Abstract
Stress hormones released in the CNS following exposure to unavoidable, aversive stimuli have been shown to alter the physiology of neurons in multiple brain regions including hippocampus, amygdala, prefrontal cortex, and ventral tegmental area. The nucleus accumbens (NAc), a motor-limbic interface linked to motivation and reward, receives inputs from each of these stress-affected brain regions, raising the possibility that its function might also be altered in response to stress. To assess potential stress-induced plasticity in the NAc, we exposed adult mice to daily cold water forced swim for 2 consecutive days and conducted electrophysiological experiments assessing glutamate receptor function in brain slices taken 18–24 h following the second swim. We found that AMPA receptor (AMPAR)/N-methyl-d-aspartate receptor (NMDAR) ratios, a measure of synaptic strength, were increased in the NAc shell but not core medium spiny neurons (MSNs) in stressed animals relative to controls. This effect was blocked by preadministration of glucocorticoid receptor (GR) antagonist RU486, suggesting that the observed changes are dependent on corticosteroid signaling. The role of corticosterone (CORT) in the observed plasticity was confirmed, because exogenous administration of 10 mg/kg CORT also enhanced AMPAR/NMDAR ratios in the NAc shell. The synaptic changes in NAc shell MSNs reflect an enhancement of AMPAR-mediated currents, as we observed increased AMPAR miniature postsynaptic current (mEPSC) amplitude following stress but no change in NMDAR mEPSCs. We hypothesize that altered information processing via plasticity of excitatory inputs might contribute to reward-related behaviors such as stress-induced reinstatement of drug seeking in animals and relapse in humans.
INTRODUCTION
A key element in the adaptive response to physical or psychological stress is the activation of the hypothalamic-pituitary-adrenal (HPA) axis. Following exposure to aversive or threatening stimuli, a series of hormones are released, beginning with corticotrophin-releasing factor (CRF) from the paraventricular nucleus of the hypothalamus, adrenocorticotropic hormone from the anterior pituitary, and glucocorticoids (GCs) from the adrenal cortex (McEwen et al. 1986). These hormones affect physiology throughout the organism, including the CNS (Sousa et al. 2008).
Stress has profound effects on brain areas associated with conscious memory and executive functions. In the hippocampus, stress can disrupt long-term potentiation (LTP) and hippocampus-dependent learning and memory via glucocorticoid receptor (GR) activation (Kim and Diamond 2002; Kim et al. 2006). In addition to deficits in plasticity and behavior, prolonged stress and GR activation can lead to dendritic and synaptic loss in hippocampal neurons (Sousa et al. 2000). Chronic and prolonged GC elevation also disrupt prefrontal cortex (PFC)-dependent tasks including spatial working memory and behavioral flexibility (Cerqueira et al. 2005; Mizoguchi et al. 2000). Paralleling these findings, chronic stress reduces PFC volume and causes dendritic reorganization in a GR-dependent fashion (Cerqueira et al. 2007a,b). The amygdala (AMG) stands in contrast to other limbic structures, as stress promotes LTP, enhances growth of dendrites, and facilitates fear conditioning (Sapolsky 2003).
There are also profound effects of stress and GCs on the mesoaccumbens dopamine (DA) system, which contributes to motivation and goal-directed behaviors (Piazza and Le Moal 1997). Either stress or increased plasma GC levels elevate DA release from ventral tegmental area (VTA) projections to the NAc (Kalivas and Duffy 1995; Piazza et al. 1996). Conversely, natural rewards,which increase DA levels in the NAc, also elevate plasma levels of GCs (Piazza and Le Moal 1997). Nonnatural reinforcers, such as psychostimulants, nicotine, and alcohol enhance DA and GC release as well (Piazza and Le Moal 1997). Interestingly, stress can affect the locomotor and positive reinforcing effects of psychostimulants (Marinelli and Piazza 2002); both stress and exogenous corticosterone administration enhance psychomotor activation and self-administration of drugs (Deroche et al. 1992; Kalivas and Stewart 1991; Mantsch et al. 1998). The predisposition to drug self-administration has been linked to differences in stress reactivity and GC-dependent differences in nicotinic acetylcholine receptor expression (Barrot et al. 1999; Fagen et al. 2007; Piazza and Le Moal 1997). Furthermore, acute exposure to cold water stress can strengthen glutamatergic synapses on VTA neurons via a GC-dependent mechanism (Saal et al. 2003).
The NAc is a key mesolimbic interface, receiving input from the hippocampus, PFC, AMG, and VTA (O'Donnell et al. 1999). Intriguingly, immediate-early gene expression is elevated in the NAc following acute or chronic exposure to stressors (Perrotti et al. 2004). The stress-associated changes in limbic plasticity, VTA DA release, and reward-related behaviors point to the NAc as a potentially important locus of stress-induced plasticity. Here we directly examine the effects of stress on glutamatergic synaptic inputs to NAc medium spiny neurons (MSNs).
