Abstract
Cellulosic biomass is available for the production of biofuel, with saccharification of the cell wall being a key process. We investigated whether alteration of arabinoxylan, a major hemicellulose in monocots, causes an increase in saccharification efficiency. Arabinoxylans have β-1,4-D-xylopyranosyl backbones and 1,3- or 1,4-α-l-arabinofuranosyl residues linked to O-2 and/or O-3 of xylopyranosyl residues as side chains. Arabinose side chains interrupt the hydrogen bond between arabinoxylan and cellulose and carry an ester-linked feruloyl substituent. Arabinose side chains are the base point for diferuloyl cross-links and lignification. We analyzed rice plants overexpressing arabinofuranosidase (ARAF) to study the role of arabinose residues in the cell wall and their effects on saccharification. Arabinose content in the cell wall of transgenic rice plants overexpressing individual ARAF full-length cDNA (OsARAF1-FOX and OsARAF3-FOX) decreased 25% and 20% compared to the control and the amount of glucose increased by 28.2% and 34.2%, respectively. We studied modifications of cell wall polysaccharides at the cellular level by comparing histochemical cellulose staining patterns and immunolocalization patterns using antibodies raised against α-(1,5)-linked l-Ara (LM6) and β-(1,4)-linked d-Xyl (LM10 and LM11) residues. However, they showed no visible phenotype. Our results suggest that the balance between arabinoxylan and cellulose might maintain the cell wall network. Moreover, ARAF overexpression in rice effectively leads to an increase in cellulose accumulation and saccharification efficiency, which can be used to produce bioethanol.
Introduction
Biofuel production from lignocellulosic biomass has attracted much attention as a method to reduce the consumption of fossil fuels, but a practical problem for use is that biofuel made from cellulosic biomass is costy because plant cell walls are very complex structures and are difficult to degrade. Therefore, for saccharification, the degradation of cell wall polysaccharides is a key process required to solve this problem.
Primary cell walls mainly consist of three polysaccharides: cellulose, hemicelluloses, and pectin. These polysaccharides determine the cell shape and mechanical strength. Lignin is a phenylpropanoid polymer deposited in the cell wall during secondary wall thickening [1]. Research on cell walls is important not only to understand mechanisms of plant growth and development but also to produce better plant material for practical use. Cell wall matrix polysaccharides generally occupy 10–30% of the dry weight of plants, and hemicellulose is responsible for cross-links between cellulose fibers. The dicot genus and monocot genus except for Commelina have type I cell walls and the hemicelluloses of members are rich in xyloglucan [2], while gramineous monocots have type II cell walls and their hemicellulose is rich in β-1,3-1,4-glucan and arabinoxylan. Arabinoxylan occupies 20–50% of the dry mass of the cell wall in primary and secondary cell walls [3] [4]. Arabinoxylans have a β-1,4-xylopyranosyl backbone and 1,3- or 1,4- α-l-arabinofuranosyl residues bonded at O-2 and/or O-3 of xylopyranosyl residues as side chains [2] [4] [5]. The xylan backbone has a 1,4-pyranose structure that is the same as cellulose or β-1,4-glucan, and forms strong hydrogen bonds with the surface of cellulose microfibrils. The arabinose side chain reportedly interrupts this hydrogen bond between arabinoxylan and cellulose, and the ratio of the addition of arabinose side chains to xylan backbone changes the wall mechanical properties [6]. Arabinose side chains can carry an ester-linked feruloyl substituent and these feruloyl groups form diferuloyl cross-links between arabinoxylans [7] [8], and in secondary cell walls, feruloyl acid is bonded to lignin polymers [9]. Thus, the arabinose side chain is the base point for diferuloyl cross-links and lignification. Although arabinofuranosyl residues are a quantifiably important constituent of plant primary and secondary cell walls, studies on this arabinose as a diferuloyl cross-link base point are lacking.
Genetic modifications of the cell wall have been reported [10], and plants with decreased hemicellulose and cellulose are generally physically weak and poorly adapted to the natural environment. For example, the cell wall network containing arabinose has been studied in dicots, and the loss of arabinose was found to be critical for plant development [11]. The Arabidopsis double mutant rgp1rgp2 and transgenic UDP-arabinopyranose murase RNAi rice plants present lethal or dwarf phenotypes [12] [13]. In this paper, we focus on the functions of arabinose residues in arabinoxylan. We modified the arabinose content in rice using arabinofuranosidase (ARAF) overexpressor, Full-length cDNA overexpressor (FOX) lines [14] [15]. Using the endogenous enzyme may contribute to improved public acceptance of GM crops.
Beyond glycosyl composition analysis, we probed for wall modifications at the cellular level by comparing histochemical cellulose staining patterns and immunolocalization patterns using antibodies raised against α-(1,5)-linked l-Ara (LM6) and β-(1,4)-linked d-Xyl (LM10 and LM11) residues. We report the effect of a decrease in arabinose content by ARAF overexpression on maintenance of the cell wall network through arabinoxylan and cellulose and saccharification efficiency for production of bioethanol.
Materials and Methods
Plant material and growth conditions
Rice plants of the control (Oryza sativa cv. Nipponbare) and the two FOX lines AY311 and CO035, which carry overexpression constructs for OsARAF1 (RAP locus: 07g0686900) and OsARAF3 (Os11g0131900), respectively, were grown in soil in a greenhouse during the natural growing season. In this environment, the temperature was 20–30°C and the light value was around 1,000 µmol s–1 m–2. All plants retained normal fertility.
The presence of the constructs in genomic DNA of these lines was confirmed by PCR. All experiments were performed with the T3 generation. Transgenic lines were selected on hygromycin-containing agar plates and tested for heritability of the expression pattern and the altered sugar trait.
Phylogenetic analysis
BLAST searches were conducted with amino acid sequences of selected genes, namely Oryza sativa (members of GH family 51 and 3), A. thaliana (ARAF1, ARAF2, XLY1, and XLY3), and H. vulgare (AXHAI and AXAHII). A multiple alignment was generated by the neighbor-joining method in ClustalX [16] using full-length sequences and then manually adjusted. The phylogenetic tree was visualized using TreeView [17].
RNA extraction and RT-PCR
Plant material was frozen in liquid nitrogen and ground with a Tissue Lyser II (Qiagen, Hilden, Germany). Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and the DNase I recombinant (Roche, Basel, Switzerland) according to the manufacturers' protocols. cDNA was synthesized with ReverTra Ace® (Toyobo, Tokyo, Japan) according to the manufacturer's protocol. For the OsARAF1-FOX line, the quantity of transcripts was determined using primer sets of OsARAF1-forward (5′-CCAGAAGGGCAGTTATTG-3′) and OsARAF1-reverse (5′-CCAGAAGGGCAGTTATTG-3′). For the OsARAF3-FOX line, the quantity of transcripts was determined using primer sets of OsARAF3-forward (5′-GCTTCTCTTCCTTCCTTCCTTGT-3′) and OsARAF3-reverse (5′- CGATACTTGATCAAACCATACAACTCCCTT-3′). As an endogenous control, the quantity of 17S rRNA transcript were determined using primer sets of 17S rRNA-forward (5′-GCAAATTACCCAATCCTGAC-3′) and 17S rRNA-reverse (5′- CTATTGGAGCTGGAATTACC-3′). The amplified cDNA fragments were separated on 2% agarose gel and the bands stained with ethidium bromide.
