Abstract
A Bacillus sp. BS061 significantly reduced disease incidence of gray mold and powdery mildew. To identify the active principle, the culture filtrate was partitioned between butanol and water. The antifungal activity against B. cinerea was evident in the butanol-soluble portion, and active substances were identified as cyclic lipopeptides, iturin A series, by nuclear magnetic resonance spectrometry (NMR) and mass analysis. Interestingly, antifungal activity against powdery mildew was observed in the water-soluble portion, suggesting that cyclic lipopeptides have no responsibility to suppress powdery mildew. This finding reveals that biocontrol agents of Bacillus origin suppress gray mold and powdery mildew through the secretion of different bioactive substances.
Keywords: Bacillus sp. BS061, Biocontrol agent, Gray mold, Lipopeptides, Powdery mildew
Gray mold and powdery mildew diseases are very common plant diseases throughout various geographical regions, and occur under a variety of growing conditions. Gray mold disease, caused by Botrytis cinerea Pers.: Fr., is one of the most serious plant diseases affecting a vegetables, ornamentals and fruit crops produced in commercial greenhouse and fields all over the world [1]. Control of B. cinerea is normally carried out by the application of fungicides. However, the growing demand of consumers worldwide for a reduction in the use of fungicides, as well as the appearance of pathogens resistant to chemical compounds has emphasized the need to find alternative methods for gray mold disease control [2]. Powdery mildew is one of the most serious plant diseases of all, worldwide, causing large yield losses in a number of crops [3]. The conventional chemical control of powdery mildew is through repeated foliar applications of combination of protectant and systemic fungicides. But the intensive use of fungicides worldwide has resulted in an increased frequency of powdery mildew pathogens with reduced sensitivity to chemical fungicides.
Biological control, as the use of a microorganism or its secretion to prevent disease, offers an attractive alternative or supplement to fungicides for the management of plant disease, without any of the negative effects of chemical control [4, 5]. However, relatively few of these antagonistic microbes have been commercialized as biocontrol agents, due to problems such as inconsistent performance in the field, lack of broad-spectrum disease suppression activity, or slower or less complete suppression when compared to chemical alternatives [6-8].
In our screening program for microorganisms with the potential to be used as microbial fungicides for the simultaneous control of gray mold and powdery mildew, an effective bacterial strain Bacillus sp. BS061 was isolated from the leaf of a plant. In a previous study, we reported the cultivation, biological control effect, and antifungal activity of Bacillus sp. BS061 [9]. In this study, the isolation and chemical structures of antifungal substances produced by Bacillus sp. BS061 are described.
A loopful of Bacillus sp. BS061, which was maintained on Mueller hinton (MH) agar plate, was transferred into a 500 mL Erlenmeyer flasks containing 200 mL MH broth. The flasks were incubated on a rotary shaker at 200 rpm/min for 12 hr at 28℃. One milliliter of this fresh culture was inoculated into 500 mL Erlenmeyer flasks containing the same medium as above. The flasks were incubated under the same conditions as described earlier for 96 hr.
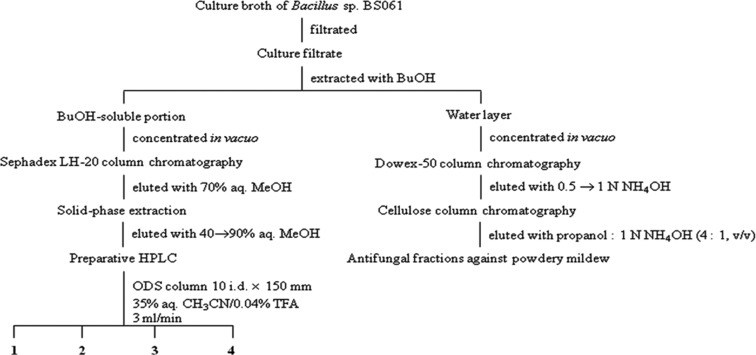
Antifungal substances were isolated and purified from the culture filtrate of Bacillus sp. BS061 by antifungal activity-guided fractionation against B. cinerea (agar diffusion method) and powdery mildew (leaf disk bioassay method), as shown in Fig. 1. The culture filtrate was partitioned twice with equal volume of butanol. The antifungal activity against B. cinerea was evident in the butanol-soluble portion, and antifungal activity against powdery mildew was observed in the water-soluble portion. The antifungal substances against B. cinerea were purified from the butanol-soluble portion by a Sephadex LH-20 (Phamarcia, Uppsala, Sweden) column chromatography eluted with 70% aqueous methanol, followed by ODS Sep-pak cartridge (Alltech, Deerfield, IL, USA) chromatography eluted with a gradient of increasing methanol concentration (40% to 95%) in water. The active fractions were monitored by the combination of antifungal activity and thin-layer chromatographic analysis using chloroform : methanol : water (65 : 25 : 4, v/v). Active substances could be detected by spraying with water. The active fraction (80% aqueous methanol eluate) was finally purified by reversed-phase high performace liquid chromatography with C18 (RP-18; Cosmosil, Kyoto, Japan) column using 35% acetonitrile containing 0.04% trifluoroacetic acid to afford four antifungal compounds 1~4 (Fig. 2).