METHODS
Ethical approval
All experiments were approved by the University of Chicago Institutional Animal Care and Use Committee and conform to methods described in the authors' Animal Care and Use protocols.
Animals and in vivo manipulations
Eight- to 10-wk-old C57BL/6J mice (20–25 g, Jackson Labs, Ben Harbor, ME) were acclimated to their home cage for >7 days before testing. For 2 days preceding brain slice electrophysiology, all animals were singly housed. Animals were stressed using once daily forced swim for 5 min in 4–6°C water for 2 consecutive days. One exposure to this stressor increases AMPA receptor (AMPAR)/N-methyl-d-aspartate receptor (NMDAR) ratios in VTA DA neurons (Saal et al. 2003). The effects of GR receptor blockade were tested by intraperitoneal administration of GR antagonist RU486 (40 mg/kg in DMSO; Tocris, Ellisville, MO) 30 min before cold water stress. The half-life of RU486 is 25–28 h, so the dose administered on day 2 was reduced to 25 mg/kg. The effects of CORT were assessed using once daily intraperitoneal injection of vehicle (50% DMSO, 50% saline) or 10 or 25 mg/kg CORT (Sigma, St. Louis, MO). Animals were killed, and brains slices were prepared 18–24 h following the final injection or swim.
Slice preparation
Chemicals were obtained from Sigma, unless otherwise specified. Animals were anesthetized using inhaled isofluorane and rapidly decapitated into ice cold sucrose-artificial cerebrospinal fluid (ACSF) with 1 mM ascorbic acid (in mM: 252 sucrose, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 20 glucose, and 25 NaHCO3). Sagittal slices (250 μM) were taken at the level of the NAc (3–4 slices per animal), transferred to a perfusion chamber with normal ACSF (sucrose replaced with 125 mM NaCl), containing 1 mM ascorbic acid, heated to 32°C, and perfused at 20 ml/min. Slices were equilibrated for 30 min to 1 h before electrophysiology. All solutions were saturated with 95% O2/5% CO2.
Electrophysiology
Cells were visualized with a fixed-stage upright microscope (Zeiss, Oberkochen, Germany) using infrared-differential interference contrast microscopy. During recording, slices received constant bath perfusion of normal ACSF (in mM: 125 NaCl, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 20 glucose, 1 NaH2PO4, and 25 NaHCO3), saturated with 95% O2/5% CO2. GABAA receptors were blocked with bicuculline (20 μM) in all experiments. NAc MSNs were identified by their small somatic diameters (<20 μm), hyperpolarized resting membrane potential (−75 to −85 mV), and lack of action potential activity. While a very small subset of striatal GABAergic interneurons might be included in the sample using these selection parameters, the density of MSNs (>90% of all striatal neurons) renders any significant contamination of the data set unlikely. Core MSNs were recorded from slices that contained both the rostral and caudal arms of the anterior commissure. Shell MSNs were recorded from medial slices with no dorsal striatal tissue (Thomas et al. 2001). Data were acquired using an Axopatch 200B amplifier and DigiData 1200 digitizer with pClamp 8.2 software (Molecular Devices, Palo Alto, CA). In all experiments, series resistance (<25 MΩ) was monitored and corrected.
For AMPAR/NMDAR ratio, AMPAR current-voltage (I-V) relationship, and GluR2-lacking AMPAR antagonist experiments, tungsten bipolar (75 μm diam, 505-μm tip spacing) stimulating electrodes (FHC, Bowdoinham, ME) were placed rostral to the NAc, at the prelimbic cortex-NAc border to stimulate PFC efferents (Thomas et al. 2001). Transmission was evoked using 0.2-ms square current pulses (0.05–3.0 mA) at 0.1 Hz until stable baseline EPSCs were obtained. Whole cell patch-clamp recordings were made using borosilicate electrodes (4–7 MΩ) containing (in mM: 117 cesium gluconate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 2.5 ATP, 0.25 GTP, 5 tetraethylammonium-Cl, and 20 glucose; pH 7.4; mOsm 280–290). To obtain AMPAR/NMDAR ratios, cells were initially held at −70 mV, and five EPSCs were evoked; cells were depolarized to +40 mV and five additional EPSCs were evoked. Evoked EPSC data were analyzed off-line, using Clampex 8.2 (Molecular Devices). AMPAR values for a cell were calculated by averaging the current traces recorded at −70 mV and measuring the amplitude and timing of the averaged peak. The +40-mV traces were also averaged, and the NMDAR current was determined by sampling a 10-ms window 40 ms after the peak AMPAR current, when the AMPA current has decayed. To assess AMPAR EPSC I-V properties, recordings were made with 0.1 mM spermine (Tocris) in the internal solution and NMDAR antagonist 50 μM d-AP5 (Tocris) in the external solution. EPSCs were evoked at multiple holding potentials (−70, −40, 0, 20, 40, and 60 mV). Rectification index values were obtained using by dividing the average peak current evoked at −70 mV by the average peak current elicited at +40 mV. The effects of stress on AMPAR subunit composition were assessed using 100–200 μM of GluR2-lacking AMPAR antagonist 1-naphthylacetyl spermine trihydrochloride (Naspm). The contribution of GluR2-lacking AMPARs was calculated by dividing the amplitude of the evoked EPSC measured following 8–10 min of Naspm application by the EPSC value obtained before drug wash in.
