Abstract
Objective
The purpose of this investigation was to examine serum vitamin D status in a population of Punjabi ancestry from Northern India with a high prevalence of type 2 diabetes (T2D) and evaluate the effects of 25(OH)D levels on cardio-metabolic traits.
Research design and methods
We assessed cardiovascular risk factors and 25(OH)D levels in 1,765 participants (887 T2D cases, 878 normoglycemic controls).
Results
76% of individuals were deficient (<50 nmol/L) in vitamin D. A higher percentage of T2D participants(83%) were vitamin D deficient compared to normoglycemic controls (68%)(p<0.0001).The prevalence of vitamin D deficiency increased progressively with body mass index (BMI) categories (p<0.0001): BMI<23 kg/m2, 65%; BMI 23–27.5 kg/m2, 75%; and BMI>27.5 kg/m2, 81%. T2D participants had significantly decreased serum 25(OH)D levels (β=−0.41, p=2.8 × 10−20). Individuals with low serum 25(OH)D had elevated fasting glucose(β=−0.18, p=0.022), BMI (β=-0.71, p=1.4 × 10−7) and systolic blood pressure (β=−1.68, p=0.006). A positive association of increased 25(OH)D with HOMA-B (β=0.17, p=8.0×10−6), and C-peptide (β=0.09, 0.017) was observed. Non-medicated, normoglycemic, non-hypertensive individuals classified as vitamin D deficient (n=289) exhibited a significant increase in fasting glucose (p=0.02) and BMI (p<0.0001) as well as a significant decrease in C-peptide (p<0.0001) and amylin (p<0.0001) compared to vitamin D sufficient controls (n=150).
Conclusions
Vitamin D deficiency appears to be a significant risk factor for T2D severity and associated cardio-metabolic risk. Early intervention may be considered to improve prevention of T2D related cardiovascular complications.
Introduction
It may not be coincidental that the prevalence of vitamin D deficiency, estimated to affect over 1 billion people worldwide [1], is increasing in conjunction with T2D, obesity, and cardiovascular disease. The ubiquitous distribution of vitamin D receptors in the body, controlled by nearly 3,000 genes [2], suggest that a deficiency could have widespread health implications. Recent studies have examined the physiological functions of vitamin D beyond its well established role in musculoskeletal health [3]. In addition to findings of oncologic [4] and immunologic [5] associations, vitamin D deficiency is associated with cardio-metabolic risk factors including T2D [6], blood pressure [7], and obesity [8].
Lo et al. [9] report that Asian Indians require twice as much UV-B exposure to produce 25(OH)D levels equal to Caucasians due to increased skin pigmentation. In addition, a cultural tendency to avoid direct sunlight may contribute to suboptimal vitamin D status although the climate in India is sunny throughout the year. Currently, 62.4 million people in India have T2D and 77.2 million have prediabetes [10], representing the country with the second highest prevalence in the world after China [11]. Asian Indians have lower body mass index (BMI) than US whites, African Americans, and Mexican Americans [12] but are termed “metabolically obese” due to disproportionally high abdominal fat, an important contributing factor in T2D. Central obesity, sedentary lifestyle, a westernized diet, and genetic predisposition are several factors contributing to the alarming increase of T2D in India and around the world. The high morbidity and mortality associated with T2D presents an overwhelming health care burden necessitating improved treatment and preventative therapy.
Vitamin D status is known to be poor among Asian Indians [13]; however, limited data exists to assess the implication of vitamin D deficiency on cardio-metabolic traits in Asian Indians. To our knowledge, this is the first large study reporting the role of 25(OH)D deficiency in North Indians who have an increased prevalence of T2D and cardiovascular diseases [14–16].
