Abstract
Insulin and glucose may influence cancer mortality via their proliferative and anti-apoptotic properties. Using longitudinal data from the nationally representative Third National Health and Nutrition Examination Survey (NHANES III;1988–1994), with an average follow-up of 8.5y to mortality, we evaluated markers of glucose and insulin concentrations, with cancer mortality, ascertained using death certificates using the National Death Index. Plasma glucose, insulin, C-peptide, and lipid concentrations were measured. Anthropometrics, lifestyle, medical and demographic information was obtained during in-person interviews. After adjusting for age, race, sex, smoking status, physical activity and body mass index, for every increase in 50 mg/dl of plasma glucose, there was a 22% increased risk of overall cancer mortality. Insulin resistance was associated with a 41% (95% confidence interval (CI)(1.07–1.87;p=0.01) increased risk of overall cancer mortality. These associations were stronger after excluding lung cancer deaths for insulin resistant individuals (HR:1.67; 95% CI:1.15–2.42;p=0.01), specifically among those with lower levels of physical activity (HR:2.06; 95% CI:1.4–3.0;p=0.0001). Similar associations were observed for other blood markers of glucose and insulin, albeit not statistically significant. In conclusions, hyperglycemia and insulin resistance may be ‘high-risk’ conditions for cancer mortality. Managing these conditions may be effective cancer control tools.
Keywords: cancer mortality, insulin, glucose control, epidemiology, longitudinal study
INTRODUCTION
Cancer is the leading cause of death in individuals under the age of 85 years [1, 2] and overall, the second leading cause of death in the US, responsible for 1 in 4 deaths [3]. It has been hypothesized that insulin resistance (characterized by hyperinsulinemia) and hyperglycemia may play a role in tumor growth by providing a conducive environment for growth of cancer cells [4]. Hyperinsulinemia and hyperglycemia result from dysregulation of insulin action at the cellular level and insulin secretion [5]. A major risk factor of this metabolic dysregulation is excess body weight [6], which has reached epidemic proportions in the US [7, 8]. It is noteworthy that excess adiposity accounts for approximately a quarter to one-third of cancers of the colon, breast, endometrium, kidney and adenocarcinomas of the esophagus [9]. There is robust evidence from a large, national prospective study (n=90,000) that obesity is associated with higher overall cancer and site-specific cancer mortality [10]. The authors estimated that a body mass index ≥25 contributed to about 90,000 cancer-related deaths in the U.S., annually. However, this study did not have the ability to investigate the potential underlying mechanisms that link obesity and cancer mortality due to the unavailability of laboratory data.
At first glance, hyperinsulinemia and hyperglycemia seem to be unrelated to cancer. However, some previous studies have evaluated diabetes or insulin resistance in relation with development of cancers. Taken together, these studies have generally found detrimental associations [11–15], and provide an impetus to further investigate these associations in human studies. Given that cancer is a major cause of death in the US [1, 2], it is important to evaluate the impact of insulin and glucose on the late effects of cancer, in addition to its development.
Previous studies that address markers of glucose or insulin metabolism, in relation with cancer morality did not have the ability to evaluate the confluence of multiple biomarkers and lifestyle [15–17] or relied on self-reported diabetes [18, 19]. Additionally, studies that utilized multiple biomarkers examined single cancer sites, specifically breast or colon cancer [20–22]. Furthermore, a recent large study assessed cancer mortality within men but not women [23]. Overall, the relationships of insulin resistance and diabetes as well as the insulin resistance syndrome (a constellation of related biochemical abnormalities, also known as the metabolic syndrome) in relation with cancer mortality are not well studied. With the recent increase in cancer prevalence and escalating rates of obesity, diabetes and insulin resistance, studies examining the interrelationships of these conditions are required to ascertain whether managing these conditions along with lifestyle changes may serve as a tool for effective prevention of disease-specific death, in the very large at-risk population of cancer patients.
In the present study, we evaluated the longitudinal associations of obesity, hyperglycemia, measures of insulin resistance and the insulin resistance syndrome in relation with overall cancer mortality, using data from the nationally representative National Health and Nutrition Examination Survey III (NHANES III) (1988–1994). We re-evaluated these relationships after excluding deaths from lung cancer (which is not associated with obesity). The availability of comprehensive data on medical history, diet, blood analyses, anthropometric measurements, and linkage with longitudinally ascertained mortality status of the participants, made it possible to investigate these associations. Specifically, the presence of data on blood biomarkers related with insulin and glucose concentrations provide a unique opportunity to study potential mechanisms underlying cancer mortality. Additionally, self-reports of physical activity, an important risk factor of cancer, allowed us to explore how the relationships of cancer mortality and insulin resistance syndrome vary with this lifestyle behavior.