Enzyme assay
Enzyme assay in each line was measured according to [18]. Mature leaves were frozen in liquid nitrogen and ground with a Tissue Lyser II (Qiagen, Hilden, Germany). The following operations were carried out at 0–4°C. Ground samples were suspended in 20 mM sodium acetate buffer (pH 5.0) containing 1 M sodium chloride for 2 h. After centrifugation at 10,000× g for 5 min, the supernatant was applied to a PD-10 column midi-Trap G-25 (GE Healthcare, Milwaukee, WI, USA) and the eluted fraction was used for the enzyme assay. The concentration of protein was determined by the method of Bradford, with bovine serum albumin as the standard [19]. Enzyme activities were determined using a reaction mixture (200 µl) consisting of protein fractions, 25 mM acetate buffer (pH 5.0), and 1 mM p-nitrophenol glycoside. After incubation at 37°C for 2 h, the reaction was terminated by the addition of 200 mM sodium carbonate (800 µl) and monitored at 420 nm. One unit of enzyme activity is defined as 1 µmol of p-nitrophenol liberated per minute at 37°C min–1.
Extraction and analysis of cell wall polysaccharides
Mature leaves of FOX lines were frozen in liquid nitrogen and ground with a Tissue Lyser II (Qiagen, Hilden, Germany) at 30 Hz for 2 min, and the resulted powder washed in 80%(v/v) EtOH. The supernatant was removed after centrifugation for 5 min at 15,000× g. The pellet was washed three times with water, three times with methanol:chloroform (MC = 1∶1v/v), and three times with acetone. A drop of phenol:acetic acid:water (PAW = 2∶1∶1v/v) was added to the pellet and mixed. Two drops of MC were added to the sample and washed with acetone. This process was repeated three times and the sample was then dried at room temperature for over 1 h. Starch was removed by digestion with amylase (2 unit/ml amylase; Wako, Osaka, Japan) in 50 mM acetate buffer at 37°C for 3 h. After reaction, the samples were centrifuged and the residues washed three times with water, MC, and acetone. After washing, the samples were air-dried for over 12 h. Alcohol-insoluble residues (AIRs) were used as the cell wall material. A total of 2 mg of AIR was hydrolyzed with 2 M trifluoroacetic acid (TFA) at 121°C for 2 h. After hydrolysis, the samples were centrifuged at 15,000× g for 5 min. The supernatant was the TFA-soluble fraction. The pellets were hydrolyzed with 72% H2SO4 at room temperature for 2 h and then diluted to 4% H2SO4 and boiled for 1 h. The H2SO4 solutions were neutralized with Ba(OH)2. Sugar in TFA-soluble and -insoluble fractions was treated with methanol:hydrogen chloride and the resulting methyl glycosides were converted into trimethylsilyl (TMS) derivatives and analyzed by gas-liquid chromatography (GC-14; SHIMADZU Kyoto, Japan). Sugar content in TFA-soluble and TFA-insoluble fractions was determined using the phenol sulfuric acid method.
Cellulose analysis
Crystalline cellulose was measured according to [20]. Briefly the samples were treated with acetic and nitric acids to remove non-cellulosic polysaccharides, and the remaining pellets were hydrolyzed with 72% sulfuric acid. Glucose content in sulfuric acid was determined by phenol sulfuric acid method.
Lignin measurement
Lignin contents in each line were measured according to [21]. Explaining briefly, mature leaves were frozen in liquid nitrogen and ground with a Tissue Lyser II (Qiagen, Hilden, Germany) at 30 Hz for 2 min. 3N HCl and 0.1 ml thioglycolic acid were added to 20 mg of AIR and heated at 80°C for 3 hours. After centrifugation, the pellet was dissolved in 1N NaOH. The solution was submitted to spectrophotomeric measurement.
pCA, FA and DFA measurement
Cell wall was treated with 1N NaOH for 12h, and acidified with HCl. pCA, FA and DFA liberated were extracted three time with diethyl ether. The extract was air-dried, then stored in the dark until determination. pCA, FA and DFA contents were determined using HPLC system (Shimadzu, Kyoto, Japan) with a column Luna C18(2) column, (150×4.60mm, Phenomenex, USA) and monitored for UV(262 nm) and fluorescence (330 and 435 nm). The column equilibrated with 5 mM ammonium acetate (pH 4.4) containing 25% methanol was eluted by a linear gradient of methanol (25–50%, 2–10 min) and 5 mM ammonium acetate (pH 4.4) containing 50% methanol (10–20 min) at flow rate of 1.0 ml/min.
Immunohistochemistry and cellulose staining
To perform immunolocalization, leaves of the control, OsARAF1-FOX, and OsARAF3-FOX lines at the same developmental stage were fixed with 4% paraformaldehyde, 0.25% glutaraldehyde, and 0.05 M phosphate buffer (pH 7.5) and embedded in 5% agar. Sections of 30 µm were cut with a microtome (VT1200S; Leica Microsystems, Nussloch, Germany). The TSATM Kit no. 12, with HRP-goat anti-rabbit IgG and Alexa Fluor 488 tyramide (Molecular Probes/Invitrogen, Eugene, OR, USA), was used according to the manufacturer's protocol. The primary antibodies LM6, LM10, and LM11 (PlantProbes, Leeds, UK) were used at a 1∶30 dilution. HRP conjugate working solution was used at a 1∶100 dilution. A negative control experiment was performed without the primary antibody. Cellulose was stained with 0.01% calcofluor white (Fluorescent Brightener 28; Sigma-Aldrich, St. Louis, MO, USA). All sections were observed using fluorescence microscopy (Leica Microsystems, Wetzlar, Germany), with UV filter A, excitation filter BP 340–380 nm, suppression filter LP 425, and an exposure time of 190 ms.
Measurement of mechanical properties
Mature leaves of 7–8 mm width of the control, OsARAF1-FOX, and OsARAF3-FOX were cut 12 cm from the leaf tip and immediately used to assess mechanical properties. Both sides of a sample were adhered to the stage, with an interval of 2 cm; the stage moved to load the middle of the sample on the sensor; and then the change of load (break force) and extension length per leaf width were monitored using EZ Graph (Shimadzu, Kyoto, Japan).
Measurement of saccharification efficiency
Mature leaves were ground with a Tissue Lyser II (Qiagen, Hilden, Germany) at 30 Hz for 2 min. An aliquot of 1.2 ml of 0.1 M sodium acetate buffer (pH 4.5) was added to 40 mg of the resulting powder and suspended. A sample of 60 µl was recovered and 40 µl sodium acetate buffer added at time 0. A total of 10 mg/ml Meiselase (Meiji, Tokyo, Japan) was added to the remaining sample and reacted at 45°C with shaking at 120 Hz. After 1–24 h, 100 µl suspensions were recovered followed by centrifugation at 15,000× g for 10 min at room temperature. Sugar content in the supernatant was determined by the phenol sulfuric acid method. The saccharification efficiency was calculated as sugar liberation (%) = µg/mg dry weight of leaves.