Fig. 1.
Purification flow chart of antifungal compounds from culture filtrate of Bacillus sp. BS061.
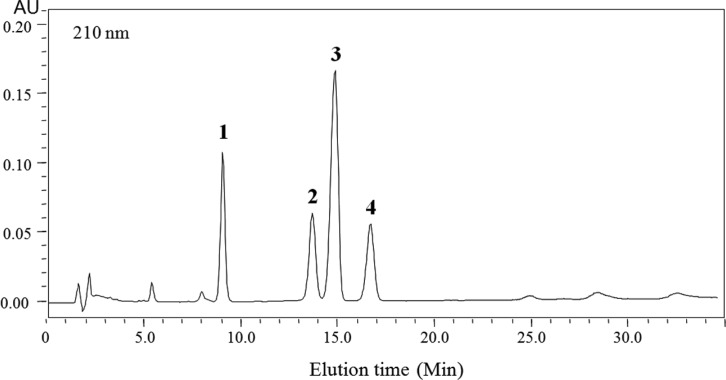
Fig. 2.
High performace liquid chromatography chromatogram of compounds 1~4 produced by Bacillus sp. BS061. Column, Microsorb C18, i.d. 2.4 × 250 mm; mobile phase, 35% aq. acetonitrile/0.04% TFA; flow rate, 1mL/min; detection, 210 nm.
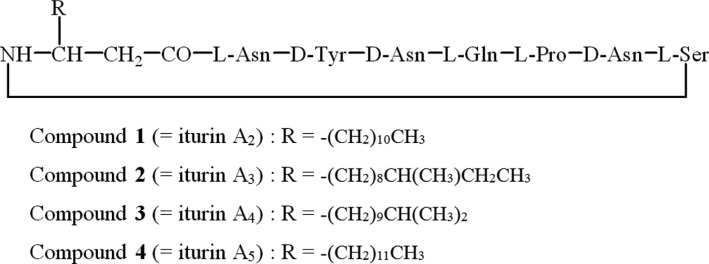
Chemical structures of compounds 1~4, showing antifungal activity against B. cinerea were determined by spectroscopic methods, as shown in Fig. 3. The electrospray ionization (ESI) mass measurement of compound 1 exhibited the pseudo-molecular ion peaks at m/z 1,065.9 [M+Na]+ in positive mode and at m/z 1,042.1 [M-H]- in negative mode, suggesting that its molecular weight was 1,043. In addition to mass analysis, 1H NMR spectrum measured in CD3OD suggested that this compound had lipopeptidic character. In 1H NMR spectrum, 1,4-disubstituted benzene protons at δ 7.05 (2H, d, J = 8.8 Hz) and 6.71 (2H, d, J = 8.8 Hz), α-protons of amino acids between δ 4.7~3.6, methylenes derived from an alkyl chain of lipid at δ 1.3~1.2, and methyl protons at δ 0.86 (3H, t, J = 6.8Hz) were observed. A literature survey based on the above results suggested that compound 1 was a member of the iturin series. Its molecular weight and NMR spectra were in good agreement with that of iturin A2, revealing that compound 1 was identical to iturin A2 [10].
Fig. 3.
Chemical structures of antifungal substances produced by Bacillus sp. BS061.
The molecular weight of compound 2 was determined to be 1,057 by the ESI mass measurement, which exhibited the molecular ion peaks at m/z 1,079.9 [M+Na]+ in positive mode and at m/z 1,056.0 [M-H]- in negative mode. In the 1H NMR spectrum measured in CD3OD, 1,4-disubstituted benzene protons at δ 7.04 and 6.70, α-protons of amino acids between δ 4.8~3.7, methylene protons derived from an alkyl chain at δ 1.4~1.2, and two methyl protons at δ 0.86, were observed. The 1H NMR spectrum was very similar to that of compound 1, except for the presence of two methyl groups. These results revealed that compound 2 was identical to iturin A3.