For analysis of miniature excitatory postsynaptic currents (mEPSCs), the internal solution contained (in mM) 142 K-gluconate, 1 KCl, 10 HEPES, 5 ATP, and 0.1 GTP; pH 7.4; mOsm 315–325, with 1 μM TTX in the bath (Alomone, Jerusalem, Israel). To analyze dual component (AMPA and NMDA) mEPSCs, slices were perfused in a Mg2+-free external solution. Cells were held at −70 mV, and mEPSCs were recorded for 2 min, after which 50 μM d-AP5 was applied for 8–10 min, and then recorded again for 2min (Kourrich et al. 2007). Average mEPSCs in the presence and absence of d-AP5 were subtracted to estimate average NMDA mEPSC amplitudes. All mEPSC data were analyzed using Minianalysis software (Synaptosoft, Decatur, GA); mEPSCs were defined as inward currents with amplitudes >5 times RMS noise.
Statistical analysis
A 4 × 1 ANOVA was used to evaluate main effects of treatment in AMPAR/NMDAR ratio datasets. Fisher's protected least significant difference (PLSD) post hoc tests were used to assess effects between treatment groups. Cumulative probability distributions were analyzed using the Kolmogorov-Smirnov (K-S) test. Student's unpaired t-test were used to assess group differences in mEPSC amplitude, mEPSC frequency, rectification index, Naspm block, and subtracted NMDA current datasets. Any individual measurements that fell beyond 2 SD from the group mean were identified as outliers and removed from the analyses. All statistical tests were performed using Systat software (SPSS, Chicago, IL). Data were averaged by animal and represented as group means ± SE.
RESULTS
To examine the effects of stress on glutamatergic transmission in the NAc, we exposed adult male C57BL/6J mice to 5-min cold water forced swim for 2 consecutive days. Eighteen to 24 h following the second swim, animals were killed for electrophysiological study. Sagittal brain slices at the level of NAc were taken and used for whole cell patch-clamp recordings.
Cold water stress enhances synaptic strength in NAc shell MSNs
The status of the glutamatergic inputs to NAc neurons was assayed by measuring the ratio of the AMPAR component of the synaptic currents to the NMDAR component (Fig. 1A). To measure the AMPAR currents, cells were held at −70 mV, and EPSCs were evoked using a bipolar stimulating electrode placed rostral to the NAc. Cells were depolarized to +40 mV, and EPSCs were evoked again. NMDAR current was defined by a 10-ms window in the EPSC recorded at +40 mV starting 40 ms after the AMPAR peak measured at −70 mV. The timing of the 10-ms window coincides with a near total decay of AMPA-mediated EPSCs, providing an uncontaminated measurement of NMDAR EPSCs. The ratio of the AMPA peak at −70 mV, and the calculated NMDA value from the +40 mV trace was used to evaluate stress-induced changes in synaptic strength in NAc MSNs.
FIG. 1.
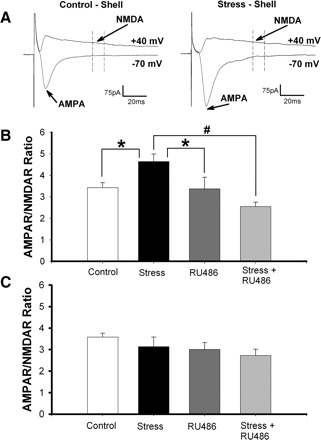
Stress increases AMPAR/NMDAR ratio in the nucleus accumbens (NAc) shell but not core. A: sample traces in control and stressed animals, showing how AMPAR/NMDAR ratios were obtained. B: averaged AMPAR/NMDAR ratios measured in the NAc shell from control animals, stressed animals, animals administered RU486, and animals administered RU486 30 min before cold water stress. The AMPAR/NMDAR ratios were increased in stressed animals compared with all other groups (*P < 0.02, #P < 0.0005). C: averaged AMPAR/NMDAR ratios measured in the NAc core under the same conditions as in B.