Materials and Methods
Participants in this diabetes-focused case-control cohort are part of the Asian Indian Diabetic Heart Study (AIDHS)/Sikh Diabetes Study (SDS) [17] (Table 1). The diagnosis of T2D was confirmed by scrutinizing medical records for symptoms, use of medications, and defining diabetes according to fasting glucose levels as defined in the American Diabetes Association guidelines [18]. BMI was calculated as (weight (kg)/height (meter2)). Waist and hip circumferences were measured with a tape measure at the abdomen and at the hip. The World Health Organizations (WHO) has recommended lower BMI thresholds for Asians [19] therefore, obesity was defined using WHO’s new guidelines [19]. Participants with BMI<23 kg/m2 were classified as normal weight, BMI between 23–27.5 kg/m2 were classified as overweight, and BMI>27.5kg/m2 were classified as obese. Individuals with type 1 diabetes (T1D), or with rare forms of T2D such as maturity-onset diabetes of young (MODYs), or secondary diabetes (e.g., due to hemochromatosis or pancreatitis) were excluded. Details of physical activity, smoking, alcohol, diet, and family history are described elsewhere [17,20]. Blood pressure was measured twice after a five minute seated rest period with the participant’s feet flat on the floor. Pulse pressure was calculated as: [systolic blood pressure (SBP)-diastolic blood pressure (DBP)] and mean arterial pressure was calculated as (2×DBP + SBP)/3. All blood samples were obtained at the baseline visit. Among diabetics, 40% were taking oral hypoglycemic agents. Of these, 9% were treated with sulphonylurea, metformin, dianil, ayurvedic, or ‘desi’ medicine, 5% were treated with insulin, and 26% were co-treated with insulin and oral agents. The remaining 60% were maintaining glycemic control with diet and exercise. All participants provided informed consent following procedures approved by Institutional Review Boards (IRBs). All SDS protocols and consent documents were reviewed and approved by the University of Oklahoma Health Sciences Center (OUHSC)’s IRB as well as the Human Subject Protection (Ethics) committees at the participating hospitals and institutes in India.
Table 1.
Prevalence of vitamin D deficiency and the association of cardio-metabolic traits with 25(OH)D.
| Combined | Males (52.6%) | Females (47.4%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | Deficient | Sufficient | p value* | Deficient | Sufficient | p value* | Deficient | Sufficient | p value* |
| (<50nmol/L) | (≥50nmol/L) | (<50nmol/L) | (≥50nmol/L) | (<50nmol/L) | (≥50nmol/L) | ||||
| Prevalence | (%) | (%) | (%) | (%) | (%) | (%) | |||
| Total (n=1,765) | 75.8 | 24.2 | p<0.0001 | 78.3 | 21.7 | p<0.0001 | 72.6 | 27.4 | p<0.0001 |
| Type 2 Diabetes | 83.51 | 16.5 | p<0.0001 | 85 | 15 | p<0.0001 | 81.6 | 18.4 | p<0.0001 |
| Normoglycemic controls | 68.01 | 32.7 | p<0.0001 | 71.5 | 28.5 | p<0.0001 | 63.5 | 36.5 | p<0.0001 |
| BMI<23kg/m2 | 65.22 | 34.8 | p<0.0001 | 68.7 | 31.3 | p<0.0001 | 59.8 | 40.2 | p=0.012 |
| BMI 23–27.5kg/m2 | 75.52,3 | 24.5 | p<0.0001 | 80.2 | 19.8 | p<0.0001 | 68.6 | 31.4 | p<0.0001 |
| BMI>27.5kg/m2 | 81.43 | 18.6 | p<0.0001 | 82.4 | 17.6 | p<0.0001 | 80.4 | 19.6 | p<0.0001 |
| Hypertensive (>140/85) | 78.24 | 21.8 | p<0.0001 | 81.8 | 18.2 | p<0.0001 | 73.8 | 26.2 | p<0.0001 |
| Non-Hypertensive | 72.84 | 27.2 | p<0.0001 | 74.1 | 25.9 | p<0.0001 | 71.6 | 28.4 | p<0.0001 |
| β (95% CI) | p value | β (95% CI) | p valued | β (95% CI) | p valued | ||||
| Type 2 Diabetes | −0.41 (−0.5, −0.3) | 2.8×10−20a | −0.5 (−0.6, −0.4) | 4.9×10−17 | −0.3 (−0.4, −0.2) | 3.6×10−7 | |||
| Metabolic Traits | |||||||||
| Fasting Glucose (mg/dL) | −0.18 (−0.3,−0.03) | 0.022b | −0.01 (−0.04, 0.01) | 0.31 | −0.03 (−0.5, −0.003) | 0.03 | |||
| Fasting Insulin (µIU/mL) | 0.04 (−0.01, 0.09) | 0.145b | 0.006 (0.07, 0.08) | 0.89 | 0.08 (0.002, 0.2) | 0.044 | |||
| C Peptide (ng/mL) | 0.09 (0.02,0.1) | 0.017b | 0.002 (0.1, 0.1) | 0.97 | 0.21 (0.09, 0.3) | 4.9×10−4 | |||