METHODS
Study population
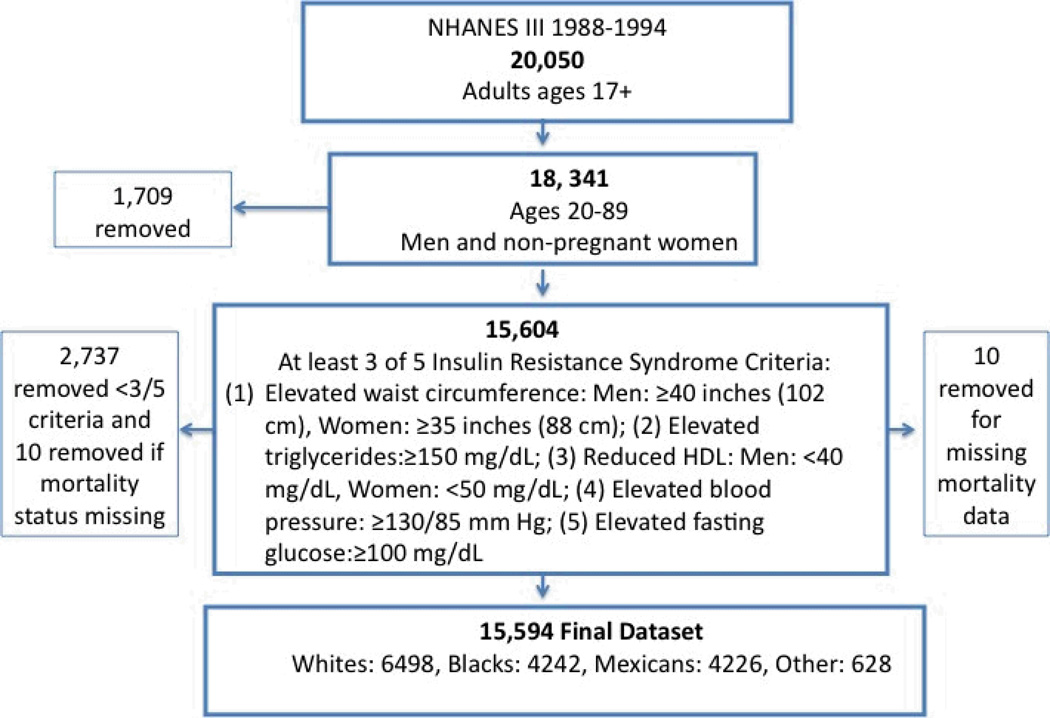
NHANES III, a nationally representative sample of the civilian non-institutionalized individuals, conducted by the National Center for Health Statistics of the Centers (NCHS) for Disease Control and Prevention in 1988 to 1994, was designed to provide detailed information on health and nutrition status of Americans. The NHANES III sample was selected through a complex, multistage probability design that allows results to be extrapolated to the entire U.S. population [24]. NHANES III participants 17+ years were eligible for mortality follow-up from the date of participation (1988–1994) through December 31, 2000. The present research included subjects aged 20 to 89 years, having sufficient data for at least 3 of 5 criteria for the insulin resistance syndrome. Individuals 20 years or older were considered young adults per the NCEP-ATP-III definition [25]. Pregnant women were excluded to avoid misclassification of body mass index (BMI) or insulin resistance syndrome. Subjects older than 89 years were excluded due to arbitrary assignment of a reported age of 90 years for confidentiality, which could result in inaccurate statistical adjustment for age in this group. The final analyses dataset consists of 15,594 individuals (Figure 1).
FIGURE 1.
Final analysis dataset for NHANES III (1988-94) participants
Data collection
The baseline survey consisted of a structured household interview and a standardized physical examination in a mobile examination center (MEC). Participant age, education level, race, alcohol use, current prescription medication and drug use including oral hypoglycemic agents and aspirin consumption, physical activity level, presence of doctor-diagnosed medical conditions including hypertension, were self-reported in a structured personal interview. Race-ethnic groups were categorized into four major ethnic groups: non-Hispanic whites, non-Hispanic Blacks, Mexican-Americans and “other”. The “other” group included Aleut, Eskimo, or American Indian, Asian or Pacific Islander. These ethnic groups were defined based on combinations of the reported race and ethnicity responses of survey participants. History of diabetes was ascertained based on self-reported doctor-diagnosed diabetes and from questions querying insulin use or use of hypoglycemic agents. Blood pressure was measured by a physician, height, weight and waist circumference were measured by trained personnel [24]. Body mass index was calculated from height and weight. Smoking status was computed as never, past and current using self-reported responses during the in-person MEC interview to questions regarding use of cigarettes, pipes and cigars, for the purpose of these analyses. The Household Adult Questionnaire queried 17 leisure time physical activities including type of activity and frequency in the past month. Estimated physical activity levels were obtained by multiplying each activity by its corresponding intensity value in metabolic equivalents (METS). These products were summed in order to produce an intensity-weighted frequency value for each participant for the current study.
During the physical examination, blood was obtained by venipuncture for all survey participants. The first venipunctures were done to collect fasting samples, with a median fasting time of approximately 11 hours. Blood lipids were measured using a Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics). Fasting plasma glucose concentrations were measured using a modified hexokinase enzymatic method (Roche Cobas Mira). The oral glucose tolerance test (OGTT) was conducted on examinees between the ages of 40–74 years who did not use exogenous insulin. The OGTT protocol included two timed venipunctures and a glucose drink. After the first venipuncture, the examinee drank the glucose drink, and a second venipuncture was performed approximately two hours later. Insulin was analyzed using a radioimmunoassay, for adults 20+ years and C-peptide levels were obtained using the C-peptide radioimmunoassay (RIA) (Berthold Multi-Crystal Gamma Counter, Berthold, Nashua, NH). Between 1991–1994, the procedure was modified to add tests for C-peptide and insulin on specimens from the second venipuncture. Examinees reporting a medical history of diabetes without the use insulin therapy were eligible for the OGTT if their fasting values were normal. Glycosylated hemoglobin was measured using the Diamat Analyzer System (Bio-Rad Laboratories, Hercules, CA). Insulin-like growth factor-1 (IGF-1) concentrations were obtained only among participants who underwent a morning examination using the IGF-I ELISA assay (Webster, Texas). Details of the NHANES III protocols have been published [26].
Exposure definitions
1. Blood glucose
Three biomarkers of blood glucose metabolism were used: 1) fasting plasma glucose, 2) non-fasting plasma glucose following the OGTT, both of which are short term indicators of blood glucose concentrations, and 3) Glycosylated hemoglobin, which is a long-term indicator of blood glucose concentrations, over ~3–4 months. A value of glycosylated hemoglobin below 6.0% is considered normal and above 7% indicates poor glycemic control.
2. Insulin
Four biomarkers of insulin metabolism were used: 1) Insulin resistance: Per the Adult Treatment Panel (ATP) III, clinical diagnosis of insulin resistance is defined as elevated fasting glucose >110 mg/dl [25]. For the purpose of these analyses individuals were also considered to be insulin resistant if they had self-reported current use of insulin or consumed oral hypoglycemic agents, which are insulin secretoguoues as previously published [27].
2) Homeostatic Model Assessment (HOMA-IR): HOMA-IR is a technique which dually measures β-cell function and insulin resistance from basal glucose and insulin or C-peptide concentrations [28]. HOMA-IR was calculated using following equation: (fasting glucose (mg/dl) × fasting insulin (µU/ml))/ 405). This technique compensates for variations in basal glucose levels [29]. A value of ≥2.8 better predicts the developing of diabetes based on a previous study [30].
3) Serum Insulin: Serum insulin is another potential marker for the measure of insulin resistance with normal fasting levels ranging from 6 – 27 µU/ml [31].
4) C-peptide: C-peptide, a product of the enzymatic cleavage of pro-insulin, is a stable biomarker pancreatic β-cell secretory activity, with a half-life 2–5 times longer than insulin. Therefore, in this study fasting and postprandial C-peptide concentrations were utilized in addition to serum insulin concentrations to assess insulin resistance. The normal reference range for C-peptide levels is 0.24 – 0.72 pmol/ml [32].
