aspergillus oryzae N159-1 were investigated. Subsequent purification and characterization of inhibitor were also performed in this study.
MATERIALS AND METHODS
Strains, enzymes, and chemicals
Thirty-four kinds of fungi from traditional fermented Korean foods were obtained from the Korea Food Research Institute, Korea.
α-Glucosidase from baker's yeast and p-nitrophenyl-α-D-glucopyranoside (pNPG) and pepsin, trypsin, pancreatin, α-amylase, maltase, trifluoroacetic acid (TFA), and ammonium formate were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Sephadex G-25 was purchased from Pharmacia Fine Chemicals (Uppsala, Sweden) and acetonitrile and water for high performance liquid chromatography (HPLC) were obtained from J. T. Baker (Phillipsburg, NJ, USA). Unless otherwise specified, all chemicals and solvents were of analytical grade.
Screening of fungi and preparation of cell extract
Fungi from several sources were cultured in potatodextrose broth at 28℃ for two days. The supernatants and cell pellets were separated by filtration for the fungi culture broths using Whatman No. 41 filter papers. In order to select the extracellular α-glucosidase inhibitor-producing fungi, α-glucosidase inhibitory activities were determined using fungi supernatants. Cell extracts were obtained by filtration of crude extracts prepared by sonication using a Whatman No. 41 filter paper and subsequent centrifuge at 15,000 ×g (10 min, 4℃). For selection of the intracellular α-glucosidase inhibitor-producing fungi, α-glucosidase inhibitory activities were determined using cell-free extracts.
Assay of α-glucosidase inhibitory activity
α-Glucosidase inhibitory activity was assayed according to the modified chromogenic method reported by Kim et al. [25], using α-glucosidase from baker's yeast. For simulation of intestinal fluid, a substrate solution of pNPG was prepared in a 0.1M potassium phosphate buffer and adjusted to pH 6.8. A 50 µL solution (0.2 units/mL, dissolved in potassium phosphate buffer, pH 6.8) of α-glucosidase was preincubated at 37℃ for 5 min with 50 µL of the respective test solution (dissolved in potassium phosphate buffer, pH 6.8, solutions of samples at 20 mg/mL concentrations). In the blank solution, the sample solution was replaced with potassium phosphate buffer. The enzymatic reaction was initiated by addition of 30 µL of pNPG (0.2 mM), and the mixture was incubated for another 20 min at 37℃. The reaction was terminated by addition of 100 µL of sodium carbonate solution (0.1M, pH 9.8).
Inhibition of α-glucosidase was determined by measuring the optical density (OD) of the p-nitrophenol released from pNPG at 405nm using an ELISA reader. The α-glucosidase inhibitory activity was calculated using the following formula:
| Inhibition ratio (%) = 100 × (OD(sample) - OD(blank))/(OD(control) - OD(blank)) |
Purification and characterization of the α-glucosidase inhibitor
Cell-free extract of Aspergillus oryzae N159-1 was fractionated stepwise with n-hexane, chloroform, ethyl acetate, butanol, and water and their α-glucosidase inhibitor activities were determined for each fraction. After ultrafiltration of active solvent fractions using the Centriprep YM-50, 30, 3 (Millipore Co., Billerica, MA, USA), we obtained 3 kDa below filtrates as active filtrate.