Cold water stress induced an increase in AMPAR/NMDAR ratios measured in the NAc shell (Fig. 1B; P < 0.02; control, 3.42 ± 0.23, n = 17 cells, 6 mice; stress, 4.64 ± 0.35, n = 16 cells, 6 mice). This is consistent with stress-induced long-term potentiation (LTP) of the excitatory synaptic inputs to these cells. Administration of the GR antagonist, RU486 (40 mg/kg), 30 min before the forced swim completely blocked the stress effects (RU486 + stress, 2.55 ± 0.20, n = 15 cells, 5 mice), suggesting that GC signaling is essential for the observed stress-induced plasticity. Treatment with RU486 alone did not affect synaptic strength in NAc shell MSNs (RU486, 3.367 ± 0.54, n = 15 cells, 5 mice). Interestingly, there was a trend toward a decrease in AMPAR/NMDAR ratio when comparing the RU486 plus stress group to either controls (P < 0.1) or to animals receiving RU486 alone (P < 0.13). This raises the possibility that, in the absence of GR activation, stress might decrease synaptic efficacy in NAc shell MSNs. In contrast to the shell, we saw no stress-induced changes in synaptic strength when recording from neurons in the NAc core (Fig. 1C; control, 3.58 ± 0.18, n = 18 cells, 6 mice; stress, 3.13 ± 0.44, n = 15 cells, 5 mice; RU486, 3.01 ± 0.32, n = 15 cells, 5 mice; RU486 + stress, 2.729 ± 0.24, n = 14 cells, 6 mice). The subregion specificity of this change was not surprising, given previous studies that showed stress and GC-mediated increases in NAc dopamine overflow only in the NAc shell (Barrot et al. 2000; Kalivas and Duffy 1995).
Exogenous corticosterone enhances synaptic strength in NAc shell MSNs
Given that GR activation is apparently necessary for stress-induced enhancement of corticoaccumbens synaptic strength, we tested the effect of exogenous CORT administration on AMPAR/NMDAR ratios in NAc shell MSNs. Mice were given intraperitoneal injections of vehicle (DMSO and sterile saline), 10 mg/kg CORT, or 25 mg/kg CORT once daily for 2 consecutive days. Eighteen to 24 h following the second injection, animals were killed for electrophysiology as described above. These experiments showed an inverted U-shaped relationship between synaptic strength and CORT dose. Specifically, mice that received 10 mg/kg CORT showed increased AMPAR/NMDAR ratios in NAc shell MSNs compared with those recorded in vehicle-injected controls (Fig. 2; P < 0.003; vehicle, 2.83 ± 0.21, n = 14 cells, 5 mice; 10 mg/kg CORT, 5.04 ± 0.62, n = 15 cells, 5 mice) and 25 mg/kg CORT-treated animals (P < 0.035; 25 mg/kg CORT, 3.65 ± 0.24, n = 14 cells, 5 mice). AMPAR/NMDAR ratios recorded from animals given 25 mg/kg CORT were not significantly different from vehicle controls (Fig. 2; P < 0.18). While the loss of CORT's ability to enhance synaptic strength at the 25 mg/kg dose was unexpected, it parallels previous findings that show primed burst-induced hippocampal LTP is facilitated by low to intermediate CORT levels but not with higher concentrations (Diamond et al. 1992).
FIG. 2.
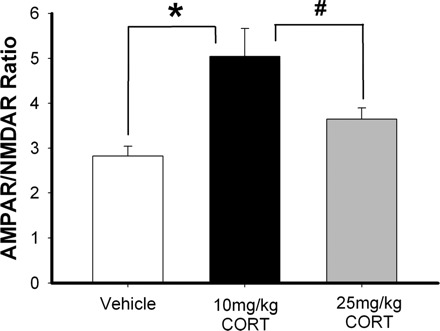
Corticosterone (CORT) increases AMPAR/NMDAR ratio of glutamatergic inputs to NAc shell medium spiny neurons (MSNs). Average AMPAR/NMDAR ratios in NAc shell MSNs measured in mice given 2 consecutive daily intraperitoneal injections of vehicle (50% DMSO, 50% saline), 10 mg/kg CORT, or 25 mg/kg CORT. The AMPAR/NMDAR ratios from animals receiving the 10 mg/kg CORT were increased compared with control and 25 mg/kg CORT-treated mice (*P < 0.003, #P < 0.035).