Results
Selection of ARAF genes from the FOX library
To select the ARAF genes of rice, we searched the Rice PIPELINE database (http://cdna01.dna.affrc.go.jp/PIPE/; [22]) and identified 16 putative ARAF genes with reference to the Carbohydrate-Active enzyme [CAZy] database (http://www.cazy.org; [23] [24]), showing that the ARAF genes are members of GH family 3 and 51. We generated a phylogenic tree using 16 rice ARAF genes, four Arabidopsis thaliana genes (AtARAF1: At3g10740, AtARAF2: At5g26120, XYL1: At5g49360, XYL3: At5g09730), and two Hordeum vulgare genes (AXHAI and AXAHII). Several enzymes of the GH family 3 and 51 have been reported to have bifunctional activity for ARAF/β-d-xylosidase. Arabidopsis XLY1 and XLY3 [25] [26] have activity to hydrolyze p-nitrophenyl-α-l-arabinofuranoside (PNP-ARAf), p-nitrophenyl-β-d-xylopyranoside (PNP-Xyl), oat spelt xylan, rye arabinoxylan, wheat arabinoxylan, and oligo-arabinoxylan [25]. ASD1 and ASD2 have only ARAF activity [27]. The barley AXAH-I and AXAH-II have ARAF activity to release arabinose from 1,5-α-l-arabinopentaose, sugar beet arabinan, wheat arabinoxylan, and larchwood (Larix) arabinogalactan, but do not release d-Xyl [28] [29]. The phylogenetic tree showed that enzymes belonging to GH family 3 have bifunctional activity for ARAF/β-d-xylosidase and those in GH family 51 seemed to only have ARAF activity (Figure 1A). Therefore, we selected genes from GH family 51 for further study.
Figure 1. Characteristics of Oryza sativa ARAF1 and ARAF3.
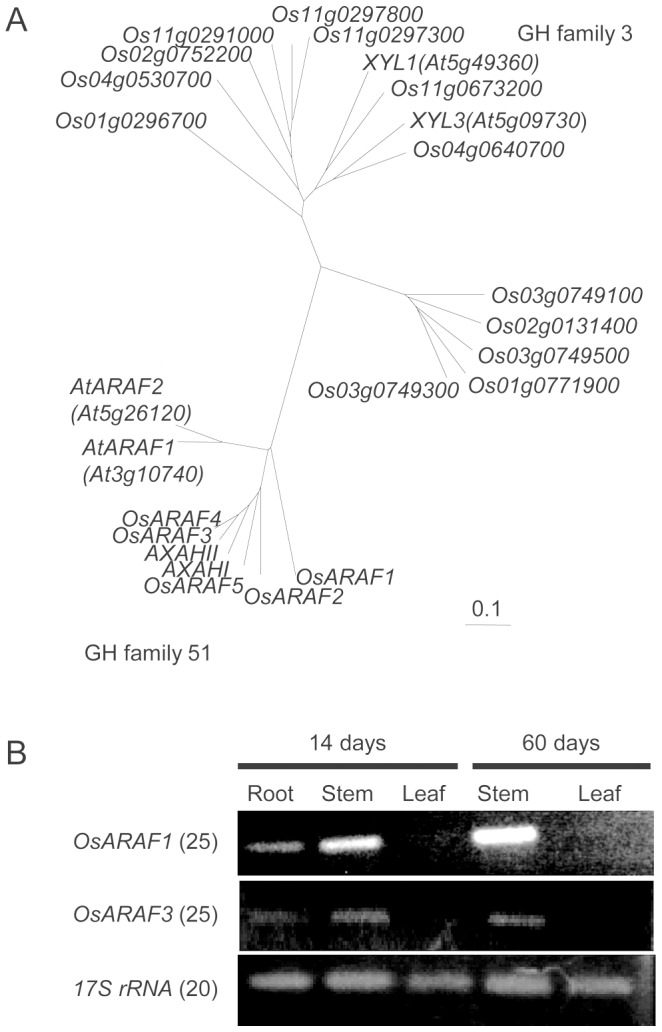
(A) Phylogenetic tree of putative ARAFs and xylosidases (members of the GH families 51 and 3) in Oryza sativa, Arabidopsis thaliana (AtARAF1: At3g10740, AtARAF2: At5g26120, XLY1: At5g49360, XYL3: At5g09730) and Hordeum vulgare (AXHAI and AXAHII). Phylogenetic trees were constructed by the neighbor-joining method in ClustalX. (B) The expression patterns of OsARAF1 and OsARAF3. RT-PCR analysis was performed using total RNA isolated from different organs of 14-day-old seedlings and 60-day-old mature plants. The numbers in parentheses indicate the numbers of PCR cycles. These experiments were performed at least twice with similar results.
For a systematic approach to analyze a gain-of-function phenotype, [14] [15] developed the FOX hunting system by using expression libraries for full-length cDNAs (fl-cDNAs) from rice at a maximum of 28,000 fl-cDNAs clones in total, and individually overexpressed the fl-cDNAs in rice driven by the maize Ubiquitin-1 gene promoter. Amoung ∼14,500 FOX-rice lines, we identified two lines overexpressing fl-cDNAs for OsARAF1 (Accession no. AK064838, Rap-ID:Os07g0686900), and OsARAF3 (AK065240, Os11g0131900) in GH family 51 and named them the OsARAF1-FOX and OsARAF3-FOX lines. We analyzed a total of 23 plants from the T1 to T3 generations; they showed essentially identical results. Since OsARAF3 has high homology to H. vulgare ARAF AXAHI and AXAHII [23], OsARAF3 was expected to cleave various arabinofuranosyl side chains, e.g., arabinoxylan and rhamnogalacturonan-I (RG-I).
The expression levels and patterns in OsARAF1 and OsARAF3 of leaf, stem, and root of immature seedlings (14 days old) and leaf and stem of mature plants (60 days old) were analyzed by reverse transcription (RT)-PCR expression analysis (Figure 1B). OsARAF1 was expressed strongly in 14- and 60-day-old stems but was not detectable in 14- and 60-day-old leaves. OsARAF3 was expressed weakly in 14- and 60-day-old leaves. These results are consistent with data retrieved from the RiceXPro (http://ricexpro.dna.affrc.go.jp; [30]). Because OsARAF1 and OsARAF3 are not expressed or only expressed weakly in leaves, we focused on their effects on leaves.
OsARAF1-FOX and OsARAF3-FOX lines were ARAF overexpressors
RT-PCR analysis showed that OsARAF1 and OsARAF3 transcripts were elevated in OsARAF1-FOX and OsARAF3-FOX, respectively (Figure 2A). ARAF activity in mature leaves, assayed using p-nitrophenyl arabinofuranoside as substrate, increased 1.7-fold in OsARAF1-FOX and OsARAF3-FOX compared to the control (Figure 2B and Figure S1A). Since some ARAFs have bifunctional activity for ARAF/β-d-xylosidase, we measured xylosidase activity using p-nitrophenyl xyloside as substrate. The xylosidase activities in OsARAF1-FOX and OsARAF3-FOX lines were almost the same as in the control (Figure 2C and Figure S1B), as predicted from the phylogenetic analysis.