3. Insulin resistance syndrome
Data from the NHANES III adult questionnaires, MEC examination, and laboratory analysis were used to ascertain the presence of the insulin resistance syndrome. Insulin resistance syndrome, as detailed in the ATP-III report [25], was defined by the presence of 3 or more, of the five criteria: 1) insulin resistance (fasting glucose ≥110 mg/dl), 2) hypertension (systolic blood pressure >130 mm Hg or diastolic blood pressure >85 mm Hg) 3) hypertriglyceridemia (triglycerides >150 mg/dL), 4) low high-density cholesterol levels (<40 mg/dL in men or <50 mg/dL in women), and 5) abdominal obesity (waist circumference >102 cm in men or >88 cm in women). For the purpose of these analyses, the ATP III definition was modified to classify individuals as insulin resistant if they self-reported current use of insulin or consumed oral hypoglycemic agents and were considered to be hypertensive if they reported use of anti-hypertensive drugs, as previously published [27].
4. Body mass index
Body mass index was used as a continuous variable in the analysis. We repeated analyses after categorizing BMI according to the World Health Organization definitions with overweight defined as a BMI between 25.0 –29.9 kg/m2 and obesity defined as ≥30 kg/m2 [33].
Outcome
Cancer mortality ascertainment
Mortality information was based upon either 1) receipt of death certificates or 2) the results from a probabilistic match between NHANES III and the NCHS, National Death Index (NDI) records. The median length of follow-up was 8.55 years. The International Classification of Diseases, Tenth Revision (ICD-10; NCHS 2006) was used to identify deaths due to malignant neoplasm (ICD-10 codes C00–C97). The source of mortality data was the NDI. Persons who were not matched to a death record were considered alive at the end of the follow-up period. Additionally, data on the age and date of death of individual was available.
Statistical methods
First, descriptive statistics were generated to evaluate risk factors, lifestyle and dietary attributes related with cancer mortality, and to identify potential confounders. Next, we used Cox proportional hazard regression analysis, using age as the time scale to compute the crude univariate relative hazard and 95% confidence intervals, of overall cancer mortality for the following exposures: plasma glucose (mg/dl; continuous), glycosylated hemoglobin (%, continuous) insulin resistance (yes/no), C-peptide (pmol/ml, continuous); insulin resistance syndrome (yes/no) and BMI (kg/m2; continuous). Follow-up for those who died from causes other than cancer or those who were considered alive were censored at the age of death.
Confounders were defined as variables that changed the crude, age-adjusted hazard ratios for cancer mortality by 10% or more when entered singly into the regression models. Potential variables tested were age (continuous; years), race or ethnicity (categorical; white, black, Mexican, other), height (continuous; cm), cardiovascular disease (categorical yes/no), smoking (categorical; never, current, past), alcohol consumption (continuous; number of drinks) and education (categorical; <12 and ≥12 years), cancer status at baseline (yes/no), use of oral hypoglycemic agents (yes/no) and aspirin use (yes/no). Variables identified as confounders, were added to the final multivariable Cox proportional regression models. Additionally, we also adjusted for variables shown to be associated with cancer mortality in previous literature [10, 34]. We tested for potential interactions of race, age and gender for the relationships of insulin resistance and cancer mortality. The level of significance was set a priori at p=0.10 to consider an interaction significant. A significance level of 0.05 was used for all the other tests. We re-analyzed the associations of all the exposures with cancer mortality after excluding lung cancer deaths, because lung cancer is not associated with obesity. All analyses were performed using SAS version 9.1 and SUDAAN version 10.
RESULTS
Participant characteristics
The baseline characteristics of the NHANES III are summarized in Table 1, which are expressed as either weighted frequencies or medians. The population had approximately 77% non-Hispanic Whites with a median age of 42 years. Approximately half the population was obese or overweight (BMI>25), and a median waist circumference of 91 cm. Twenty-two percent of the population had insulin resistance syndrome defined per the ATP III criteria, and 11.5% were insulin resistant. In this dataset, n=525 (weighted frequency of 2.4%) of the population had died from cancer at the end of the follow-up period till 2000, with the highest deaths from lung cancer (n=152). A quarter of the patients who had died of cancer reported doctor-diagnosed cancer during their in-person baseline interview.
TABLE 1.
Characteristics of the NHANES 1988–1994 (N=15,594) study participants for select variables1
| % | Mean | Std. Error | Minimum | Median | Maximum | |
|---|---|---|---|---|---|---|
| DEMOGRAPHIC INFORMATION | ||||||
| Non-Hispanic Whites | 76.8 | |||||
| Non-Hispanic Blacks | 10.4 | |||||
| Mexican-Americans | 5.2 | |||||
| Other Races/Ethnicities | 7.8 | |||||
| Sex (men) | 48.7 | |||||
| Age (years) | 45.0 | 0.14 | 20.0 | 42.0 | 89.0 | |
| ANTHROPOMETRIC MEASURES | ||||||
| Body mass index (wt(kgs)/ht2(meter) | 26.6 | 0.04 | 11.7 | 25.6 | 79.6 | |
| Waist circumference (cm) | 92.0 | 0.12 | 57.5 | 91.1 | 174.1 | |
| BLOOD ANALYSES | ||||||
| Triglycerides (mg/dl) | 143.7 | 0.98 | 22.0 | 111.0 | 3616.0 | |
| High Density Lipoprotein (mg/dl) | 50.5 | 0.12 | 8.0 | 48.0 | 196.0 | |
| Fasting Serum Insulin (µU/mL) | 11.3 | 0.14 | 1.8 | 8.3 | 2367.0 | |
| Non-Fasting Serum Insulin (µU/mL) | 58.3 | 0.88 | 2.7 | 44.7 | 823.0 | |
| Fasting Glucose (mg/dl) | 99.0 | 0.24 | 35.4 | 93.1 | 642.6 | |
| Non-Fasting Glucose (mg/dl) | 141.5 | 0.865 | 33.7 | 122.9 | 755.1 | |
| Glucose >110 mg/dl | 10.67 | |||||
| Insulin Resistance | 11.5 | |||||
| Insulin Resistance Syndrome | 22.2 | |||||
| Glycosylated Hemoglobin >7% | 6.2 | |||||
| C-Peptide (pmol/ml) | 0.7 | 0.004 | 0.02 | 0.59 | 12.8 | |
| Non-Fasting C-Peptide | 3.3 | 0.025 | 0.02 | 3.1 | 15.4 | |
| Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)2 | 56.9 | 1.78 | 5.3 | 34.2 | 16481.0 | |
| Insulin-Like Growth Factor 1(IGF-1) | 270.3 | 1.35 | 11.2 | 259.3 | 863.8 | |
| Insulin-Like Growth Factor Binding Protein 3 (IGFBP3) | 4447.9 | 12.5 | 969.0 | 4462.0 | 9835.0 | |
| CANCER MORTALITY | % (N)3 | |||||
| Overall | 2.4 (525) | |||||
| Breast Cancer | 1.5 (30) | |||||
| Colon Cancer | 2.2 (66) | |||||
| Lung Cancer | 9.0 (152) | |||||
| Prostate Cancer | 1.5 (46) | |||||
| Cancer Mortality from Female | 2.5 (47) | |||||
| Cancers4 | ||||||
| Individuals with self-reported doctor-diagnosed history of cancer at baseline, who died of cancer | 24.9 | |||||
Data is presented as weighted frequencies (percentages) for categorical variables and as weighted means (SE), median, minimum and maximum values for continuous variables
HOMA-IR was calculated using following equation: (Fasting glucose (mg/dl) × Fasting insulin (µU/ml))/ 405. This technique compensates for variations in basal glucose levels [29]. A value of ≥ 2.8 better predicts the developing of diabetes and correlates with the hyperglycemic-hyperinsulinemic clamp method, is considered to be abnormal, based on a previous study [30].