Cold water stress increases AMPA mEPSC amplitude in NAc shell MSNs
AMPAR/NMDAR ratios are a useful metric for assessing changes in synaptic strength, but the observed changes can result from alterations in AMPARs, NMDARs, or both. To identify any stress-induced changes in AMPA-mediated currents in the MSNs, mEPSCs were recorded in the presence of TTX (1 μM) at −70 mV (Fig. 3A). Cumulative probability plots (Fig. 3B) and frequency histograms (Fig. 3C) of AMPAR mEPSC amplitudes show a shift to larger events in the recordings from NAc shell MSNs of stressed animals relative to controls (K-S test; P < 0.022; control, n = 10 cells, 5 mice; stress n = 10 cells, 5 mice). Consistent with these findings, mean mEPSC amplitude was larger in stressed mice (Fig. 3C, inset; P < 0.02) Comparison of interevent interval distributions (Fig. 3, D and E) and mean mEPSC frequencies (Fig. 3E, inset) showed no differences between groups, suggesting that stress does not affect presynaptic release probability. These data support the hypothesis that stress enhances synaptic strength in NAc shell MSNs by increasing the number or function of AMPARs on the plasma membrane. Similar results have been previously reported in hippocampal slices, where exogenous CORT enhances mEPSC amplitude without affecting event frequency (Karst and Joels 2005).
FIG. 3.
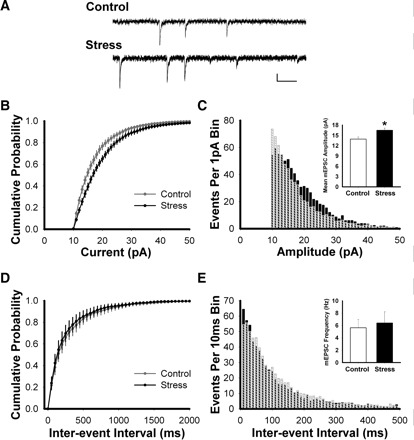
AMPAR miniature excitatory postsynaptic currents (mEPSCs) are larger in the NAc shell from stressed animals. A: sample traces showing typical mEPSCs at −70 mV. Scale bar: 10 pA, 100 ms. B: cumulative amplitude distributions of mEPSCs (1-pA bins) measured in the NAc shell MSNs, showing a shift toward larger events following stress relative to control (Kolmogorov-Smirnov test; P < 0.022). C: frequency histogram showing distribution of mEPSC amplitudes (1-pA bins) in control (crosshatched bars) and stressed (black bars) animals. Inset: average mEPSC amplitude is increased in the NAc shell of stressed animals relative to controls (t-test; P < 0.02). D: cumulative interevent interval distributions of mEPSCs (50-ms bins) in the NAc shell of control and stressed mice. E: frequency histogram showing distribution of mEPSC interevent intervals (10-ms bins). Inset: average mEPSC frequency is similar in NAc shell MSNs in control and stressed mice (t-test; P > 0.73).
Cold water stress reduces AMPA current rectification in NAc shell MSNs
Subunit composition determines the rectification and ion permeability characteristics of individual AMPARs (Burnashev et al. 1992; Verdoorn et al. 1991). Specifically, GluR2 subunits undergo a high efficacy posttranscriptional RNA editing process that leads to the replacement of an uncharged glutamine residue with a charged arginine residue in the second transmembrane segment of the protein (Sommer et al. 1991). AMPARs that contain edited GluR2 subunits have a linear I-V relationship and low permeability to divalent cations, whereas GluR2-lacking AMPARs show inward rectification and permeability to Ca2+ (Burnashev et al. 1992; Verdoorn et al. 1991). To assess if stress-induced enhancement of AMPA currents in NAc shell MSNs was accompanied by changes in rectification, we bath applied d-AP5 (50 μM) to block NMDARs, and voltage clamped the cells with spermine (0.1 mM) in the internal solution to limit possible dilution of intracellular polyamines. EPSCs were evoked at holding potentials ranging from −70 to +40 mV (Fig. 4A). We calculated the ratio between the peak current at −70 and +40 mV to determine the AMPAR current rectification index. Using this method, we found that AMPAR currents in the NAc shell MSNs of stressed animals showed less rectification compared with controls (Fig. 4B; P < 0.05; control, 3.14 ± 0.42, n = 14 cells, 5 mice; stress, 2.198 ± 0.14, n = 14 cells, 6 mice).
FIG. 4.