Figure 2. Characteristics of the OsARAF1-FOX and OsARAF3-FOX lines.

(A) RT-PCR analysis of transcripts in mature leaves from the control, OsARAF1-FOX and OsARAF3-FOX lines. The levels of OsARAF1 and OsARAF3 transcripts were higher in each FOX line. 17S Ribosomal RNA-specific primers were used as controls. The numbers in parentheses indicate the numbers of PCR cycles. These experiments were performed at least twice with similar results. (B) Relative ARAF activities in OsARAF1-FOX and OsARAF3-FOX leaves determined using 4-nitrophenyl-α-l-arabinofuranide as a substrate. Activity is expressed as a ratio of the activity in each FOX line to that in the control leaves. Error bars indicate the SD (n = 3). Letters in each panel indicate significant differences at P<0.05 (Tukey's test). Black, white and gray columns indicate the control, OsARAF1-FOX and OsARAF3-FOX lines, respectively. (C) Relative xylosidase activities in OsARAF1-FOX and OsARAF3-FOX leaves determined using 4-nitrophenyl-β-d-xylopyranoside as a substrate. Activity is expressed as a ratio of the activity in each FOX line to that in the control leaves. Error bars indicate the SD (n = 3). Letters in each panel indicate significant differences at P<0.05 (Tukey's test). Black, white and gray columns indicate the control, OsARAF1-FOX and OsARAF3-FOX lines, respectively.
OsARAF1-FOX and OsARAF3-FOX lines, however, showed no visible phenotype (Figure 3). Vegetative parameters, plant height, stem diameter, and number of tillers, and reproductive parameters, fertility rate, number of rachis-branches, and spike length, were unchanged in OsARAF1-FOX and OsARAF3-FOX lines compared to the control (data not shown). Two FOX lines did not show high lodging.
Figure 3. The phenotypes of the control (A), OsARAF1-FOX (B) and OsARAF3-FOX (C) in 60-day-old plant after sowing.
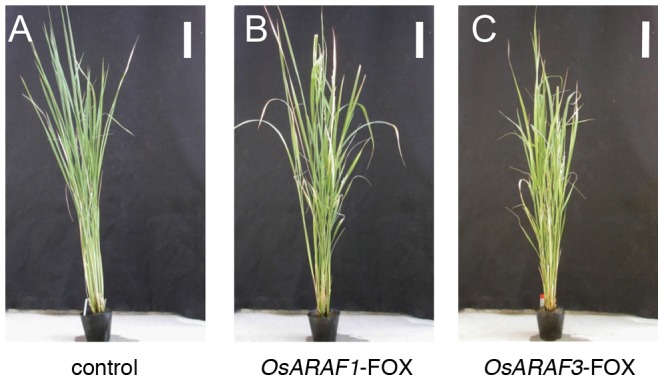
The growth levels of FOX lines were very similar to those of the control, and all FOX lines had normal fertility. Bar = 10 cm.
Effects of overexpression of ARAF on cell wall composition
We determined the glycosyl composition of cell walls prepared from mature leaves of OsARAF1 and OsARAF3 lines. The arabinose content in the TFA-soluble fraction, which was mainly derived from the side chain of arabinoxylan, in OsARAF1-FOX and OsARAF3-FOX lines decreased to 75.4% and 81.6% of that in the control, respectively (Table 1). The xylose content, which was derived from the main chain of arabinoxylan, also decreased in both lines. The glycosyl residue composition of cell walls isolated from leaves of the control and FOX lines was determined to establish whether overexpression of arabinofuranosidase affected the cell wall polymer content.
Table 1. Monosaccharide compositions of TFA-soluble fractions.
| control | OsARAF1-FOX | OsARAF3-FOX | |
| Ara | 41.9±2.6 a | 31.6±5.6 b | 33.8±6.6 b |
| Rha | 2.7±1.4 a | 2.5±0.9 a | 2.5±0.5 a |
| Fuc | N.D. | N.D. | N.D. |
| Xyl | 153.2±7.4 b | 131.6±15.9 a | 135.6±11.8 a |
| Man | 11.2±4.3 a | 9.8±3.4 a | 14.9±4.2 a |
| Gal | 11.8±4.3 a | 11.4±3.4 g | 12.9±4.2 a |
| Glc | 128.3±15.2 a | 65.1±29.9 b | 90.0±18.4 ab |
| GalA | 2.3±1.0 a | 1.4±1.5 a | 2.2±1.0 a |
| GlcA | 7.3±1.9 a | 5.8±2.6 a | 9.1±3.0 a |
| total | 351.7±125 a | 283.6±28.3 b | 323.7±18.5 ab |
The monosaccharide compositions of alcohol-insoluble residues (AIRs) in mature leaves of the control, OsARAF1-FOX and OsARAF3-FOX were determined by GC. The values are means ± SD (n = 12). Different letters within the same column indicate significant differences among means (P<0.05) as determined by Tukey's test. N.D. means “not detected”.
The glucose content in the TFA-insoluble fraction in OsARAF1-FOX and OsARAF3-FOX increased to 128.2% and 134.2% that of the control, respectively (Table 2). The cellulose content was determined using the acetic/nitric acid method. The analysis revealed that the amount of sugar in the acetic/nitric acid-insoluble fraction of OsARAF3-FOX was about 120.0% that of the control (Figure 4A). The amount of lignin was slightly higher in OsARAF1-FOX and OsARAF3-FOX (Figure 4B). These results indicate that overexpression of OsARAF1 and OsARAF3 caused a decrease in arabinoxylan and increases in cellulose and lignin. However, the other cell wall sugars and phenolics were almost the same among the control, OsARAF1-FOX, and OsARAF3-FOX lines (Table 1, Table 2 and Figure 4C).
Table 2. Monosaccharide compositions of TFA-insoluble fractions.
| control | OsARAF1-FOX | OsARAF3-FOX | |
| Ara | N.D. | N.D. | N.D. |
| Rha | N.D. | N.D. | N.D. |
| Fuc | N.D. | N.D. | N.D. |
| Xyl | N.D. | N.D. | N.D. |
| Man | 27.8±5.8 a | 28.6±13.0 a | 36.4±17.4 a |
| Gal | 2.2±2.5 a | 4.3±11.4 a | 2.4±3.5 a |
| Glc | 370.3±28.1 a | 474.6±40.0 b | 497.0±32.4 b |
| GalA | N.D. | N.D. | N.D. |
| GlcA | N.D. | N.D. | N.D. |
| total | 400.3±31.3 a | 508.5±36.8 b | 535.7±32.1 b |
The monosaccharide compositions of alcohol-insoluble residues (AIRs) in mature leaves of the control, OsARAF1-FOX and OsARAF3-FOX were determined by GC. The values are means ± SD (n = 12). Different letters within the same column indicate significant differences among means (P<0.05) as determined by Tukey's test. N.D. means “not detected”.
Figure 4. Cell wall component analysis of the control, OsARAF1-FOX and OsARAF3-FOX.