N represent actual unweighted number of cancer mortality cases in the analyses dataset
Female cancers include breast, ovarian, endometrial and cervical cancer
We evaluated correlations between anthropometric measurements (BMI and waist circumference) and the blood biomarkers, which are described in Table 1. In summary, all the biomarkers were positively and significantly correlated with the anthropometric measures and the coefficients ranged between 0.12 and 0.38 for both BMI and waist circumference. The correlation coefficient for fasting C-peptide with both the anthropometric measurements was 0.51–0.56. We observed a negative correlation between physical activity with both anthropometric measurements (r=0.1 for BMI and waist circumference).
Associations of markers of insulin and glucose concentrations, and BMI with cancer mortality
1. Blood glucose
Fasting glucose
The associations of plasma glucose concentrations and cancer mortality were evaluated by treating glucose as a continuous variable in the Cox proportional regression models. For every increase in 50 mg/dl in fasting plasma glucose concentrations, the hazard ratio for mortality from all cancers combined increased by 22% after adjusting for age and 21% after adjusting for age, race, and physical activity, smoking status, sex and body mass index (HR:1.22; 95% CI: 1.06– 1.39). The increase in risk for cancer mortality persisted after excluding lung cancer deaths (HR:1.25; 95% CI: 1.11–1.41). The associations of plasma glucose and cancer mortality were re-evaluated after excluding individuals who achieved glycemic control by using oral hypoglycemic agents or exogenous insulin (n=949). The HRs for overall cancer mortality were greater than one (HR:1.05; 95 CI: 0.84–1.31). The associations were stronger after excluding lung cancer deaths (HR:1.25 95% CI 1.03–1.52) (Table 2).
Table 2.
Hazard Ratio (HR) (95% CI) for measures of glucose and insulin control, and body mass index in relation with cancer mortality in NHANES III (1988–1994) participants 20–89 years (N=15,594)
| N (cancer mortality) |
Hazard Ratio (95% CI) for overall cancer mortality |
N (cancer mortality) |
Hazard Ratio (95% CI) for all cancers excluding lung cancer |
|
|---|---|---|---|---|
| Fasting Glucose (mg/dl) per 50 Units (mg/dl)1 | ||||
| Age-Adjusted | 532 | 1.22 (1.06 – 1.42) | 372 | 1.24 (1.10 – 1.41) |
| Adjusted Model2 | 459 | 1.22 (1.06 – 1.39) | 330 | 1.25 (1.11– 1.41) |
| After Excluding Oral Hypoglycemic Agent/ Insulin (OHA) (N=14618) | ||||
| Age-adjusted | 468 | 1.08 (0.88–1.32) | 334 | 1.27 (1.07 – 1.53) |
| Adjusted Model2 | 409 | 1.05 (0.84 – 1.32) | 293 | 1.25 (1.03 – 1.52) |
| Non-Fasting Glucose (mg/dl) per 50 mg/dl1 (N=6636) | ||||
| Age-Adjusted | 263 | 1.09 (0.96 – 1.17) | 174 | 1.15 (1.02 – 1.22) |
| Adjusted Model2 | 231 | 1.18 (1.01 – 1.19) | 154 | 1.21 (1.03 – 1.26) |
| After Excluding Oral Hypoglycemic Agent/ Insulin (OHA) (N=14618) | ||||
| Age-adjusted | 250 | 1.05 (0.94 – 1.18) | 165 | 1.13 (1.01 – 1.27) |
| Adjusted Model2 | 220 | 1.11 (1.0 −1.21) | 145 | 1.16 (1.03 – 1.30) |
| Glycosylated Hemoglobin per 2%3 | ||||
| Age-Adjusted | 520 | 1.22 (0.97 – 1.53) | 370 | 1.13 (0.89 – 1.44) |
| Adjusted Model6 | 458 | 1.22 (0.96– 1.55) | 329 | 1.19 (0.94 – 1.51) |
| After Excluding Oral Hypoglycemic Agent/ Insulin (OHA) (N=14618) | ||||
| Age-adjusted | 466 | 1.04 (0.8 – 1.36) | 332 | 1.16 (0.83 – 1.62) |
| Adjusted Model6 | 408 | 1.02 (0.88 – 1.34) | 292 | 1.20 (0.86 – 1.66) |
| Insulin Resistance (yes/no)4 | 2541 11.5 | |||
| Age-Adjusted | 524 | 1.45 (1.11 – 1.89) | 373 | 1.74 (1.24 – 2.43) |
| Adjusted Model2 | 461 | 1.41 (1.07 – 1.87) | 331 | 1.67 (1.15 – 2.42) |
| After Excluding Oral Hypoglycemic Agent/ Insulin (OHA) (N=14,618) | ||||
| Age-adjusted | 469 | 1.31 (0.96 – 1.80) | 334 | 1.80 (1.24 – 2.62) |
| Adjusted Model2 | 410 | 1.26 (0.90 – 1.76) | 293 | 1.71 (1.12 – 2.61) |
| HOMA-IR per 5 Units | ||||
| Age-Adjusted | 578 | 1.04 (0.96 – 1.13) | 368 | 1.04 (0.96 – 1.13) |
| Adjusted Model2 | 455 | 1.06 (0.96 – 1.13) | 326 | 1.05 (0.98 – 1.14) |
| Fasting Insulin (µU/mL)5 | ||||
| Age-Adjusted | 518 | 0.99 (0.99 – 1.01) | 368 | 1.00 0.96 – .03) |
| Adjusted Model2 | 455 | 1.00 (0.97 – 1.03) | 326 | 1.01 (0.97 – 1.04) |
| Non-Fasting Insulin (µU/mL) (N=3372) | ||||
| Age-Adjusted | 105 | 1.00 (0.99–1.01) | 71 | 1.00 (1.0–1.01) |
| Adjusted Model2 | 95 | 1.00 (0.99–1.01) | 64 | 1.01 (1.0 – 1.01) |
| C-peptide per 1 Unit (pmol/ml) | ||||
| Age-Adjusted | 464 | 1.09 (0.92 – 1.29) | 330 | 1.18 (0.99 – 1.41) |
| Adjusted Model2 | 405 | 1.05 (0.87 – 1.28) | 289 | 1.15 (0.94 – 1.41) |