In order to increase α-glucosidase inhibitory activity, the active filtrate of 3 kDa below was digested with 1% pepsin, trypsin, pancreatin, α-amylase, and maltase at their optimal reaction pH and temperature. Then, the reactions were inactivated by heating in boiling water at 100℃ for 10 min. After removal of precipitate by centrifuge at 10,000 rpm, for 25min at 4℃, the supernatant was filtered and lyophilized. α-Glucosidase inhibitory activity of the re-solubilized solution was determined. The active hydrolyzed fraction was concentrated by lyophilization and then applied to a Sephadex G-25 column (3.0 × 35 cm) equilibrated with distilled water and eluted with distilled water at a flow rate of 1.3mL/min. The active fraction was lyophilized and was applied to a strong cation exchange (SCX) solid-phase extraction column (Hypersep SCX; Thermo Scientific Co., Milford, MA, USA), equilibrated with 10 mM ammonium formate, and eluted with ammonium formate gradients, increasing from 5 mM to 200 mM. The active fraction obtained was then applied to a reverse phase-high performance liquid chromatography (RP-HPLC) (Vydac 218TP54, C18 column, 5 µm, 4.6 × 250 mm; Discovery Science Co., Deerfield, IL, USA) equilibrated column with acetonitrile and its absorbance was monitored at 280. A linear gradient was applied with 0.1% TFA in water from 0% to 50% acetonitrile (v/v) with a flow rate of 0.8 mL/min. The active fractions were collected and lyophilized immediately. The active fraction was subjected to size exclusion chromatography with water under isocratic conditions with a flow rate of 1.0 mL/min and the purified α-glucosidase inhibitor was then obtained.
Molecular weight and amino acid sequence determination by mass spectrometry
The purified angiotensin-converting enzyme inhibitor was solubilized in disaster water and eluted on a ZORBAX 300SB-C18 column (1 × 150 mm, 3.5 µm; Agilent, Santa Clara, CA, USA) at a flow rate of 35 µL/min. Subsequently, the peptides were eluted from the column by application of a gradient 0~95% acetonitrile for 45 min at the same flow rate. All mass spectrometry (MS) and tandem mass spectrometry (MS/MS) spectra in the Hybrid Quadrupole-TOF LC-MS/MS Mass Spectrometer (CA 94404; AB Sciex Instruments, Foster City, CA, USA) were obtained in ESI+ MS/MS. For peptide identification, the MS/MS spectra were also performed using a De-novo sequencing program.
Determination of inhibition pattern on α-glucosidase
α-Glucosidase inhibitor was added to each reaction mixture according to the procedure reported by Bush et al. [26] with some modifications. Enzyme activity was measured using different concentrations of the substrate. The kinetics of α-glucosidase in the presence of the inhibitor was determined by Lineweaver-Burk plots.
Statistical analysis
Each experiment was performed at least three times, and all quantitative data were expressed as mean ± SD values.
RESULTS AND DISCUSSION
Screening of the α-glucosidase inhibitor-producing fungi
Thirty four kinds of fungi isolated from traditional fermented Korean foods were cultured at 30℃ for two days and their α-glucosidase inhibitory activities were then determined (Table 1). Extracellular α-glucosidase inhibitory activities were very weak or even not detected for all 34 different kinds of fungi. α-Glucosidase inhibitory activities were detected when we used cell-free extracts for determination of intracellular inhibitory activity. In particular, cell-free extract prepared from Aspergillus oryzae N159-1 showed the highest α-glucosidase inhibitory activity of 48.3%. Therefore, Aspergillus oryzae N159-1 was selected for further study of intracellular α-glucosidase inhibitor produced from fungi. The filamentous fungus, especially Aspergillus oryzae, has been used in production of traditional fermented food, such as sake, makgeolli (rice wine), miso, meju (soybean paste), and shoyu (soy sauce) [27-29]. Aspergillus oryzae also produces a variety of enzymes, such as amylases and proteases, and acts on the nutrients of soy to break down carbohydrate and protein, forming koji [30]. A. oryzae is listed as a GRAS (i.e., generally regarded as safe) strain by the U.S. Food and Drug Administration.
Table 1.
α-Glucosidase inhibitory activities of various fungi from fermented Korean foods

n.d, not detected.
To date, production of intracellular α-glucosidase inhibitor by Aspergillus oryzae N159-1 has not been reported. Therefore, α-glucosidase inhibitor purified from A. oryzae in this study may be a useful candidate for application to prevention of obesity.