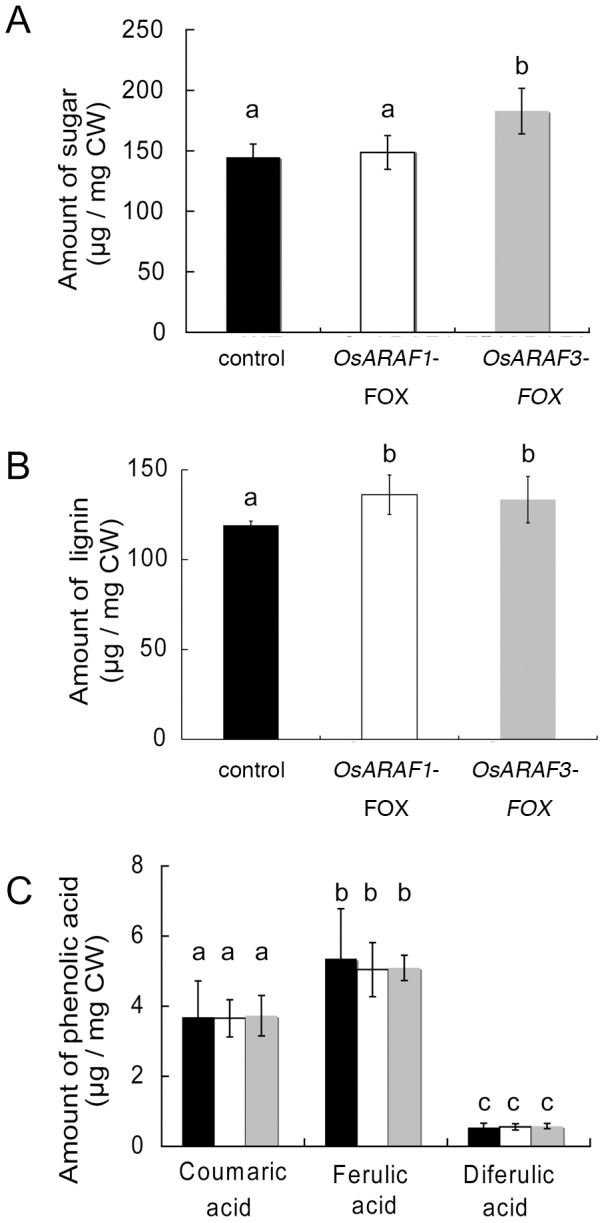
The amounts of sugar in the acetic/nitric acid-insoluble fraction (A) and the amounts of lignin (B) and phenolic acids released by mild alkaline hydrolysis (C) from AIRs in the control, OsARAF1-FOX and OsARAF3-FOX lines are shown Black, white and gray symbols indicate the control, OsARAF1-FOX and OsARAF3-FOX, respectively. Error bars indicate the SD (n = 4). Different letters in each panel indicate significant differences at P<0.05 (Tukey's test).
Overexpression of ARAF caused changes in localization of cell wall components
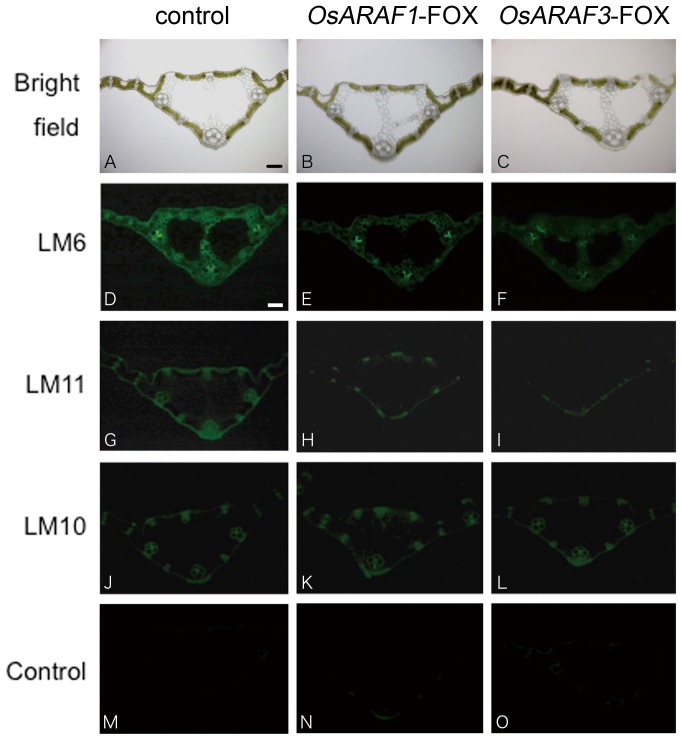
We further determined the distribution of cell wall sugars using monoclonal antibodies against cell wall polysaccharide epitopes and Calcofluor White. The monoclonal antibody LM6 labels arabinan and arabinose side chains in arabinoxylan epitopes ([31]; University of Georgia: http://www.ccrc.uga.edu/~mao/wallmab/Antibodies/antib.htm). LM11 labeled the arabinoxylan and LM10 labeled the xylan epitope [32]. When probed with LM6, OsARAF1 and OsARAF3 had fewer signals in all tissues (Figure 5D–F). Moreover, LM11 stained the wall of vascular bundles and the epidermis of the control, but few signals were detected in OsARAF1 and OsARAF3 lines (Figure 5G–I). However, LM10 label had the same signal intensity among the control, OsARAF1-FOX, and OsARAF3-FOX lines (Figure 5J-L).
Figure 5. Immunofluorescent labeling of the control and FOX lines with arabinoxylan related antiibodies.
Immunohistochemistry on sections of agar-embedded mature leaves of the control (D, G, J and M), OsARAF1-FOX (E, H, K and N) and OsARAF3-FOX (F, I, L and O) line were observed. The sections were labeled with the monoclonal antibodies LM6 (D–F), LM11 (G–l) and LM10 (J–L). Sections were observed under bright-field illumination (A–C). The micrographs (M–O) show the negative control performed without the first antibody step. All experiments were performed at least twice with similar results. Bars = 100 µm.
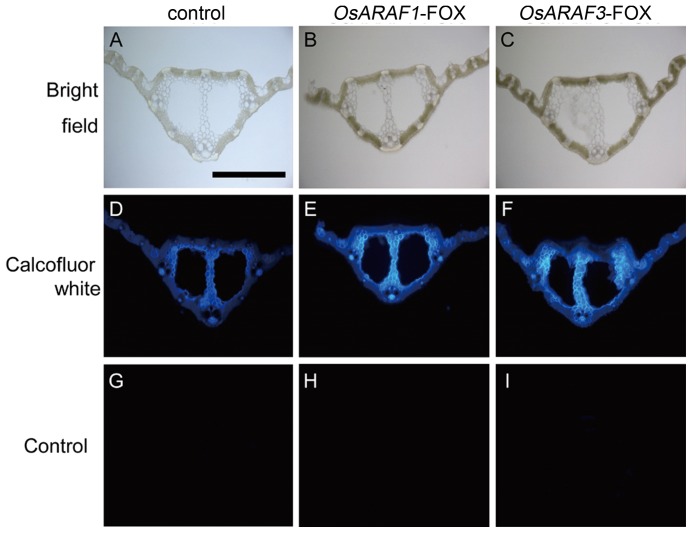
In OsARAF1 and OsARAF3, a high intensity of Calcofluor staining was observed in the midrib and phloem (Figure 6D–F). Calcofluor White is reported to stain β-1, 4-glucan (cellulose), β-1, 3-glucan (callose), and mannan [33] [34]. Because callose is present in specific cell wall components [35], and the resulting glycosyl composition showed very little mannan (Table 1 and Table 2), Calcofluor White should stain cellulose.