| Non-Fasting C-Peptide (pmol/ml) (N=3360) | ||||
| Age-Adjusted | 103 | 0.96 (0.76 – 1.21) | 69 | 1.10 (0.81 – 1.5) |
| Adjusted Model2 | 93 | 0.99 (0.76 – 1.28) | 62 | 1.11 (0.77 – 1.6) |
| Insulin Resistance Syndrome (yes versus no) | ||||
| Frequency of Insulin Resistance syndrome (yes) | ||||
| Age-Adjusted | 524 | 1.04 (0.77 – 1.41) | 373 | 1.16 (0.85 – 1.58) |
| Adjusted Model6 | 461 | 1.01 (0.72 – 1.42) | 331 | 1.16 (0.8 – 1.67) |
| Body Mass Index per 5 Units (kg/m2)7 | ||||
| Age-Adjusted | 524 | 0.93 (0.83 – 1.04) | 373 | 1.01 (0.89 – 1.14) |
| Adjusted Model6 | 461 | 0.92 (0.80 – 1.05) | 331 | 0.96 (0.83 – 1.11) |
Analyses were restricted to those individuals with values between 50 – 400 mg/dl
Adjusted for age, race, sex, smoking status, physical activity and body mass index
Analyses were restricted to those individuals with values <14%
Insulin resistance was defined as blood glucose >110 mg/dl and includes individuals who consumed oral hypoglycemic agents or insulin users
Insulin levels were restricted to those individuals with values <32 µU/ml
Adjusted for age, race, sex, smoking status, physical activity
BMI was restricted to those individuals with values < 50
In order to assess whether the observed detrimental associations persisted among individuals without a cancer diagnosis, we excluded persons with a self-reported history of cancer at baseline (n=588) and re-analyzed these relationships. The direction of the associations remained unchanged among individuals with no history of cancer, after adjusting for age, sex, smoking status, physical activity and body mass index, in the whole population (HR:1.21; 95% CI:1.04–2.74), and after excluding lung cancer deaths (HR:1.28; 95% CI:1.12–1.47). Additionally, we evaluated these associations among people with longer durations of follow-up (9–12 years) from baseline to mortality (n=7,552). We noted that the detrimental direction of the associations between fasting glucose and cancer mortality persisted among individuals with a longer follow-up, after adjusting for covariates (HR: 1.15; 95% CI:0.97–1.37). Furthermore, the associations were stronger after exclusion of lung cancer deaths from the analyses (HR: 1.31; 95% CI:1.11–1.55).
Non-fasting glucose
Because previous studies have suggested that non-fasting versus fasting glucose concentrations have better predictive value [4, 22], the associations of non-fasting plasma glucose concentrations in relation with cancer mortality were also evaluated in the present study. The associations observed for non-fasting glucose were similar to those observed with fasting plasma glucose, with hazard ratios greater than one for all cancers combined (HR:1.15; 95% CI: 1.01–1.19) and after excluding lung cancer (HR:1.21; 95% CI: 1.03–1.26).
Glycosylated hemoglobin
Next, glycosylated hemoglobin, was examined in relation with cancer mortality. Glycosylated hemoglobin concentrations were treated as a continuous variable in the regression model. There was a borderline significant 22% increased risk of dying from cancer for 2% increments in this analyte, after adjusting for age only (HR:1.22; 95% CI: 0.97–1.53) and after adjusting for age, race and physical activity, smoking status, sex and BMI (HR:1.22; 95% CI: 0.96–1.55). The detrimental associations persisted when we repeated the analyses after excluding lung cancer mortality cases (HR:1.19; 95% CI: 0.94–1.51), and after limiting analyses to those who did not use oral hypoglycemic agents or exogenous insulin, although not statistically significant (HR:1.02; 95% CI: 0.88–1.34).
We tested for potential interactions between the associations of all the markers of blood glucose concentrations and cancer mortality. However, none of the observed relationships differed by age or sex, and no other significant interactions were noted.
2. Insulin
We evaluated the associations for four different measures of insulin concentrations in relation with overall cancer mortality and after excluding lung cancer deaths. The age-adjusted risk of dying of all cancers combined was 45% higher among individuals with insulin resistance (HR:1.45; 95% CI: 1.11–1.89 and 41% higher (HR:1.41; 95% CI: 1.07–1.87) after adjusting for age, race and physical activity, smoking status, sex and BMI. These associations were stronger after excluding lung cancer deaths (HR:1.67; 95% CI: 1.15–2.42). Furthermore, in order to remove the potential bias of controlled diabetes, we excluded individuals who were either on oral hypoglycemic agent or insulin treatment (n=949). The associations between insulin resistance and cancer mortality persisted (HR:1.26; 95% CI: 0.90–1.76).