| Amylin (pg/mL) | 0.019 (−0.002,0.04) | 0.071b | 0.004 (0.03, 0.03) | 0.82 | 0.04 (0.009, 0.06) | 0.009 | |||
| HOMA-B | 0.17 (0.09,0.2) | 8.0×10−6b | 0.09 (0.001, 0.19) | 0.05 | 0.2 (0.1, 0.3) | 1.4×10−5 | |||
| Obesity | |||||||||
| BMI (kg/m2) | −0.71 (−0.9, −0.4) | 1.4×10−7c | −0.6 (−0.9, −0.3) | 3.8×10−4 | −0.8 (−1.2, −0.4) | 8.7×10−5 | |||
| Cardiovascular Traits | |||||||||
| Systolic BP (mmHg) | −1.68 (−2.9, −0.4) | 0.006b | −3.2 (-4.9, −1.5) | 1.8×10−4 | −1.0 (−2.9, 0.7) | 0.23 | |||
| Diastolic BP (mmHg) | 0.32 (0.3,0.9) | 0.35b | 0.2 (1.2, 0.8) | 0.75 | 0.3 (0.6, 1.2) | 0.56 | |||
| Pulse Pressure mmHg) | −1.85 (−2.8, −0.9) | 1.2×10−4b | −3.1 (−4.3, −1.8) | 2.3×10−6 | −1.0 (−2.4, 0.4) | 0.17 | |||
Prevalence *Chi-square analysis:
T2Dvs Normoglycemic (p<0.0001);
BMI<23 vs BMI 23–27.5 (p<0.0001);
BMI 23–27.5 vs BMI>27.5 (p<0.0001);
Hypertensive vs Non-Hypertensive (p<0.0001);
Multiple Regression a Logistic regression adjusted for BMI, age, gender;
Linear regression adjusted for T2D, age, BMI and gender;
Linear regression adjusted for T2D, age and gender;
Gender was excluded as co-variant
The selection of controls was based on a fasting glycemia<100.8 mg/dL or a 2 hr. glucose<141.0 mg/dL. Subjects with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) were excluded from the study. Insulin was measured by radio-immuno assay (Diagnostic Products, Cypress, USA). Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as: fasting insulin (εIU/mL)×fasting glucose (mmol/L)/22.5. HOMA-B assessed β-cell function using the following formula: 20×fasting insulin (εIU/mL)/fasting glucose (mmol/L)−3.5. Serum was obtained from fasting blood samples to quantify 25(OH)D levels in a total of 1,765 individuals using standard monoclonal antibody based ELISA kits from ALPCO Diagnostics (Salem, NH, USA). Samples were run in duplicate following the manufacturer’s instructions. Vitamin D status was classified as deficient<50 nmol/L or sufficient ≥50 nmol/L according to recommendations of the Institute of Medicine, Food and Nutrition Board [21] and severely deficient when <30 nmol/L. C-peptide and amylin measures were simultaneously quantified using Millipore’s Magnetic MILLIPLEX Human Metabolic panel (St. Charles, Missouri) and analyzed on a Bio-plex 200 multiplex analysis system (Bio-Rad Hercules, CA).
Statistical Analysis
Statistics were performed using SPSS for windows statistical package (SPSS INC., Chicago, USA). Serum 25(OH)D levels were analyzed as a categorical variable deficient [<50 nmol/L] and sufficient [≥50 nmol/L] or a continuous variable after log transformation. As a categorical variable, difference in frequency distribution between vitamin D deficient participants and vitamin D sufficient participants were analyzed using χ2-test. As a continuous variable, multivariate logistic regressions tested association of log transformed serum 25(OH)D levels with T2D adjusting for age, BMI and gender. Multivariate linear regressions were performed to test association between log transformed serum 25(OH)D as the independent variable with quantitative traits and biomarkers related to T2D (adjusted for T2D, age, BMI and gender), obesity (adjusted for T2D, age, gender) and cardiovascular risk (adjusted for T2D, age, BMI, gender) as the dependent variable. Variables with skewed distributions were log transformed (fasting glucose, fasting insulin, C-peptide, and amylin). Untransformed measures were reported as arithmetic means while arithmetic means of the transformed variables were retransformed into the original measurement scale and reported as geometric means. Independent t-tests were used to analyze the association between serum 25(OH)D (continuous variable) with BMI tertiles in participants with and without T2D and to evaluate the effect of vitamin D deficiency (categorical variable) on mean levels of quantitative traits (continuous variable).