Figure 6. Calcofluor white staining for cellulose of mature leaves of the control and FOX lines.
The section of agar-embedded mature leaves of the control (D), OsARAF1-FOX (E) and OsARAF3-FOX (F). Sections were observed under bright-field illumination (A–C). The micrographs (G–I) show negative controls performed without calcofluor white. Bars = 500 µm. All experiments were performed at least twice with similar results.
Overexpression of ARAF affects the improvement of mechanical strength
Glycosyl composition analysis and histochemical observation showed that overexpression of ARAF caused a decrease in arabinose and xylose content and an increase in cellulosic glucose in the cell wall. The altered cell wall composition may influence plant mechanical properties, and thus we examined differences in mechanical properties between the control, OsARAF1-FOX, and OsARAF3-FOX leaves using the creep meter test. The breaking force and extension of leaves was similar among the control, OsARAF1-FOX, and OsARAF3-FOX. The extension length was slightly increased in OsARAF3-FOX (Figure 7). These results indicate that overexpression of ARAF may affect the improvement of mechanical properties of the leaf.
Figure 7. Mechanical properties of leaves of the control, OsARAF1-FOX and OsARAF3-FOX lines.
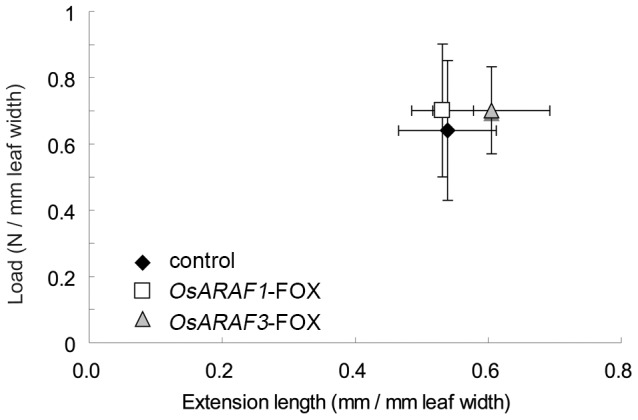
The break force is expressed per leaf width. The horizontal axis shows the extension length. Black, white and gray symbols indicate the control, OsARAF1-FOX and OsARAF3-FOX, respectively. Error bars indicate the SD (n = 8).
Overexpression of ARAF led to an increase in saccharification efficiency
The increase in cellulosic glucose content in the cell wall of OsARAF1-FOX and OsARAF3-FOX lines implied that FOX lines produce more glucose in saccharification compared to the control. We measured sugar liberation from OsARAF1-FOX and OsARAF3-FOX mature leaves (Figure 8A and B). The rates of saccharification were almost the same for 1 h in the control, but longer reaction times led to a significantly higher saccharification rate in FOX lines than in the control, and the amount of liberated sugar after 24 h increased by 46.4% and 69.6%, respectively, compared to the control. Because the amount of starch in mature leaves accounted for only about 2% of the total sugar content (data not shown), the increase in saccharification efficiency does not appear to be influenced by starch.
Figure 8. Saccharification of the control OsARAF1-FOX and OsARAF3-FOX lines in dry leaves.
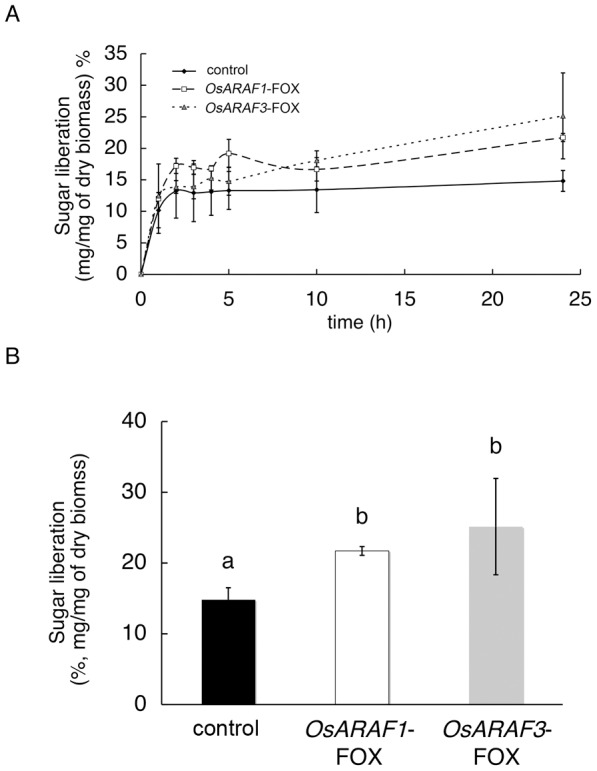
Time-dependent saccharification (A) and saccharification efficiency after 24 h (B) were measured. Error bars indicate the SD (n = 3). Black, white and gray columns indicate the control, OsARAF1-FOX and OsARAF3-FOX, respectively. Different letters in the same column indicate significant differences at P<0.05 (Tukey's test).
Discussion
We analyzed ARAF-overexpressed rice plants. Arabinose content in the cell walls of the two ARAF-overexpressed plants decreased to 75% and 80% of that in the control, and the amount of cellulosic glucose increased to 128.2% and 134.2% of that of control. We did not use fungal ARAF for which the exact activity against multiple substrates is known because overexpression of fungal ARAF might have resulted in lethality due to xylan degradation and inhibition of cell wall synthesis. The use of fungal cell wall hydrolases was previously shown to result in a low yield [36]; therefore, we used the endogenous arabinofuranosidases. OsARAF1-FOX and OsARAF3-FOX lines showed no morphological phenotype and some improvement of mechanical strength as the control (Figure 3 and 7). In addition, overexpression caused an increase in saccharification efficiency (Figure 8).
Arabinoxylans are major non-cellulosic polysaccharides in primary and secondary walls of grass cell walls. They are hydrogen-bonded to themselves and cellulose microfibrils to form cellulose–hemicellulose networks. Ferulic acids are ester-linked to arabinose side chains of arabinoxylan to form diferulate cross-linked arabinoxylan. In monocots, most arabinose residues exist as side chains of arabinoxylans. In secondary cell walls, ester-linked ferulic acid is incorporated into lignin to form an arabinoxylan–lignin complex [9]. Hence, arabinose residues are thought to be the base point for cross-linking between arabinoxylan and lignin [37]. In growing tissues, arabinoxylan content in cell walls increases slowly and arabinoxylans are highly branched with arabinosyl residues and are removed during growth [38] [39].