To explore whether modest exposure to hyperinsulinemia influences the risk of cancer deaths, the analyses were repeated using the cutoff as 100 mg/dl of plasma glucose (instead of 110mg/dL), and the risk persisted (HR adjusted for age only: 1.23; 95% CI: 0.95–1.58) and after adjustment of covariates (HR: 1.17 (0.91–1.51). The risk was stronger after excluding lung cancer deaths in the age-adjusted (HR: 1.39 (1.06–1.82) and multivariate models (HR: 1.36 (1.00–1.86). To investigate whether these associations persisted before a cancer diagnosis, we excluded persons who reported having a history of cancer at baseline. Similar to the results for fasting blood glucose concentrations, the detrimental associations persisted among individuals who were free of cancer at baseline (HR:1.27; 95% CI:1.00–1.81) after adjusting for covariates, and after excluding lung cancer deaths (HR:1.60; 95% CI:1.08–2.36). Next, we re-analyzed these relationships among people with longer durations of follow-up (9–12 years) to mortality (n=7,606). We noted that the detrimental effect of the associations between fasting glucose and cancer mortality persisted (HR: 1.18; 95% CI:0.89–1.56) after adjusting for covariates, and were stronger after exclusion of lung cancer deaths from the analyses (HR: 1.80; 95% CI:1.26–2.56).
In this dataset, physical activity was a major risk factor of cancer mortality. Therefore, we explored the relationship of insulin resistance by level of physical activity (above and below median METs). As shown in Table 3, the HR for cancer mortality was significantly higher among individuals with lower physical activity levels (HR:1.60; 95% CI: 1.20–2.12), and these associations were stronger after excluding lung cancer deaths (HR:2.06; 95% CI: 1.41–3.01). It is noteworthy that these associations appeared to be attenuated among individuals with higher physical activity levels, although the effect modification was not statistically significant.
Table 3.
HR (95% CI) Exploring the associations with Insulin resistance among people with high and low physical activity for all cancers combined and after excluding lung cancer
| Insulin Resistance (yes/no) |
LOW PHYSICAL ACTIVITY (Below median METS1) |
HIGH PHYSICAL ACTIVITY (Above median METS1) |
||
|---|---|---|---|---|
| Overall cancer mortality | excluding lung cancer | Overall cancer mortality | excluding lung cancer | |
| Age-Adjusted | 1.63 (1.21 – 2.20) | 2.06 (1.42 – 2.94) | 1.19 (0.75.–1.88) | 1.27 (0.71 – 2.28) |
| Adjusted Model2 | 1.60 (1.20 – 2.12) | 2.06 (1.41 – 3.01) | 1.10 (0.71 – 1.70) | 1.16 (0.66 – 2.06) |
Median for total Metabolic Equivalents (METS) is 45 METS
Adjusted for age, race, sex, smoking status, physical activity and body mass index
Next, we evaluated cancer mortality in relation with HOMA-IR, plasma insulin, and C-peptide and consistent with the direction of association observed for insulin resistance. Although not statistically significant, the HRs for HOMA-IR (HR:1.06; 95% CI: 0.96–1.13) and C-peptide (HR:1.05; 95% CI: 0.87–1.28) were >1. The associations were similar after excluding lung cancer deaths (HR:1.05; 95% CI: 0.98–1.14 and HR:1.15; 95% CI: 0.94–1.41 respectively). No associations were noted between fasting and non-fasting insulin concentrations and cancer mortality (HR:1.00; 95% CI: 0.97–1.03 and HR:1.00; 95% CI: 0.99–1.01). No significant interactions were noted for the associations of all markers of insulin metabolism and cancer mortality.
3. Insulin resistance syndrome
To assess the potential synergistic influence of the obesity, derangements in insulin and glucose metabolism, hypertension and lipid derangements, we evaluated the associations and cancer mortality and the insulin resistance syndrome per the modified ATP III definition. We noted no associations between insulin resistance syndrome and cancer mortality for all cancer sites combined (HR:1.01; 95% CI: 0.72–1.42). Although not statistically significant, the HR for cancer mortality was 16% higher among individuals with the insulin resistance syndrome after excluding lung cancer deaths (HR:1.16; 95% CI: 0.8–1.67). After excluding persons with self-reported history of cancer at baseline (n=588), these associations were unchanged (data not shown).
4. Body mass index
We assessed the associations of cancer mortality and excess adiposity, measured as BMI. Similar to the associations of the insulin resistance syndrome, we noted no associations between BMI (treated as a continuous variable) and mortality from all cancers combined (HR:0.92; 95% CI: 0.80–1.05), even after excluding lung cancer (HR:0.96; 95% CI: 0.83–1.11). These associations were re-analyzed using BMI groups per the WHO classification and no associations were noted between categories of BMI and cancer mortality in the overall population as well as after excluding lung cancer deaths (data not shown). Additionally, the lack of association persisted after removing individuals with self-reported history of cancer at baseline.
DISCUSSION
Our results using data from the nationally representative NHANES III (1988–1994) survey support the hypothesis that disturbances in insulin and glucose metabolism are associated with an increased risk of cancer mortality. These relationships were stronger after excluding individuals who died of lung cancer, which is a particularly important endpoint because lung cancer is not associated with adiposity in the existing literature [10]. The detrimental effect of the associations persisted after excluding individuals who achieved glycemic control pharmacologically and after excluding individuals with a diagnosis of cancer at baseline, and among individuals with a longer follow-up to mortality of approximately 9–12 years. We observed similar non-significant detrimental associations with higher percentages of glycosylated hemoglobin, a marker of long-term glycemic control. Finally, in exploratory analyses among people who reported high and low levels of physically active, we observed that the risk of overall cancer mortality doubled among individuals who did not engage in at least a moderate level of physical activity.
The results of the current study are consistent with the majority of previous studies that have evaluated diabetes status in relation with cancer. For example in a large prospective study in the US (n= 467,922 men and 588,321 women), self-reported diabetes was significantly associated with death from multiple cancers in both men and women with risk estimates ranging from 1.20–2.19 in multivariate analyses [18]. Similarly, a Japanese population-based study (n=15,724) reported that having diabetes was associated with a 88% higher risk of dying from all cancers among women [19]. Two other studies found a higher risk of death from endometrial cancer [35] and prostate cancer [36] among persons with diabetes versus those without diabetes. Additionally, elevated glycosylated hemoglobin concentrations were associated with poorer 5-year survival for colorectal cancer and a 2-fold increased risk of colorectal cancer progression compared to individuals with better glycemic control [16]. It is noteworthy that the majority of these studies [18, 19] did not have the ability to evaluate markers of plasma glucose or insulin concentrations, and relied on self-reported diabetes status.