Optimal conditions for production of α-glucosidase inhibitor
Culture conditions for production of intracellular α-glucosidase inhibitor from Aspergillus oryzae N159-1 were investigated. Optimal medium for maximal production of α-glucosidase inhibitor was obtained in tryptic soy broth medium and its α-glucosidase inhibitory activity was 65.9% (IC50, 10.1mg/mL). Intracellular α-glucosidase inhibitory activity of various cultures from yeast extract-malt extract, yeast-peptone-dextrose, and potato-dextrose medium was also high, at 52.1%, 49.1%, and 48.1%, respectively. However, LB and MRS media were not effective for production of α-glucosidase inhibitor from Aspergillus oryzae N159-1 (data not shown). The maximal cell growth was reached at seven days of cultivation, whereas maximal production of the α-glucosidase inhibitor was obtained at five days of cultivation (Fig. 1).
Fig. 1.
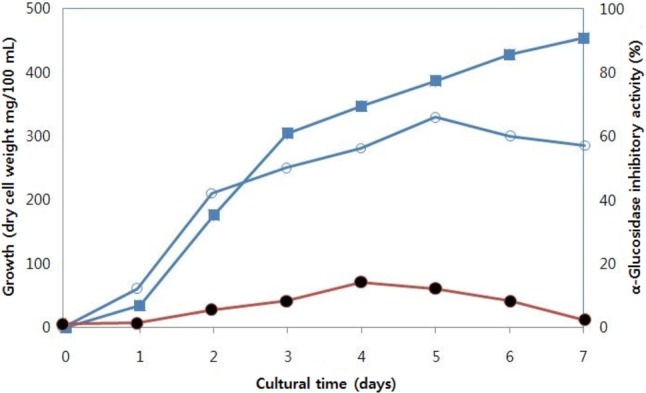
Effect of cultural periods on production of α-glucosidase inhibitor from Aspergillus oryzae N159-1. ▪, cell weight; ○, α-glucosidase inhibitory activities of cell-free extracts; •, α-glucosidase inhibitory activities of culture supernatants.
Purification of the intracellular α-glucosidase inhibitor from Aspergillus oryzae N159-1
To elucidate characteristics of the α-glucosidase inhibitor of Aspergillus oryzae N159-1, the active intracellular α-glucosidase inhibitor from systematic water extracts was purified as described in the Material and Methods section.
The α-glucosidase inhibitory activities of the filtrates ranging from 50 kDa to 3 kDa size cut-off by ultrafiltration were determined. The 3 kDa-below filtrates showed the highest α-glucosidase inhibitory activity of 69.9% (IC50, 7.7mg/mL). The 3 kDa-below filtrates were treated with various enzymes, such as pepsin, under optimal reaction conditions and their α-glucosidase inhibitory activity was determined. Treatment with pepsin resulted in increased α-glucosidase inhibitory activity, suggesting that the α-glucosidase inhibitor of Aspergillus oryzae N159-1 is a peptide compound. After Sephadex G-25 column chromatography of the active pepsin hydrolysates, an active fraction (F-2) with α-glucosidase inhibitory activity of IC50 4.7 mg/mL was obtained. The active fraction (F-2) was then applied to SCX solid-phase extraction chromatography and eluted by 10~200 mM ammonium formate gradient. The active fraction (F-2-3) eluted from the 50 mM ammonium formate concentration was recovered with inhibitory activity of 4.0mg/mL of IC50. When the active fraction was applied to RP-HPLC using a Vydac protein/peptide reverse-phase 218T P54 column, peaks were separated into two active fractions, F-2-3-1 and F-2-3-2. The F-2-3-2 fraction showed higher α-glucosidase inhibitory activity (IC50, 3.2mg/mL) than the F-2-3-1 fraction. The active fraction (F-2-3-2) was subjected to size-exclusion chromatography. Finally, one peak was eluted showing α-glucosidase inhibitory activity of 3.1mg/mL of IC50 obtained with 1.9% of solid yield (Table 2, Fig. 2).
Table 2.