Results
Prevalence of vitamin D deficiency
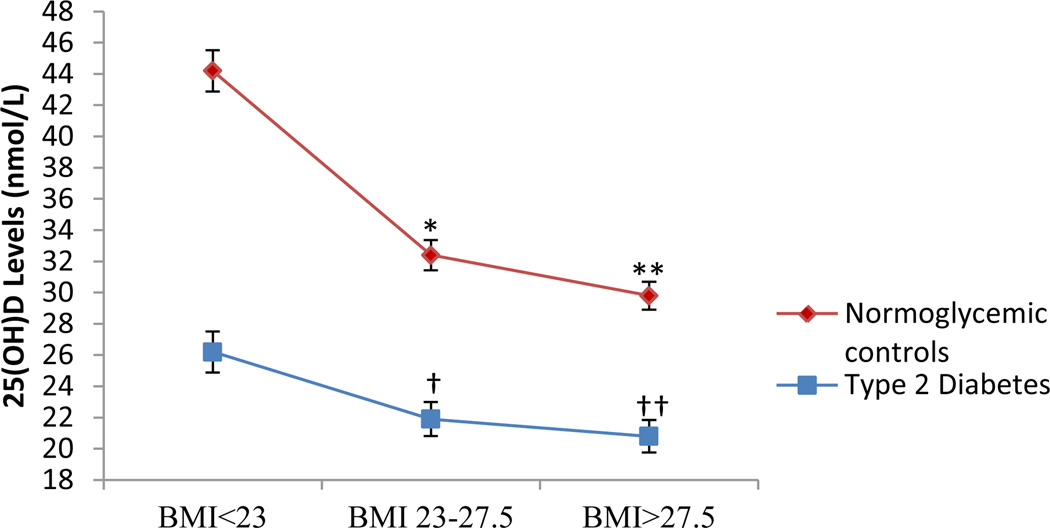
25(OH)D was quantified in a total of 1,765 participants with a mean age of 52.7 ± 12.4 years. Among the participants, 52.6% were male, 50.2% had T2D, 21.6% were normal weight (BMI<23 kg/m2), 36.4% overweight (BMI 23–27.5 kg/m2) and 42.0% were obese (BMI>27.5 kg/m2). Table 1 summarizes the vitamin D status of our population. A total of 75.8% of participants were classified as vitamin D deficient (<50 nmol/L) and only 24.2% of individuals had sufficient (≥50 nmol/L) 25(OH)D levels (p<0.0001). Of particular note was the increased prevalence of vitamin D deficiency in T2D, obese, and hypertensive participants. The T2D cases had a significantly higher prevalence of vitamin D deficiency (83.5%) when compared to normoglycemic controls (68%) (p<0.0001). Similarly, a significant linear increase (p<0.0001) in the frequency of vitamin D deficient individuals was observed (65.2%, 75.5%, 81.4%) as the BMI increased (<23 kg/m2, 23–27.5 kg/m2, >27.5 kg/m2), respectively. Figure 1 highlights the significant decrease in serum 25(OH)D levels as BMI increases in the entire AIDHS/SDS cohort stratified by T2D. Serum 25(OH)D levels were markedly reduced in over-weight and obese individuals even without T2D.
Figure 1.
*BMI<23 vs BMI 23–27.5 (p<0.0001), **BMI 23 vs BMI>27.5 (p<0.0001);
†BMI<23 vs BMI 23–27.5 (p<0.0001), ††BMI 23 vs BMI>27.5 (p<0.0001)
Association of BMI Tertiles with 25(OH)D in participants with and without T2D.