Not all previous studies reported associations between diabetes and cancer mortality. In a meta-analyses diabetes was not associated with cancer mortality (p=0.69) [37]. However, this study did not evaluate insulin resistance and restricted analyses to diabetes status in existing patients. Similarly, another study did not observe any differences between cancer mortality rates in people with diabetes versus those without diabetes, which were confirmed from medical records [17].
Our report of increased risk of cancer mortality among insulin resistant individuals, adds to the limited existing evidence noting similar detrimental associations. There is robust evidence from two large prospective studies suggesting 50% to a 3-fold increased risk of mortality from colorectal cancer among participants with insulin resistance or associated biochemical abnormalities after adjusting for covariates [20] [22]. In a prospective cohort study evaluating the late effects of cancer among 512 women with breast cancer, insulin resistance was associated with a ~3 fold increased risk of death from the disease after adjusting for tumor and treatment- related variables [21]. Similarly, in a 10-year prospective Korean study (n=1,298,385), individuals with higher versus lower fasting serum glucose concentrations (proxy for insulin resistance) were ~30% more likely to die of cancer (all cancers combined), and these detrimental associations persisted across all body mass index categories, suggesting an independent role of insulin beyond excess body weight [15]. However, one study did not note associations between increased non-fasting insulin concentrations and cancer mortality [13].
Several biological mechanisms might explain the observed relationships between disturbed insulin and glucose metabolism and cancer mortality in the current study. It has been well documented that glucose increases cancer cell growth, with up-regulation of cell proliferation factors and delayed apoptosis [38] [39] [40]. Insulin resistance, characterized by higher circulating levels of insulin, is associated with poor glycemic control. Insulin is an anabolic hormone that may stimulate cell proliferation and cancer progression via multiple pathways by increasing production of IGF-1 via the growth hormone mechanism (reviewed in [41]) making more IGF-1 available to bind to its receptors on normal and cancer cells. This receptor-bound IGF-1 modulates cell proliferation and survival [42]. Normally, insulin stimulates liver uptake of glucose and the storage of glucose as glycogen, or enhances its metabolism via the glycolytic pathway. However, insulin fails to suppress gluconeogenesis in laboratory studies, implying further glucose availability for cellular metabolism of cancer cells [43]. Another factor common to insulin resistance [44, 45], hyperglycemia [46–48] and cancer is inflammation [49, 50]. An increase in pro-inflammatory factors produce a cellular environment, which makes it conducive to oncogenesis, cancer progression and mortality [51–53]. Studies have reported an increased risk of cancer mortality among subjects with joint exposure to inflammation and disturbances in glucose metabolism [47, 48].
Despite the potential biological mechanisms of insulin in cancer biology, associations were either not observed or were not significant for HOMA-IR, fasting C-peptide and fasting insulin concentrations, and overall cancer mortality, or after excluding cases of lung cancer deaths. Previous studies investigating cancer risk or mortality, have shown the limited predictive value of fasting concentrations biomarkers of the insulin-glucose axis, and reported associations that were limited to plasma obtained in a non-fasting state [54] [55]. One study investigating risk of colon cancer observed similar findings for insulin as well as for C-peptide [56], suggesting that postprandial insulin may be a better measure for the association with cancer risk than fasting insulin levels. We speculate that this might be one explanation for the lack of associations observed for some of the markers of insulin metabolism, in this sample. Based on the findings of previous studies that suggest that non-fasting values are more appropriate choices, we also performed analyses in the current study sample using non-fasting insulin and C-peptide values. However, we did not have sufficient power to detect these associations if they did exist, because the measurements of non-fasting insulin and C-peptide were done in a subsample of individuals aged 40–74 among those who participated in the study in the second phase of NHANES III (n=3,747; ~20% of the total sample eligible for this study).
Finally, the insulin resistance syndrome was not associated with cancer mortality. This may be explained by the fact that insulin resistance syndrome comprises of 3–5 components, which may negate the effects of in the independent component, suggesting that the confluence of these factors is not important in cancer deaths. However, non-significant detrimental associations were observed after excluding lung cancer among people with versus without insulin resistance syndrome and warrants further explanation. One previous large study using the Chicago Heart Association Detection Project in Industry population reported a 50% increased risk of colon cancer mortality among participants having the insulin resistance syndrome (≥3 of 5 biochemical abnormalities) [22]. Another large study among men reported a 56% greater risk of death from all cancers combined among individuals with metabolic syndrome [23]. Similarly, an approximate 3-fold increased risk was observed in one study among men and women with metabolic syndrome [20]. However, they speculated that elevated blood glucose might be the component “driving” the associations [20].
Differently from previous studies that report associations between BMI and cancer deaths [10, 12] we did not observe any associations between increased BMI or waist circumference, and overall cancer mortality in the whole population, or after excluding individuals who died of lung cancer. We explored these relationships separately by sex to check for gender differences, however, the associations were null. It is not uncommon to have shifts in body composition among those who develop cancer. Lack of information of BMI status after recruitment at baseline among the cancer patients and the inability to adjust for this may be a potential explanation for the null findings, although we were not able to identify a definitive explanation for these results.
Some weaknesses of this study must be noted. Due to a limited number of cancer deaths, we did not have the ability to evaluate site-specific cancers in relations with markers of insulin and glucose metabolism. Although, cancer deaths ascertained from death certificates agree well with cause of death from autopsies [57], there could be potential misclassification of the underlying cause of death on the death certificates, which were used to ascertain disease-specific mortality. Evidence suggests that this type of misclassification may attenuate the observed relationships [58]. However, studies have been published using NHANES III mortality data [59, 60]. Cancer mortality was ascertained longitudinally in NHANES III, but there was only one measure of the exposures of interest, taken approximately 8.5 years before mortality follow-up, causing potential bias toward the null. This single measurement would likely attenuate any associations as it fails to detect new individuals who may have developed insulin resistance in the follow-up period and does not account for individuals who may have made lifestyle changes to improve their glycemic profile. Finally, the results of this study might be an underestimation of the problem, because the exposure data were collected in 1988–1994 and obesity and diabetes rates have increased in the past two decades [61, 62].
In conclusion, the results from this large longitudinal nationally representative study adds to the existing epidemiologic evidence that hyperglycemia and insulin resistance may increase the risk of cancer morality. With the rising number of cancer survivors in the US and projected doubling by 2050 [3], as well as increasing prevalence of insulin resistance and diabetes, it is time to consider management of these modifiable risk factors, through healthier lifestyles, specifically physical activity as an important cancer control tool. The body of existing evidence and the current study support a need for updated clinical guidelines among the very large at-risk population of cancer patients to prevent disease-specific mortality. The findings of this research supports designing lifestyle intervention trials, aimed at the eventual development of individualized treatment and support the management of disturbances of the glucose-insulin axis and the integration of lifestyle alterations into cancer management.