Summary of the purification of α-glucosidase inhibitor from Aspergillus oryzae N159-1
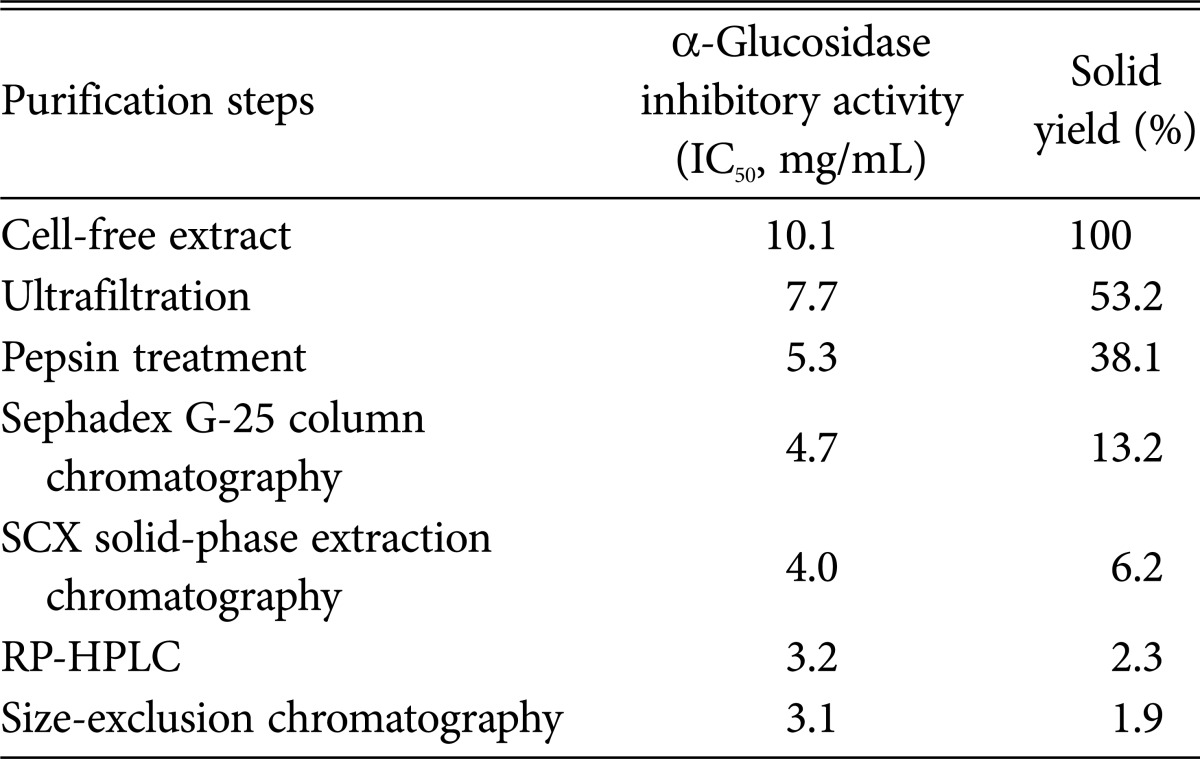
SCX, strong cation exchange; RP-HPLC, reverse phase-high performance liquid chromatography.
Fig. 2.
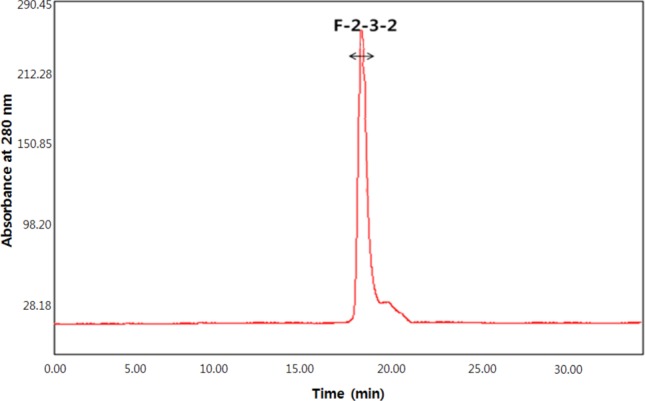
Size exclusion chromatogram of the active fraction from reverse phase-high performance liquid chromatography.