ARAFs are defined as enzymes that catalyze the hydrolysis of terminal, nonreducing α-l-arabinofuranoside residues. However, several enzymes in family 51 are capable of hydrolyzing both l-Ara and d-Xyl from a variety of substrates in vitro, and therefore may be considered as bifunctional ARAF/β-d-xylosidase enzymes. For example, barley ARA-I, Arabidopsis XYL3 and ARAF1, and alfalfa MsXyl1 have bifunctional enzyme activity [25] [26] [40] [41]. However, our results showed that OsARAF1 and OsARAF3 may have only ARAF activity and no xylosidase activity (Figure 2B and C). And, lignin complexes with arabinoxylan are mediated by ferulic acid [9]. In OsARAF1-FOX and OsARAF3-FOX, the amounts of ferulic acid were similar to that in the control. These results suggest that OsARAF1 and OsARAF3 do not possess the ability to cleave the linkage of the arabinose residue to ferulic acid. Expression of OsARAF1 and OsARAF3 was higher in growing organs and lower in mature organs (Figure 1B). These results indicate that arabinoxylans are turned over during cell wall development in growing organs.
Arabinose residues are a quantifiably important constituent in various cell wall components, not only arabinoxylan but pectin RG-I and glycoprotein [2] [42]. Glycosyl composition analysis of cell walls and immunohistochemistry showed that arabinosyl residues in arabinoxylans decreased in OsARAF1-FOX and OsARAF3-FOX lines. The xylan in OsARAF1-FOX and OsARAF3-FOX also decreased in the hemicellulose fraction (Table 1). This result suggests that the xylan main chain decreases by reducing the side chain arabinose in FOX lines. The ratios of arabinose to xylose in OsARAF1-FOX and OsARAF3-FOX were 1∶3, the same as that in the control (Table 1). These results indicate that a change occurred in the quantity rather than the structure of arabinoxylan in the FOX lines as compared to the control. The amounts of arabinose side chains may influence the xylan backbone extension, and arabinose residues may be important in the formation of the cell wall network. On the other hand, overexpression of AtARAF1 leads to increases in the secondary cell wall, especially xylan [43]. In any case, arabinofuranosidase overexpression may cause modification of the secondary cell wall.
Neither OsARAF1-FOX nor OsARAR3-FOX affected plant growth (Figure 3). The increase in cellulose may compensate for the reduction in arabinoxylan (Table 1, Table 2 and Figure 6). Additionally, amount of crystalic cellulose in OsARAF3-FOX increased compared to the control (Figure 4A). Staining with Calcofluor White increased in the midrib and phloem of FOX lines, which is associated with the increased mechanical strength (Figure 6D–F and Figure 7). From these results, it appears that rice might maintain plant body strength by maintaining a balance between cellulose and hemicellulose. In fact, OsARAF1-FOX and OsARAR3-FOX lines showed slightly increase of mechanical strength compared to the control (Figure 7). In dicots, xyloglucan is hydrogen-bonded to cellulose microfibrils. In xylogulcanase-overexpressed poplar, the amount of cellulose increased [44] and led to the generation of abnormal tensile stress. In this study, ARAF-overexpressed rice had a lower arabinoxylan content, and so it was expected to have a similar phenotype; however, no influence on growth occurred (Figure 3). Unlike dicots, rice has interspersed vascular bundles, which have less influence on retaining the strength of the plant body, so an increase of cellulose may have a positive effect on growth.
OsARAF1-FOX and OsARAF3-FOX lines had increased saccharification efficiency (Figure 8A and B). In industrial processes, plant biomass is treated with hemicellulase and cellulase because cellulose covers the cell wall matrix. We expected that the increased saccharification efficiency of FOX lines would depend on decreases in hemicellulose and substituted xylan [45]. The saccharification efficiency after 24 h reaction increased in FOX lines (Figure 8A), which may have been caused not only by an increase in accessibility of the cellulose due to a decrease in hemicelluloses but also an increase in cellulose content. In cell walls, glucose is contained in cellulose and β-1,3-1,4-glucan. The hydrolysis of β-1,3-1,4-glucan is accelerated by growth regulators; in mature leaves, the β-1,3-1,4-glucan level is low [2]. The amount of lignin increased slightly in OsARAF1-FOX and OsARAF3-FOX lines, but this didn’t influence saccharification efficiency. Lignin complexes with arabinoxylan and is mediated by ferulic acid [9]. In this study, the decrease in arabinoxylan had little effect on cell wall network assembly and growth. The small change in cell wall components led to higher cellulose content in the plant but had no effect on growth. And, in previous studies, few examples of increases in cellulose content in cell walls were reported. Hence, our report provides an important contribution to the study of cell wall biosynthesis and future technology. The modification cell walls in rice through ARAF overexpression improves saccharification efficiency, and these FOX rice lines could prove useful for producing cellulosic bioethanol.
Supporting Information
Enzyme activity in WT, OsARAF1 -FOX and OsARAF3 -FOX. (A) ARAF activity using 4-nitrophenyl-α-l-arabinofuranide as substrate in WT, OsARAF1-FOX and OsARAF3-FOX leaves. (B) Xylosidase activity using 4-nitrophenyl-β-D-xylopyranoside as substrate in WT, OsARAF1-FOX and OsARAF3-FOX leaves. Black, white, and gray columns indicate the WT, OsARAF1-FOX, and OsARAF3-FOX lines, respectively. Black, white, and gray columns indicate WT, OsARAF1-FOX, and OsARAF3-FOX, respectively.