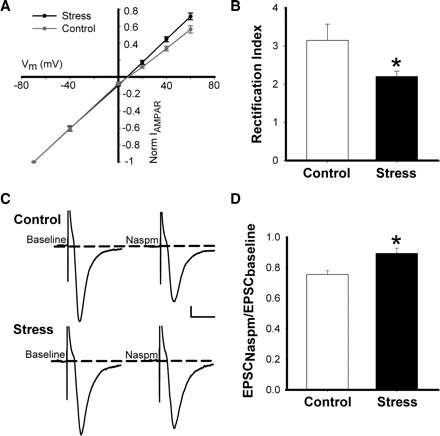
Stress increases the linearity of the AMPAR receptor I-V relationship in NAc shell MSNs. A: AMPAR current-voltage relationship for neurons shown in B. B: rectification index (I−70mV/I+40mV) showing reduced AMPAR current rectification in stressed animals relative to controls (*P < 0.05). C: sample traces showing the effects of 1-naphthylacetyl spermine trihydrochloride (Naspm; 100–200 μM) on evoked AMPAR EPSCs recorded at −70 mV from NAc MSNs of control and stressed mice. Scale bar: 25 pA, 30 ms. D: the effects of Naspm application on evoked AMPAR EPSCs reported as EPSC amplitude following 8 to 10 min drug wash in normalized to baseline EPSC amplitude. Naspm-mediated reduction of AMPAR EPSC amplitudes was greater in controls than in stressed mice (*P < 0.01).
To further study the effects of stress on NAc shell MSN glutamate receptor subunit composition, we used Naspm, a blocker of GluR2-lacking AMPARs. Neurons were held at −70 mV, and EPSCs were evoked at 0.1 Hz. After recording stable baseline currents, 100–200 μM Naspm was applied to the slice for 8–10 min, and EPSCs were evoked again (Fig. 4C). Comparing the ratio of the evoked current amplitudes in the presence and absence of Naspm shows more GluR2-lacking receptors in control relative to stressed mice (Fig. 4D; P < 0.01; control, 0.76 ± 0.03, n = 11 cells, 6 mice; stress, 0.89 ± 0.02, n = 8 cells, 5 mice). Together, these data suggest that following 2 consecutive days of cold water stress, there is a net increase surface expression of AMPARs that contain GluR2 subunits in NAc shell MSNs. Interestingly, this finding parallels data from a recent study showing that exposure of cultured hippocampal neurons to CORT leads to increased synaptic incorporation of GluR2-containing AMPARs via a GC-dependent mechanism (Groc et al. 2008).
Cold water stress does not alter NMDAR mEPSC amplitude in NAc shell MSNs
To evaluate potential changes in NMDAR function in NAc shell MSNs following cold water stress, we used a subtracted averages technique that has been used previously to assess NMDAR-mediated current amplitude (Kourrich et al. 2007; Thomas et al. 2001). Slices were bathed in an external solution containing TTX to block action potentials and zero external Mg2+ to unblock NMDARs. NAc shell MSNs were patched, and 2 min of mEPSC activity was recorded (Fig. 5A). Excitatory synaptic currents recorded under these conditions are mediated by both AMPARs and NMDARs. Competitive NMDAR antagonist d-AP5 (50 μM) was bath-applied for 8–10 min. Following blockade of NMDAR activity, another 2 min of mEPSCs were recorded (Fig. 5A). Events recorded before d-AP5 treatment were averaged for each cell, yielding an average dual-component mEPSC. By averaging the mEPSCs recorded after NMDAR block and subtracting this trace from the dual-component mEPSC trace obtained from the same cell, we generated a subtracted average NMDAR mEPSC (Fig. 5B). This subtracted trace represents the average contribution to NMDAR-mediated currents to mEPSCs recorded in the absence of extracellular Mg2+. Using this method, we observed no difference in NMDA current amplitude in control versus stressed mice (Fig. 5C; P < 0.5; control, 3.492 ± 0.39 pA, n = 8 cells, 4 mice; stress, 3.80 ± 0.30pA, n = 8 cells, 4 mice). Together with the observed increase in AMPAR mEPSC amplitude, these data suggest that the stress-induced changes in AMPAR/NMDAR ratios in NAc shell MSNs are driven by enhanced AMPAR number and/or function.
FIG. 5.

Stress has no effect on NMDAR mEPSCs. A: sample traces of mEPSCs from NAc shell MSNs, recorded in 0 Mg2+ external solution, before and after d-AP5 (50 μM). Scale bar: 10 pA, 100 ms. B: example of averaged mEPSCs before (black) and after (dark gray) d-AP5 in a representative MSN, with the subtracted average NMDAR mEPSC (light gray). C: average of subtracted average NMDAR mEPSC amplitudes showing no difference between control and stressed mice (P < 0.5).