Several α-glucosidase inhibitors from plants and microbes, such as Grateloupia elliptica [23], Nelumbo nucifera [22], Pine bark [25] and Streptomyces sp. [20], Bacillus sp. [21], and Ganoderma lucidum [24] have been reported. However, in this study, the purified α-glucosidase inhibitor showed superior inhibitory activity, compared with these plants or microbes.
Molecular weight and amino acid sequence of the purified α-glucosidase inhibitor
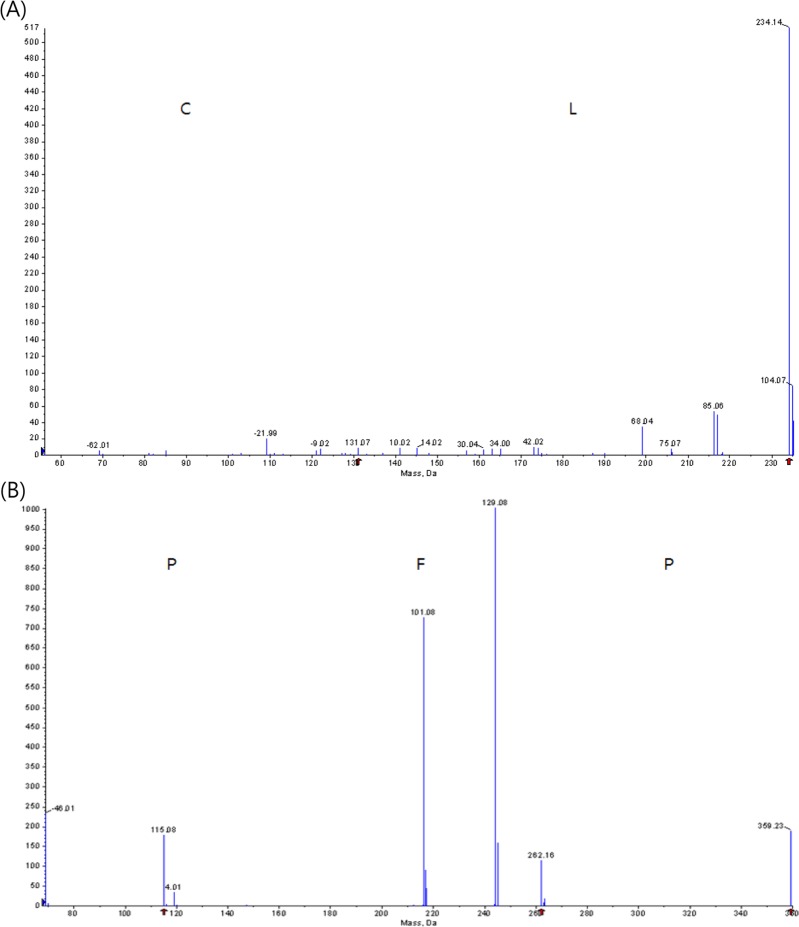
Analysis of the purified α-glucosidase inhibitor was performed using a Hybrid Quadrupole-TOF LC-MS/MS Mass Spectrophotometer and two types of peptides, which had sequences with Cys-Leu and Pro-Phe-Pro and nine sugars were obtained (Fig. 3). Among them, two peptides, P-1 (Cys-Leu) and P-2 (Pro-Phe-Pro), were chemically synthesized and their inhibitory activities were determined. The P-2 peptide showed superior inhibitory activity of IC50 value of 3.1 mg/mL to that of P-1 peptide (IC50, 12.1 mg/mL). The molecular weight of the purified α-glucosidase inhibitor, P-2 peptide, was estimated as 360.1 Da (Fig. 3).
Fig. 3.
Molecular mass and amino acid sequences of the purified α-glucosidase inhibitor peptides using liquid chromatography-tandem mass spectrometry (LC-MS/MS). A, Cys-Leu; B, Pro-Phe-Pro.