The ESI mass measurements of compounds 3 and 4 exhibited the same molecular ion peaks at m/z 1079.9 [M+Na]+ in positive mode, and at m/z 1056.0 [M-H]- in negative mode, suggesting the molecular weight of 1,057, which was also same as that of compound 2. Their 1H NMR spectra were almost same as that of compounds 1 and 2 except for the presence of methyl groups. Compound 3 showed two overlapping methyl doublets and compound 4 exhibited one methyl triplet. These results established compounds 3 and 4 as iturin A4 and iturin A5, respectively.
The water-soluble fraction showing antifungal activity against powdery mildew was percolated through a column of Dowex 50WX4 (Sigma-Aldrich, St. Louis, MO, USA), previously conditioned with 1 N HCl and then rinsed with water. After washing of the column with water, antifungal substances were eluted with 0.5 N and 1.0 N NaOH, and were then concentrated. Active fractions against powdery mildew were subjected to a cellulose column chromatography eluted with propanol-1.0 N ammonium hydroxide aqueous solution (4 : 1, v/v) to provide active fractions. The active fractions showed positive reaction to ninhydrin spray reagent revealing a pink color. Antifungal substances against powdery mildew in the water phase were very polar compounds and soluble in water, but their chemical structures still remain to be investigated.
Although we are not able to determine the chemical structures of antifungal substances against powdery mildew, this finding suggested that the antifungal substance responsible for reducing the disease incidence of powdery mildew is not a cyclic lipopeptide such as an iturin or fengycin. To the best of our knowledge, antifungal substances of Bacillus strains against powdery mildew and gray mold have mostly been identified as cyclic lipopeptides, mainly iturins and fengycins. So, we confirmed the polarity of the active compound using the commercialized biocontrol agents by solvent partitioning with butanol. As a result, they also exhibited the antifungal activity in the butanol-soluble portion against gray mold and in the aqueous phase against powdery mildew (data not shown). This finding revealed that at least cyclic lipopeptides have no responsibility to suppress powdery mildew. Antifungal substance of Bacillus strains against powdery mildew still remains to be investigated.
ACKNOWLEDGEMENTS
This work was supported by the Technology Development Program for Bio-industry, Ministry for Food, Agriculture, Forestry and Fisheries and by a grant (PJ008976062013) from the Agenda project of the Rural Development Administration (RDA), Republic of Korea.
References
- 1.Lee JP, Lee SW, Kim CS, Son JH, Song JH, Lee KY, Kim HJ, Jung SJ, Moon BJ. Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea. Biol Control. 2006;37:329–337. [Google Scholar]
- 2.Zhang H, Wang L, Dong Y, Jiang S, Cao J, Meng R. Postharvest biological control of gray mold decay of strawberry with Rhodotorula glutinis. Biol Control. 2007;40:287–292. [Google Scholar]
- 3.Kiss L. A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Manag Sci. 2003;59:475–483. doi: 10.1002/ps.689. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen BJ, Zidack NK, Larson BJ. The role of Bacillus-based biological control agents in integrated pest management systems: plant diseases. Phytopathology. 2004;94:1272–1275. doi: 10.1094/PHYTO.2004.94.11.1272. [DOI] [PubMed] [Google Scholar]
- 5.Raupach GS, Kloepper JW. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology. 1998;88:1158–1164. doi: 10.1094/PHYTO.1998.88.11.1158. [DOI] [PubMed] [Google Scholar]
- 6.Larkin RP, Roberts DP, Gracia-Garza JA. Biological control of fungal diseases. In: Hutson D, Miyamoto J, editors. Fungicidal activity: chemical and biological approaches to plant protection. New York: Wiley; 1998. pp. 141–191. [Google Scholar]
- 7.Meyer SL, Roberts DP. Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J Nematol. 2002;34:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts DP, Lohrke SM, Meyer SL, Buyer JS, Bowers JH, Baker CJ, Li W, de Souza JT, Lewis JA, Chung S. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Prot. 2005;24:141–155. [Google Scholar]
- 9.Kim YS, Song JG, Lee IK, Yeo WH, Yun BS. Bacillus sp. BS061 suppresses powdery mildew and gray mold. Mycobiology. 2013;41:108–111. doi: 10.5941/MYCO.2013.41.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao YK, Lin HY, Wu WS, Tzeng YM. Evaluation of HPLC and MEKC methods for the analysis of lipopeptide antibiotic iturin A produced by Bacillus amyloliquefaciens. Int J Appl Sci Eng. 2008;6:85–96. [Google Scholar]