DISCUSSION
These data indicate that stress enhances synaptic strength in the NAc shell, a structure thought be involved in motivation and reward. Enhancement of AMPAR-mediated excitatory drive to MSNs in the accumbens might contribute to the effects of stress on reward-related behaviors. We observed an increase in AMPAR/NMDAR ratios in NAc shell, but not core MSNs following 2 days of cold water stress. This increase was blocked by intraperitoneal preadministration of GR antagonist RU486, implicating GC signaling as a necessary component in the demonstrated change. Furthermore, CORT was shown to be sufficient to enhance corticoaccumbens synaptic strength because intraperitoneal injections of exogenous CORT also increased AMPAR/NMDAR ratios in NAc shell MSNs. We showed that the stress-induced change in AMPAR/NMDAR ratio was accompanied by increased AMPAR mEPSC amplitude without a corresponding change in mEPSC frequency. Additionally, AMPAR EPSCs from stressed animals were shown to have increased linearity in their I-V relationship and reduced Naspm sensitivity, relative to controls, implying that stress increased the number or function of GluR2-containing AMPARs in the NAc shell. Although our control I-V data differ somewhat from those reported in some previous studies (Conrad et al. 2008; Kourrich et al. 2007), there are important differences in species, animal age, and/or preparation that may account for this divergence. Notably, our experiments are done in adult animals, and recent publications have shown robust changes in EPSC properties in NAc MSNs during postnatal development (Kasanetz and Manzoni 2009; Zhang and Warren 2008). Together these data suggest that the observed change in the AMPAR/NMDAR ratio following stress was driven by increased number or function of synaptic AMPARs. Stress had no effect on NMDAR mEPSC amplitude in our experiments, suggesting that the shown changes in NAc shell MSN synaptic strength are driven primarily by enhancement of AMPA-mediated currents.
Previous studies have shown that stress can affect the physiology of the NAc. Acute restraint stress elevates c-Fos protein expression in numerous reward-related brain regions, including NAc shell and core (Perrotti et al. 2004). In the same study, chronic stress increased ΔFosB expression throughout the accumbens (Perrotti et al. 2004). These data have potential relevance to our study, because ΔFosB has been shown to increase GluR2 expression in the NAc (Kelz et al. 1999).
Our demonstration that cold water stress selectively enhances synaptic strength in the NAc shell is paralleled by data from previous studies showing shell-specific effects of stress and stress hormones. In rats, in vivo microdialysis has shown that DA levels are elevated in the NAc shell, but not the core, during the 20 min following foot shock stress (Kalivas and Duffy 1995). DA overflow into the NAc is also induced following administration of cocaine or morphine (Barrot et al. 1999). Adrenalectomy (ADX) blunts the DA increase in the shell following drug injection, whereas DA overflow in the core is unaffected (Barrot et al. 2000). Stress differentially affects Fos-like immunoreactivity (Fos-LI) in the rat striatum, with NAc shell showing a larger increase than either NAc core or caudate-putamen (Barrot et al. 1999). Fos-LI is also elevated in rat NAc following an injection of cocaine, a DA reuptake inhibitor (Barrot et al. 1999). Although cocaine-induced Fos-LI is seen in both the shell and core, ADX selectively reduces the increase in NAc shell (Barrot et al. 2000). ADX, however, does not affect D1 receptor agonist-induced Fos-LI in the NAc shell (Barrot et al. 2000). Together, these data suggest that GC regulation of shell-projecting VTA DA neurons may contribute to the plasticity reported here.
The NAc is essential for the expression of addiction-like behaviors in animal models. Moreover, perturbations to an animal's stress state or GC levels can affect drug self-administration, locomotor sensitization to psychostimulants, and reinstatement of drug seeking (Marinelli and Piazza 2002). Our data are interesting in the context of drug addiction, because withdrawal from cocaine induces similar synaptic changes to those we reported following cold water stress (Kourrich et al. 2007). In mouse brain slices taken after 2 wk of withdrawal from a sensitizing regimen of cocaine, AMPAR currents are enhanced at PFC-NAc shell synapses (Kourrich et al. 2007). Similarly, rats sensitized to cocaine have increased surface expression of AMPARs, 21 days after the final drug injection (Boudreau and Wolf 2005; Boudreau et al. 2007). Interestingly, viral-mediated gene transfer experiments in mouse have shown that increased expression of GluR2 in NAc enhances the rewarding effects of cocaine (Kelz et al. 1999). The ability of both stress and cocaine withdrawal to increase AMPAR-mediated transmission in the NAc is paralleled by the ability of both stress and extended withdrawal to enhance reinstatement of drug seeking (Shaham et al. 2003). Additionally, intra-NAc infusions of AMPA induce reinstatement in animals that have undergone extinction training from drug self administration, further suggesting that NAc AMPARs are critical for reinstatement (Suto et al. 2004). Therefore we hypothesize that GR activation and withdrawal might similarly contribute to the motivational effects of psychostimulants and reinstatement of drug seeking via enhanced AMPAR-mediated neurotransmission in NAc shell MSNs.