Inhibition pattern on chemical substrate, pNPG
The inhibitory pattern of the purified α-glucosidase inhibitor against chemical substrate, pNPG, was investigated according to Bush et al. [26] with some modifications and determined using a Lineweaver-Burk plot (Fig. 4). The purified α-glucosidase inhibitor showed a mixed inhibitory pattern to pNPG. The same result was reported by Kim et al. [23] against 2,4,6-tribromophenol and 2,4-dibromophenol.
Fig. 4.
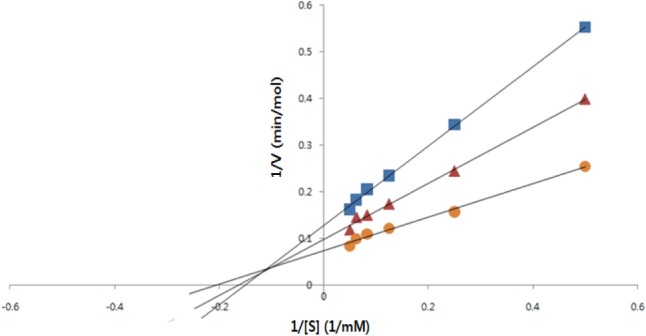
Lineweaver-Burk plot of α-glucosidase inhibition of purified α-glucosidase inhibitor from Aspergillus oryzae N159-1 at different concentrations of pNPG. ▪, 1.0 mg of inhibitor; ▴, 0.5 mg of inhibitor; •, control.
References
- 1.Shin MK, Han SH. Effects of lotus (Nelumbo nucifera Gaertner) leaf powder on lipid concentrations in rats fed high fat diet rats. Korean J Food Cult. 2006;21:202–208. [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 3.Carr KD, Park TH, Zhang Y, Stone EA. Neuroanatomical patterns of Fos-like immunoreactivity induced by naltrexone in food-restricted and ad libitum fed rats. Brain Res. 1998;779:26–32. doi: 10.1016/s0006-8993(97)01074-3. [DOI] [PubMed] [Google Scholar]
- 4.Wurtman JJ, Wurtman RJ. D-fenfluramine selectively decreases carbohydrate but not protein intake in obese subjects. Int J Obes. 1984;8(Suppl 1):79–84. [PubMed] [Google Scholar]
- 5.Heraief E, Burckhardt P, Wurtman JJ, Wurtman RJ. Tryptophan administration may enhance weight loss by some moderately obese patients on a protein-sparing modified fast (PSMF) diet. Int J Eat Disord. 1985;4:281–292. [Google Scholar]
- 6.Trichopoulou A, Gnardellis C, Benetou V, Lagiou P, Bamia C, Trichopoulos D. Lipid, protein and carbohydrate intake in relation to body mass index. Eur J Clin Nutr. 2002;56:37–43. doi: 10.1038/sj.ejcn.1601286. [DOI] [PubMed] [Google Scholar]
- 7.Wurtman JJ, Wurtman RJ, Growdon JH, Henry P, Lipscomb A, Zeisel SH. Carbohydrate craving in obese people: suppression by treatments affecting serotoninergic transmission. Int J Eat Disord. 1981;1:2–15. [Google Scholar]
- 8.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2:282–286. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doucet E, Tremblay A. Food intake, energy balance and body weight control. Eur J Clin Nutr. 1997;51:846–855. doi: 10.1038/sj.ejcn.1600497. [DOI] [PubMed] [Google Scholar]
- 10.Flatt JP. McCollum Award Lecture, 1995: diet, lifestyle, and weight maintenance. Am J Clin Nutr. 1995;62:820–836. doi: 10.1093/ajcn/62.4.820. [DOI] [PubMed] [Google Scholar]
- 11.Miura M, Gomyo T. Influences of commercial spices on α-amylase, α-glucosidase. J Jpn Soc Food Technol. 1996;43:157–163. [Google Scholar]
- 12.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UK Prospective Diabetes Study 44) Diabetes Care. 1999;22:960–964. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 13.Fatmawati S, Shimizu K, Kondo R. Ganoderol B: a potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053–1055. doi: 10.1016/j.phymed.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Wehmeier UF, Piepersberg W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Appl Microbiol Biotechnol. 2004;63:613–625. doi: 10.1007/s00253-003-1477-2. [DOI] [PubMed] [Google Scholar]