(PDF)
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (18075004 to H. Iwai), a Grant-in-Aid for Scientific Research on Innovative Areas (24114006 to S. Satoh and H. Iwai) and grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, GMA0007 to S. Satoh) and AMR0001 to H. Ichikawa, and Green Technology Project, EF1004 to H. Ichikawa. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants. Plant J 3: 1–30. [DOI] [PubMed] [Google Scholar]
- 3. Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Physiol Plant Mol Biol 61: 263–289. [DOI] [PubMed] [Google Scholar]
- 4. Fincher GB (2009) Revolutionary Times in Our Understanding of Cell Wall Biosynthesis and Remodeling in the Grasses. Plant Physiol 149: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47: 445–476. [DOI] [PubMed] [Google Scholar]
- 6. Hoije A, Sternemalm E, Heikkinen S, Tankanen M, Gatenholm P (2008) Material properties of films from enzymatically tailored arabinoxylans. Biomacromolecules 9: 2042–2047. [DOI] [PubMed] [Google Scholar]
- 7. Ishii T (1991) Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydr Res 219: 15–22. [DOI] [PubMed] [Google Scholar]
- 8. Ishii T (1997) Structure and functions of feruloylated polysaccharides. Plant Sci 127: 111–127. [Google Scholar]
- 9. Iiyama K Lam TBT, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, et al. (2010) Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci USA 107: 616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwai H, Ishii T, Satoh S (2001) Absence of arabinan in the side chains of the pectic polysaccharides strongly associated with cell walls of Nicotiana plumbaginifolia non-organogenic callus with loosely attached constituent cells. Planta 213: 907–915. [DOI] [PubMed] [Google Scholar]
- 12. Drakakaki G, Zabotina O, Delgado I, Robert S, Keegstra K, et al. (2006) Arabidopsis reversibly glycosylated polypeptides 1 and 2 are essential for pollen development. Plant Physiol 142: 1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konishi T, Aohara T, Igasaki T, Hayashi N, Miyazaki Y, et al. (2011) Down-regulation of UDP-arabinopyranose mutase reduces the proportion of arabinofuranose present in rice cell walls. Phytochemstry 72: 1962–1968. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura H, Hakata M, Amano K, Miyao A, Toki N, et al. (2007) A genome-wide gain-of function analysis of rice genes using the FOX-hunting system. Plant Mol Biol 65: 357–371. [DOI] [PubMed] [Google Scholar]
- 15. Hakata M, Nakamura H, Iida-Okada K, Miyao A, Kajiwara M (2010) Production and characterization of a large population of cDNA-overexpressing transgenic rice plants using Gateway-based full-length cDNA expression libraries. Breed Sci 60: 575–585. [Google Scholar]
- 16. Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 17. Page RDM (1996) TreeView: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358. [DOI] [PubMed] [Google Scholar]
- 18. Kotake T, Tsuchiya K, Aohara T, Konishi T, Kaneko S, et al. (2006) An a-L-arabinofuranosidase/b-D-xylosidase from immature seeds of radish Raphanus sativus L. J Exp Bot 57: 2353–2362. [DOI] [PubMed] [Google Scholar]
- 19. Bradford M (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 20. Brendel O, Iannetta PPM, Stewart D (2000) A rapid and simple method to isolate pure alpha-cellulose. Phytochem Anal 11: 7–10. [Google Scholar]
- 21. Suzuki S, Suzuki Y, Yamamoto N, Hattori T, Sakamoto M, et al. (2009) High-throughput determination of thioglycolic acid lignin from rice. Plant biotech 26: 337–340. [Google Scholar]
- 22. Yazaki J, Kojima K, Suzuki K, Ishimoto N, Kikuchi S (2004) The Rice PIPELINE: a unification tool for plant functional genomics. Nucl Acids Res 32: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: HJ Gilbert G Davies B Henrissat and B Svensson, Recent Advances in Carbohydrate Bioengineering. Cambridge: The Royal Society of Chemistry. 3–12.
- 24. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, et al. (2009) The Carbohydrate-Active EnZymes database CAZy: an expert resource for glycogenomics. Nucl Acids Res 37: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minic Z, Rihouey C, Do CT, Lerouge P, Jouanin L (2004) Purification and characterization of enzymes exhibiting d-xylosidase activities in stem tissues of Arabidopsis. Plant Physiol 135: 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Minic Z, Do CT, Rihouey C, Morin H, Lerouge P (2006) Purification functional characterization cloning and identification of mutants of a seed-specific arabinan hydrolase in Arabidopsis. J Exp Bot 57: 2339–2351. [DOI] [PubMed] [Google Scholar]
- 27. Fulton LM, Cobbett SC (2003) Two α-l-ARAF genes in Arabidopsis thaliana are differentially expressed during vegetative growth and flower development. J Exp Bot 54: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 28. Lee RC, Burton RA, Hrmova M, Fincher GB (2001) Barley arabinoxylan arabinofuranohydrolases: purification characterization and determination of primary structures from cDNA clones. Biochem J 356: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laidlaw HK, Lahnstein J, Burton RA, Fincher GB, Jobling SA (2012) Analysis of the arabinoxylan arabinofuranohydrolase gene family in barley does not support their involvement in the remodelling of endosperm cell walls during development. J Exp Bot 63: 3031–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato Y, Antonio BA, Namiki N, Takehisa H, Minami H, et al. (2010) RiceXPro: a platform for monitoring gene expression in japonica rice grown under natural field conditions. Nucl Acids Res 39: 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willats WG, Steele-King CG, Marcus SE, Knox JP (1999) Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J 20: 619–628. [DOI] [PubMed] [Google Scholar]
- 32. McCartney L, Marcus SE, Knox JP (2005) Monoclonal Antibodies to Plant Cell Wall Xylans and Arabinoxylans. J Histochem Cytochem 53: 543–546. [DOI] [PubMed] [Google Scholar]
- 33. Maeda H, Ishida N (1967) Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem 62: 276–278. [DOI] [PubMed] [Google Scholar]
- 34. Wood PJ, Fulcher RG, Stone BA (1983) Studies on the specificity of interaction of cereal cell wall components with Congo Red and Calcofluor Specific detection and histochemistry of 1→3.1→4.- β-d-glucan. J Cereal Sci 1: 95–110. [Google Scholar]
- 35.Chen XY, Kim JY (2009) Callose synthesis in higher plants. Plant Signal Behav 4: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taylor II LE, Dai Z, Decker SR, Brunecky R, Adney WS, et al. (2008) Heterologous expression of glycosyl hydrolases in planta: a new departure for biofuels. Trends in Biotechnology 26: 413–424. [DOI] [PubMed] [Google Scholar]
- 37. Fincher GB (2009) Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol 149: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carpita NC (1984) Cell wall development in maize coleoptiles. Plant Physiol 76: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibeaut DM, Pauly M, Bacic A, Fincher GB (2005) Changes in cell wall polysaccharides in developing barley Hordeum vulgare coleoptiles. Planta 221: 729–738. [DOI] [PubMed] [Google Scholar]
- 40. Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB (2003) Bifunctional Family 3 Glycoside Hydrolases from Barley with α-l -Arabinofuranosidase and β-d-Xylosidase Activity. CHARACTERIZATION PRIMARY STRUCTURES AND COOH-TERMINAL PROCESSING. J Biol Chem 278: 5377–5387. [DOI] [PubMed] [Google Scholar]
- 41. Xiong JS, Balland-Vanney M, Xie ZP, Schultze M, Kondorosi A, et al. (2007) Molecular cloning of a bifunctional β-xylosidase/α-L-arabinosidase from alfalfa roots: heterologous expression in Medicago truncatula and substrate specificity of the purified enzyme. J Exp Bot 58: 2799–2810. [DOI] [PubMed] [Google Scholar]
- 42. Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure biosynthesis and oligogalacturonide-related signaling. Phytochemistry 57: 929–967. [DOI] [PubMed] [Google Scholar]
- 43. Chávez Montes RA, Ranocha P, Martinez Y, Minic Z, Jouanin L, et al. (2008) Cell wall modifications in Arabidopsis plants with altered α-l-arabinofuranosidase activity Plant Physiol. 147: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baba K, Park YW, Kaku T, Kaida R, Takeuchi M, et al. (2009) Xyloglucan for generating tensile stress to bend tree stem. Mol Plant 2: 893–903. [DOI] [PubMed] [Google Scholar]
- 45. Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, et al. (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA 107: 17409–17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enzyme activity in WT, OsARAF1 -FOX and OsARAF3 -FOX. (A) ARAF activity using 4-nitrophenyl-α-l-arabinofuranide as substrate in WT, OsARAF1-FOX and OsARAF3-FOX leaves. (B) Xylosidase activity using 4-nitrophenyl-β-D-xylopyranoside as substrate in WT, OsARAF1-FOX and OsARAF3-FOX leaves. Black, white, and gray columns indicate the WT, OsARAF1-FOX, and OsARAF3-FOX lines, respectively. Black, white, and gray columns indicate WT, OsARAF1-FOX, and OsARAF3-FOX, respectively.
(PDF)