This study showed that cold water stress can enhance synaptic strength in NAc shell MSNs via a GR-dependent mechanism. Although it is well-established that stress and GCs alter DA transmission and fos expression in the NAc (Barrot et al. 1999, 2000; Kalivas and Duffy 1995; Perrotti et al. 2004), this is the first demonstration of stress-induced changes to NAc shell AMPAR function. Here, we showed a novel cellular substrate linking stress and reward. Better understanding of the neuroadaptations following exposure to environmental stressors will hopefully advance our understanding of the mechanisms underlying stress and reward-related pathologies.
GRANTS
This work was supported by National Institutes of Health Grants T32 DA-015918 to D. S. McGehee and GM-07839 to M. Campioni and the Pritzker Fellowship in Neuroscience to M. Campioni.
REFERENCES
- Barrot 1999.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci 11: 1155–1166, 1999 [DOI] [PubMed] [Google Scholar]
- Barrot 2000.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12: 973–979, 2000 [DOI] [PubMed] [Google Scholar]
- Boudreau 2007.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci 27: 10621–10635, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau 2005.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25: 9144–9151, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev 1992.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8: 189–198, 1992 [DOI] [PubMed] [Google Scholar]
- Cerqueira 2007a.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 27: 2781–2787, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira 2005.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25: 7792–7800, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira 2007b.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex 17: 1998–2006, 2007b [DOI] [PubMed] [Google Scholar]
- Conrad 2008.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche 1992.Deroche V, Piazza PV, Maccari S, Le Moal M, Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res 584: 309–313, 1992 [DOI] [PubMed] [Google Scholar]
- Diamond 1992.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 2: 421–430, 1992 [DOI] [PubMed] [Google Scholar]
- Fagen 2007.Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced nicotinic receptor function and drug abuse vulnerability. J Neurosci 27: 8771–8778, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc 2008.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci 11: 868–870, 2008 [DOI] [PubMed] [Google Scholar]
- Kalivas 1995.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675: 325–328, 1995 [DOI] [PubMed] [Google Scholar]
- Kalivas 1991.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16: 223–244, 1991 [DOI] [PubMed] [Google Scholar]
- Karst 2005.Karst H, Joels M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. J Neurophysiol 94: 3479–3486, 2005 [DOI] [PubMed] [Google Scholar]
- Kasanetz 2009.Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol 101: 2516–2527, 2009 [DOI] [PubMed] [Google Scholar]
- Kelz 1999.Kelz MB, Chen J, Carlezon WA Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401: 272–276, 1999 [DOI] [PubMed] [Google Scholar]
- Kim 2002.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3: 453–462, 2002 [DOI] [PubMed] [Google Scholar]
- Kim 2006.Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: synaptic plasticity and memory. Stress 9: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- Kourrich 2007.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 27: 7921–7928, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch 1998.Mantsch JR, Saphier D, Goeders NE. Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. J Pharmacol Exp Ther 287: 72–80, 1998 [PubMed] [Google Scholar]
- Marinelli 2002.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci 16: 387–394, 2002 [DOI] [PubMed] [Google Scholar]
- McEwen 1986.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev 66: 1121–1188, 1986 [DOI] [PubMed] [Google Scholar]
- Mizoguchi 2000.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci 20: 1568–1574, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell 1999.O'Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann NY Acad Sci 877: 157–175, 1999 [DOI] [PubMed] [Google Scholar]
- Perrotti 2004.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci 24: 10594–10602, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza 1997.Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 25: 359–372, 1997 [DOI] [PubMed] [Google Scholar]
- Piazza 1996.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA 93: 8716–8720, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal 2003.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582, 2003 [DOI] [PubMed] [Google Scholar]
- Sapolsky 2003.Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res 28: 1735–1742, 2003 [DOI] [PubMed] [Google Scholar]
- Shaham 2003.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20, 2003 [DOI] [PubMed] [Google Scholar]
- Sommer 1991.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67: 11–19, 1991 [DOI] [PubMed] [Google Scholar]
- Sousa 2008.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev 57: 561–570, 2008 [DOI] [PubMed] [Google Scholar]
- Sousa 2000.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97: 253–266, 2000 [DOI] [PubMed] [Google Scholar]
- Suto 2004.Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology 29: 2149–2159, 2004 [DOI] [PubMed] [Google Scholar]
- Thomas 2001.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4: 1217–1223, 2001 [DOI] [PubMed] [Google Scholar]
- Verdoorn 1991.Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252: 1715–1718, 1991 [DOI] [PubMed] [Google Scholar]
- Zhang 2008.Zhang L, Warren RA. Postnatal development of excitatory postsynaptic currents in nucleus accumbens medium spiny neurons. Neuroscience 154: 1440–1449, 2008 [DOI] [PubMed] [Google Scholar]