- 15.Murai A, lwamura K, Takada M, Ogawa K, Usui T, Okumura J. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 2002;71:1405–1415. doi: 10.1016/s0024-3205(02)01844-1. [DOI] [PubMed] [Google Scholar]
- 16.Reese ET, Parrish FW. Nojirimycin and d-glucono-1,5-lactone as inhibitors of carbohydrases. Carbohydr Res. 1971;18:381–388. [Google Scholar]
- 17.Asano N, Oseki K, Tomioka E, Kizu H, Matsui K. N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr Res. 1994;259:243–255. doi: 10.1016/0008-6215(94)84060-1. [DOI] [PubMed] [Google Scholar]
- 18.Leonhardt M, Hrupka B, Langhans W. New approaches in the pharmacological treatment of obesity. Eur J Nutr. 1999;38:1–13. doi: 10.1007/s003940050040. [DOI] [PubMed] [Google Scholar]
- 19.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 20.Do JH, Joo HK. α-D-Glucosidase inhibitor from Streptomyces sp. (I) Identification of the strain. Korean J Appl Microbiol Bioeng. 1989;17:202–206. [Google Scholar]
- 21.Kim HS, Lee JY, Hwang KY, Cho YS, Park YS, Kang KD, Seong SI. Isolation and Identification of Bacillus sp. producing α-glucosidase Inhibitor 1-deoxynojirimycin. Korean J Microbiol Biotechnol. 2011;39:49–55. [Google Scholar]
- 22.Ono Y, Hattori E, Fukaya Y, Imai S, Ohizumi Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharmacol. 2006;106:238–244. doi: 10.1016/j.jep.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Kim KY, Nam KA, Kurihara H, Kim SM. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69:2820–2825. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Kim SD, Nho HJ. Isolation and characterization of α-glucosidase inhibitor from the fungus Ganoderma lucidum. J Microbiol. 2004;42:223–227. [PubMed] [Google Scholar]
- 25.Kim YM, Wang MH, Rhee HI. A novel α-glucosidase inhibitor from pine bark. Carbohydr Res. 2004;339:715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Bush K, Herny PR, Slusarchyk DS. Muraceins: muramyl peptides produced by Norcardia orientalis as angiotensin-converting enzyme inhibitor. I. Taxonomy, fermentation and biological properties. J Antibiot (Tokyo) 1984;37:330–335. doi: 10.7164/antibiotics.37.330. [DOI] [PubMed] [Google Scholar]
- 27.Bennett JW. Aspergillus and koji: history, practice and molecular biology. SIM News. 2001;51:65–71. [Google Scholar]
- 28.Yang HJ, Kwon DY, Kim MJ, Kang S, Park S. Meju, unsalted soybeans fermented with Bacillus subtillis and Aspergilus oryzae, potentiates insulinotropic actions and improves hepatic insulin sensitivity in diabetic rats. Nutr Metab (Lond) 2012;9:37. doi: 10.1186/1743-7075-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang IT, Kang MG, Yi SH, Lim SI, Kim HR, Ahn BH, Lee JS. Physiological functionality of nuruk, makgeolli and cheonggukjang made with fungi and bacteria isolated from Korea traditional fermented foods. Korean J Mycol. 2012;40:164–173. [Google Scholar]
- 30.Marui J, Matsushita-Morita M, Tada S, Hattori R, Suzuki S, Amano H, Ishida H, Yamagata Y, Takeuchi M, Kusomoto K. Enzymatic properties of the glycine D-alanine aminopeptidase of Aspergillus oryzae and its activity profiles in liquid-cultured mycelia and solid-state rice culture (rice koji) Appl Microbiol Biotechnol. 2012;93:655–659. doi: 10.1007/s00253-011-3610-y. [DOI] [PubMed] [Google Scholar]