Association of vitamin D deficiency with cardio-metabolic traits
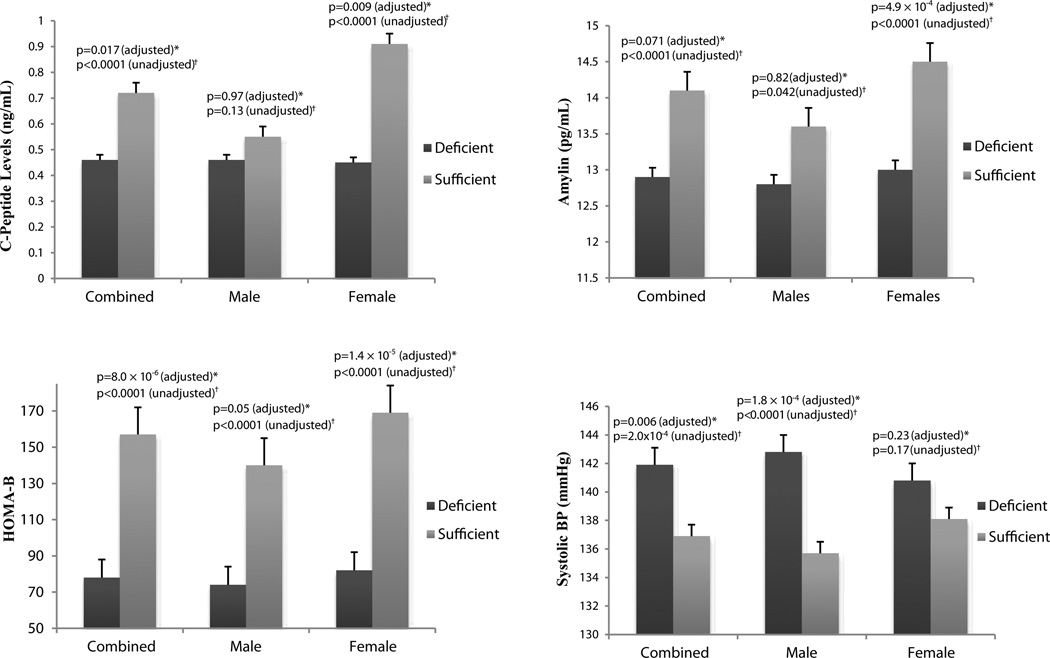
Multivariate regression analyses were performed to assess the impact of vitamin D status on T2D, obesity and related traits and biomarkers (Table 1). We report a highly significant, inverse association of serum 25(OH)D with T2D (β=−0.41, p=2.8×10−20) and BMI (β=−0.71, p=1.4×10−7) in males and females combined. Other traits related to T2D and β-cell function showing gender-specific associations with 25(OH)D levels in female participants include fasting glucose (β=−0.03, p=0.03), fasting insulin (β=0.08, p=0.044),C-peptide (β=0.21, p=4.9×10−4), and amylin (β=0.04, p=0.009). HOMA-B was significantly elevated with increased vitamin D levels in female participants (β=0.2, p=1.4 × 105).The association of serum 25(OH)D with quantitative traits related to cardiovascular function were restricted to male participants. No association was observed between 25(OH)D levels and diastolic blood pressure. We also tested the association of serum 25(OH)D with high density lipoproteins (HDL), low density lipoproteins (LDL), total cholesterol (TC), and triglycerides (TG). No significant associations were found (Supplemental Table 1). Figure 2 illustrates these highly significant gender-specific associations of C-peptide, amylin, HOMA-B and systolic blood pressure by comparing mean levels of these quantitative traits in vitamin D deficient and vitamin D sufficient participants.
Figure 2.
*Multivariate regression analysis showing association of 25(OH)D with quantitative trait adjusted for T2D, age, BMI and gender (combined only), †Independent t test comparing mean levels of quantitative traits in vitamin D deficient (<50nmol/L) versus vitamin D sufficient (≥ 50nmol/L) participants
Vitamin D deficient (<50 nmol/L) gender specific associations of C-peptide, amylin, HOMA-B and systolic blood pressure.
Impact of medication on vitamin D deficiency
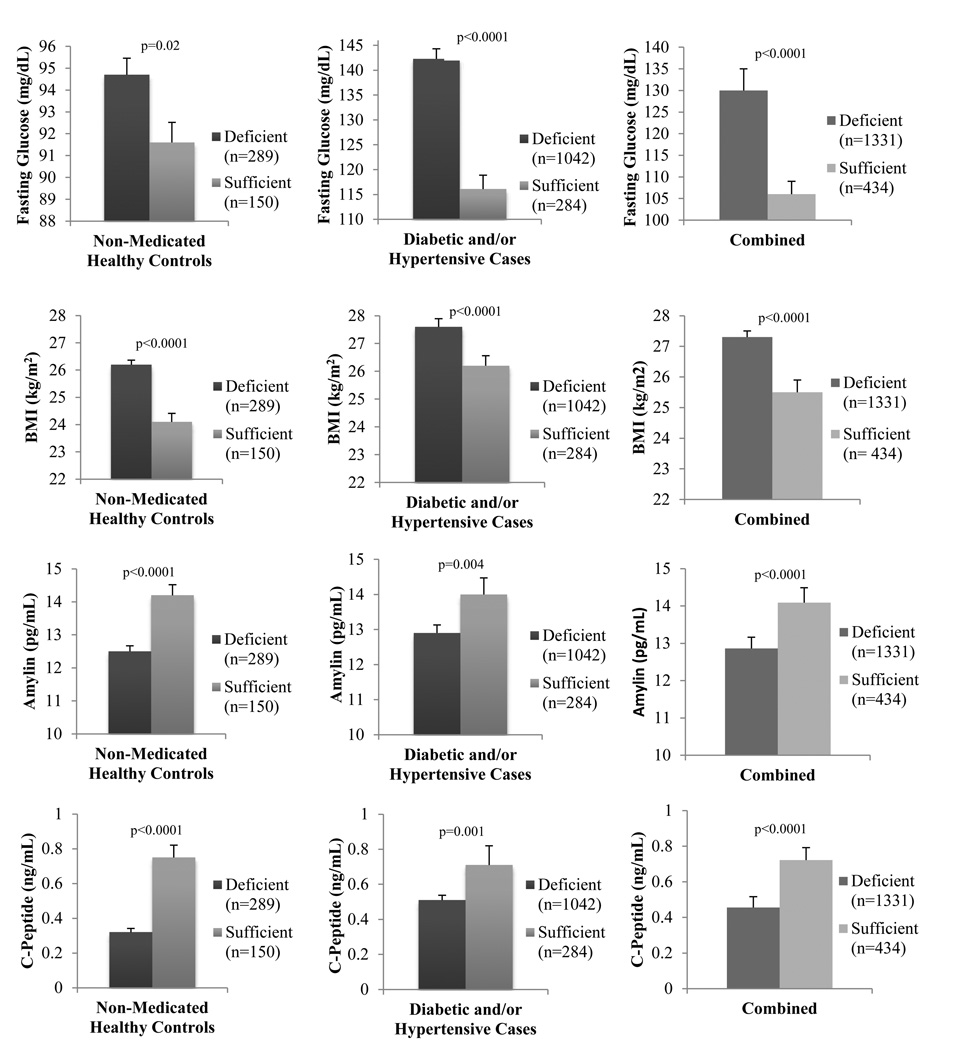