Acknowledgments
Financial support: This study was supported by the National Institutes of Health Award 1RO3CA132127 and the Cancer Institute of New Jersey Core Grant and Cancer Institute of New Jersey core grant NCI CA-72720-10
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics,2008. CA: a cancer journal for clinicians. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.US Mortality Data. 1960–2004, National Center for Health Statistics. Centers for Disease Control and Prevention. 2006 [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics,2006. CA Cancer J Clin. 2006 Mar-Apr;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995 Mar;6(2):164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension. Parallels between human disease and rodent models. Diabetes Care. 1991 Mar;14(3):195–202. doi: 10.2337/diacare.14.3.195. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004 Aug 23;23(38):6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 7.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults,1999–2002. JAMA. 2004 Jun 16;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States,1999–2004. Jama. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 9.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002 Aug 11;(Suppl 2):S94–S100. [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US. adults. N Engl J Med. 2003 Apr 24;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Komninou D, Ayonote A, Richie JP, Jr, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003 Apr;228(4):396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 12.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis;epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008 Sep;116(Suppl 1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 13.Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003 May;12(5):412–418. [PubMed] [Google Scholar]
- 14.Rinaldi S, Rohrmann S, Jenab M, et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3108–3115. doi: 10.1158/1055-9965.EPI-08-0495. [DOI] [PubMed] [Google Scholar]
- 15.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. Jama. 2005 Jan 12;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci. 2008 Sep;53(9):2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 17.Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 2009;48(3):361–367. doi: 10.1080/02841860802637765. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004 Jun 15;159(12):1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 19.Oba S, Nagata C, Nakamura K, Takatsuka N, Shimizu H. Self-reported diabetes mellitus and risk of mortality from all causes, cardiovascular disease, and cancer in Takayama: a population-based prospective cohort study in Japan. J Epidemiol. 2008;18(5):197–203. doi: 10.2188/jea.JE2008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001 Sep;10(9):937–941. [PubMed] [Google Scholar]
- 21.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002 Jan 1;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 22.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002 Apr;11(4):385–391. [PubMed] [Google Scholar]
- 23.Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009 Feb 26; doi: 10.1016/j.ejca.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center of Health Statistics. Hyattsville, MD: National Center of Health Statistics; 1995. Plan and Operation of the Third National Health and Nutrition Examination Survey,1988–1994. [Google Scholar]
- 25.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III)1988–1994. Hyattsville, MD: US. Department of Health and Human Services, Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 27.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey,1999 to 2004. J Am Coll Surg. 2008 Dec;207(6):928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama H, Emoto M, Fujiwara S, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment are useful indexes of insulin resistance in type 2 diabetic patients with wide range of fasting plasma glucose. J Clin Endocrinol Metab. 2004 Mar;89(3):1481–1484. doi: 10.1210/jc.2003-031374. [DOI] [PubMed] [Google Scholar]
- 30.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008 Winter;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 31.Mack R, Skurnick B, Sterling-Jean Y, Pedra-Nobre M, Bigg D. Fasting insulin levels as a measure of insulin resistance in American blacks. J Med. 2003;34(1–6):31–38. [PubMed] [Google Scholar]
- 32.Bonser AM, Garcia-Webb P. C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci. 1984;19(4):297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- 33.Geneva: World Health Organization; 1995. World Health Organization Physical status: the use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 34.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics,2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 35.Papanas N, Giatromanolaki A, Galazios G, Maltezos E, Sivridis E. Endometrial carcinoma and diabetes revisited. Eur J Gynaecol Oncol. 2006;27(5):505–508. [PubMed] [Google Scholar]
- 36.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005 Dec;41(18):2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008 Dec 17;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009 Jan;121(1):29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Okumura M, Yamamoto M, Sakuma H, et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002 Oct 21;1592(2):107–116. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 40.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001 Sep;21(17):5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001 Nov 131;(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 42.Grossman E, Messerli FH, Boyko V, Goldbourt U. Is there an association between hypertension and cancer mortality? Am J Med. 2002 Apr 15;112(6):479–486. doi: 10.1016/s0002-9343(02)01049-5. [DOI] [PubMed] [Google Scholar]
- 43.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009 Feb;119(2):315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999 Apr;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 45.Choi KM, Ryu OH, Lee KW, et al. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2006 Jul 25; doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ. Association between Circulating White Blood Cell Count and Long-Term Incidence of Age-related Macular Degeneration. Am J Epidemiol. 2006 Nov 16; doi: 10.1093/aje/kwk022. [DOI] [PubMed] [Google Scholar]
- 47.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004 Oct;14(9):686–695. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. Jama. 2004 Feb 4;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 49.Il'yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005 Oct;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 50.Bahia L, Aguiar LG, Villela N, et al. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics. 2006 Oct;61(5):433–440. doi: 10.1590/s1807-59322006000500010. [DOI] [PubMed] [Google Scholar]
- 51.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004 May;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 52.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005 May;115(5):911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 20. [DOI] [PubMed] [Google Scholar]
- 53.Madan AK, Tichansky DS, Coday M, Fain JN. Comparison of IL-8, IL-6 and PGE(2) formation by visceral (omental) adipose tissue of obese Caucasian compared to African-American women. Obes Surg. 2006 Oct;16(10):1342–1350. doi: 10.1381/096089206778663652. [DOI] [PubMed] [Google Scholar]
- 54.Michaud DS, Wolpin B, Giovannucci E, et al. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):2101–2109. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 55.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 56.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 2005 Oct;29(10):1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 57.National Center for Health Statistics 2007 NHANES III Linked Mortality Public-Use File: Detailed Notes for Selected Variables. 2007 [Google Scholar]
- 58.Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981 Mar;71(3):242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008 Aug 11;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007 Sep 1;166(5):518–526. doi: 10.1093/aje/kwm124. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q, Wang Y, Huang ES. Changes in racial/ethnic disparities in the prevalence of Type 2 diabetes by obesity level among US adults. Ethn Health. 2009 Apr 9;:1–19. doi: 10.1080/13557850802699155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007 Nov;45(5):348–352. doi: 10.1016/j.ypmed.2007.07.020. [DOI] [PubMed] [Google Scholar]