To assess the impact of medication on vitamin D status among diabetic patients, we stratified the data based on medication use (Supplemental Table 2). Of the 887 diabetic cases, 359 (40%) were taking medication to control glucose levels at the time of inclusion into this study. We observe significantly higher 25(OH)D levels with improved metabolic and cardiovascular traits among non-medicated, diabetic patients. While this may seem unexpected, significantly earlier age-of-onset of T2D (p<0.0001) and longer duration of T2D (p<0.0001) in medicated individuals was associated with lower 25(OH)D levels when compared to the non-medicated group. Furthermore, significantly higher fasting glucose in treated participants suggests uncontrolled T2D. To further assess the impact of medication and disease status on 25(OH)D levels, we stratified the data to compare non-medicated healthy participants (normoglycemic and non-hypertensive, n=434) with diseased participants (n=1331). Mean levels of fasting glucose and BMI were significantly lower whereas C-peptide and amylin were significantly higher in individuals classified as having sufficient serum 25(OH)D levels regardless of disease status (Figure 3).
Figure 3.
Impact of vitamin D deficiency (<50nmol/L) on non-medicated healthy controls and diabetic patients.
Conclusions
In this investigation, we report a very high prevalence of vitamin D deficiency in this Punjabi diabetic cohort from Northern India. The inverse association of serum 25(OH)D with T2D and related metabolic traits complements previous reports [6] and the proposed actions of vitamin D deficiency-mediated decrease in insulin secretion and sensitivity [22]. Consistent with reports from other epidemiological studies, we also observed a significant association of low serum 25(OH) D with obesity [23,24] and increased systolic blood pressure [25]. Due to our case-control study design, we cannot determine evidence for a causal relationship between vitamin D deficiency and declining cardio-metabolic traits related to T2D; however, low serum 25(OH)D in healthy, non-medicated controls was significantly associated with increased fasting glucose and BMI as well as decreased C-peptide and amylin (Figure 3). These results suggest vitamin D deficiency may increase susceptibility to T2D and therefore provide rationale for investigating preventive measures, such as improving vitamin D intake, as part of an overall strategy to improve glycemic outcomes in the Punjabi community.
Our findings of a significant negative association of fasting glucose as well as a significant positive association of HOMA-B with serum 25(OH)D add to the mounting evidence that proposes vitamin D plays a critical role in insulin secretion [26,27]. The biologically active form of vitamin D [1,25–(OH)2D] may directly stimulate insulin release by binding to the vitamin D receptors of β-cells, thus influencing pathways such as calcium-mediated insulin secretion or by affecting other genes associated with β-cell metabolism and development. While we did not observe a significant association of insulin resistance, assessed by HOMA-IR, with serum 25(OH)D, studies suggest that vitamin D may directly enhance insulin response of peripheral tissues by stimulating expression of insulin receptors. In addition to these direct actions, vitamin D may indirectly regulate calcium homeostasis in β-cells and insulin-responsive tissues [22]. Since calcium is critical for skeletal muscle function [28], increased vitamin D status may improve muscle function [29] thus increasing insulin sensitivity.
One of the strengths of our study is the assessment of serum biomarkers related to β-cell function, such as C-peptide and amylin with 25(OH)D. C-peptide, secreted in equimolar concentration to insulin, is widely considered a better marker of residual β-cell function than insulin due to its longer half-life [30]. In addition to C-peptide, amylin, a glucoregulatory hormone, is co-secreted with insulin in response to food intake to complement insulin-dependent maintenance of postprandial glucose homeostasis [31]. Interestingly, the negative association of insulin, C-peptide, and amylin with 25(OH)D was only seen in females. The higher prevalence of females with T2D in South Asian populations, in part accounted for by previous pregnancies with gestational diabetes [32], may influence the observed association in females but not in males. Another plausible explanation for the gender-specific associations found in this population is the restricted use of alcohol. Due to cultural and religious reasons, a vast majority of Punjabi females do not consume alcohol. Alcohol has been shown to increase systolic blood pressure [33,34] which could account for increased systolic blood pressure and pulse pressure observed in males. However, adjusting for the confounding effect of alcohol with systolic blood pressure did not diminish the significant association observed with 25(OH)D in males, indicating that alcohol could be a compounding factor in addition to other factors.
Our study supports the decreased bioavailability of serum vitamin D observed in obese individuals from previous studies [23,24]. Wortsman et al. [24] found obesity did not affect the skin’s ability to produce vitamin D, but may have altered the release of vitamin D into the circulation from adipose stores due to increased subcutaneous fat. As a fat soluble molecule, decreased 25(OH)D levels among obese individuals may be due to enhanced uptake in adipose tissue and metabolic clearance [23]. We did not measure parathyroid hormone, a limitation in our study since hyperparathyroidism, secondary to hypovitaminosis D is augmented by obesity [35] and could be responsible for gender-specific associations. Additionally, vitamin D deficiency is proposed to increase cardiovascular disease through secondary increase in parathyroid hormone which activates the renin-angiotensin system and stimulates systemic and vascular inflammation [36]. Further limitations of our study include the case-control study design and lack of nutritional status of participants as vitamin D levels are influenced by dietary factors. We also did not adjust for seasonal changes which may or may not influence this population since India is below 35°N latitude making it possible to synthesize vitamin D in the skin throughout the year [37]. However, strength of our study includes a relatively large and well characterized cohort from a relatively homogenous population available with demographic, clinical, and metabolic traits.
Cardiovascular disease remains the leading cause of death among individuals with T2D. A growing body of evidence is emerging that may implicate vitamin D deficiency as an important risk factor for hypertension. Our study reports a significant increase in systolic blood pressure and pulse pressure in vitamin D deficient males. One proposed mechanism has linked vitamin D deficiency to cardiovascular disease in T2D patients. Oh et al. [38] found that the active form of vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with T2D. In addition, a direct protective effect of vitamin D on the endothelium is possible since vitamin D deficiency has been associated with impaired endothelial function [39].
Based on the data, it is reasonable to conclude that vitamin D deficiency may increase development of risk factors that could lead to T2D and cardiovascular disease. Furthermore, overwhelming evidence exists to suggest decreased 25(OH)D levels seem to increase the severity of T2D. This could be the reason that individuals with low vitamin D in our cohort showed poor response to diabetic treatment (Supplemental Table 2). Since the Punjabi are known to be susceptible to the metabolic syndrome, T2D, and early cardiovascular disease, vitamin D deficiency may put them at additional risk, making a strong case for early intervention. Well-designed observational studies are needed to assess the use of vitamin D supplementation as a preventative agent.
Acknowledgments
This work was partly supported by NIH grants-R01DK082766 funded by the National Institute of Diabetes and Digestive and Kidney Diseases and NOT-HG-11-009 funded by National Human Genome Research Institute (NHGRI), USA. We thank the participants and research staff, and the Phen X Rising Consortium (NHGRI) who made the study possible.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Author Contribution
Conceived and designed the experiments: DKS. Performed the experiments: TRB, LFB. Analyzed the data: TRB, LFB. Contributed reagents/materials/analysis tools: DKS. Wrote the paper: TRB, PRB, DKS. Guarantors: TRB and DKS.
References
- 1.Hollick MF, Chen TC. Vitamin D deficiency a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:10805–10868. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010;10:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaney RP. Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr. 2003;78:912–919. doi: 10.1093/ajcn/78.5.912. [DOI] [PubMed] [Google Scholar]
- 4.Davis CD. Vitamin D and cancer: current dilemmas and future research needs. Am J Clin Nutr. 2008;88:565S–569S. doi: 10.1093/ajcn/88.2.565S. [DOI] [PubMed] [Google Scholar]
- 5.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, et al. VitaminD Deficiency and Supplementation and Relation to Cardiovascular Health. Am J Cardiol. 2011;109:359–363. doi: 10.1016/j.amjcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Renzaho A, Halliday JA, Nowson C. Vitamin D, obesity, and obesity-related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27:868–879. doi: 10.1016/j.nut.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Lo CW, Paris PW, Holick MF. Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. Am J Clin Nutr. 1986;44:683–685. doi: 10.1093/ajcn/44.5.683. [DOI] [PubMed] [Google Scholar]
- 10.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 11.International Diabetes Federation. IDF Diabetes Atlas. (5thedn) Brussels, Belgium: International Diabetes Federation; 2011. [PubMed] [Google Scholar]
- 12.Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20:482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Harinarayan CV, Ramalakshmi T, Venkataprasad U. High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pac J Clin Nutr. 2004;13:359–364. [PubMed] [Google Scholar]
- 14.Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Medical Genetics. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanghera DK, Been LF, Ralhan S, Wander GS, Mehra NK, et al. Genome-Wide Linkage Scan to Identify Loci Associated with Type 2 Diabetes and Blood Lipid Phenotypes in the Sikh Diabetes Study. PLoS One. 2011;6:e21188. doi: 10.1371/journal.pone.0021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun TR, Been LF, Singhal A, Worsham J, Ralhan S, et al. A Replication Study of GWAS-Derived Lipid Genes in Asian Indians: The Chromosomal Region 11q23.3 Harbors Loci Contributing to Triglycerides. PLoS One. 2012;7:e37056. doi: 10.1371/journal.pone.0037056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanghera DK, Bhatti JS, Bhatti GK, Ralhan SK, Wander GS, et al. The Khatri Sikh Diabetes Study (SDS): study design, methodology, sample collection, and initial results. Hum Biol. 2006;78:43–63. doi: 10.1353/hub.2006.0027. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Been LF, Ralhan S, Wander GS, Mehra NK, Singh J, et al. Variants in KCNQ1 increase type II diabetes susceptibility in South Asians: a study of 3,310 subjects from India and the US. BMC Med Genet. 2011;24:18–24. doi: 10.1186/1471-2350-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross CA, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D Washington. Washington D.C.: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 22.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. Review: The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 24.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya A, Forman JP. Vitamin D and hypertension: current evidence and future directions. Hypertension. 2010;56:774–779. doi: 10.1161/HYPERTENSIONAHA.109.140160. [DOI] [PubMed] [Google Scholar]
- 26.Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokineinduced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 27.Chui KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 28.Berchtold M, Brinkmeirer H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182–190. doi: 10.1111/j.1600-0838.2009.01016.x. ( Link http://www.ncbi.nlm.nih.gov/pubmed/19807897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32:301–305. doi: 10.2337/dc08-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young AA. mylin’s physiology and its role in diabetes. A Curr Opin Endocrinol Diabetes. 1997;4:282–290. [Google Scholar]
- 32.Girgis CM, Gunton JE, Cheung NW. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: a prospective study and review of the literature. ISNR Endocrinol. 2012 doi: 10.5402/2012/341638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin X, He J, Frontini MG, Ogden LG, Motsamai OL, et al. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 34.McFadden CB, Brensinger CM, Berlin JA, Townsend RR. Systematic review of the effect of daily alcohol intake on blood pressure. Am J of Hypertens. 2005;18:276–286. doi: 10.1016/j.amjhyper.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, et al. The relationship between obesity and serum 1,25-Dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 37.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 38.Oh J, Weng S, Felton SK, Bhandare S, Riek A, et al. 1,25(OH)2 Vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ertek S, Akgül E, Cicero AF, Kütük U, Demirtaş S, et al. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch Med Sci. 2012;8:47–52. doi: 10.5114/aoms.2012.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]