Abstract
GABAC receptors (GABACRs) are widely expressed in the mammalian subcortical visual system, particularly in the retina and superior colliculus (SC). GABACRs are composed of specific ρ1–3 subunits the expression of which varies among visual structures. Thus ρ1 subunits are most abundant in retina, and their loss eliminates GABACR expression and function. In the SC, ρ2 subunit expression may be equal to or stronger than ρ1 subunit expression; however, results across studies vary considerably. To more directly assess the expression of GABACR subunits, we characterized inhibition in the SC of wild-type (WT) and GABAC ρ1 Null mice that lack expression of GABAC ρ1 subunits. We used whole cell patch-clamp recordings and evaluated GABACR-mediated modulation of electrically evoked post synaptic currents using either agonists or antagonists in WT mice. In GABAC ρ1 Null stratum griseum superficiale (SGS) cells, inhibitory postsynaptic currents were shorter in duration and their excitatory postsynaptic currents (EPSCs) were longer, indicating that a slow GABACR-mediated inhibitory component was reduced in each case. In contrast to retina, GABACR-mediated currents in the SC were altered but not eliminated in GABAC ρ1 Null mice. In the majority of SC cells in GABAC ρ1 Null mice, GABACR activation could still be induced to alter EPSC peak amplitudes in putative interneurons and in many projection neurons. These results, compared with previously published data, indicate a fundamental difference between retina and SC in the control of GABACR expression and subunit composition.
INTRODUCTION
At least three pharmacologically distinct receptor types mediate the postsynaptic action of γ-aminobutyric acid (GABA), the ionotropic GABAA and GABAC receptors (GABAARs and GABACRs), and the metabotropic GABABRs (Bormann 2000; Chebib and Johnston 1999). GABACRs differ significantly from GABAARs in a number of their biophysical and pharmacological properties. For example, GABACRs are composed of specific ρ1–ρ3 subunits, have a 10-fold higher affinity for receptor agonists and show smaller current conductance that do not desensitize in the presence of agonists (Bormann 2000; Bormann and Feigenspan 1995; Chebib and Johnston 1999). Furthermore, in contrast to the ubiquitous presence of GABAARs, throughout the CNS, the expression of GABACRs is restricted to a few structures, most of which are part of the subcortical visual system (Boué-Grabot et al. 1998; Frazao et al. 2007; Rozzo et al. 2002; Wegelius et al. 1998).
In the retina, GABACRs are primarily expressed at bipolar cell terminals (Feigenspan et al. 1993; Enz et al. 1996; Lukasiewicz 1996; Wässle et al. 1998), where they modulate information transfer to ganglion cells (Dong and Werblin 1998; Flores-Herr et al. 2001; Lukasiewicz and Werblin 1994; Pan and Lipton 1995; Sagdullaev et al. 2006). Elimination of ρ1 subunit expression leads to a complete loss of both GABACR expression and GABACR-mediated function (McCall et al. 2002). As a consequence, retinal bipolar cells in GABAC ρ1 Null mice lack GABACR-mediated feedback currents without compensatory changes in other inhibitory inputs (Eggers et al. 2007) and related components of the electroretinogram are strongly enhanced in GABAC ρ1 Null mice (McCall et al. 2002).
Outside the retina, GABACR expression is particularly strong in the stratum griseum superficiale (SGS), the superficial layer of the superior colliculus (SC). In rat, activation of GABACRs reduces the activity of GABAergic SGS interneurons, resulting in increased activity of projection cells (Pasternack et al. 1999; Schmidt et al. 2001). This suggests a specific function of GABACRs in the control of local feed forward inhibition to SGS projection cells.
Attempts to define the subunit composition of GABACRs in retina and SC have produced conflicting results. Some studies report much higher expression of ρ1 relative to ρ2 subunits in retina and, higher expression of ρ2 relative to ρ1 in SC (Greka et al. 2000; Wegelius et al. 1998; McCall, unpublished observations). Other studies did not find significant differences in their expression between retina and SC (Boué-Grabot et al. 1998). Whether or not differences in the subunit density exist between the retina and other brain structures, expression of the ρ1 subunit seems to be required to form functional GABACRs in the retina, and therefore GABACR-mediated inhibition is eliminated in the absence of ρ1 expression (McCall et al. 2002).
If the rules that govern GABACR assembly in retina are similar to those in extraretinal structures, we also would expect a loss of GABACR currents in SGS cells in GABAC ρ1 Null SC. To address this hypothesis and elucidate the GABACR subunit composition in SC, we characterized and compared GABACR-mediated effects on postsynaptic responses in wild-type (WT) and GABAC ρ1 Null SGS cells. Our results demonstrate that in the SGS of the SC functional GABACRs form in the absence of ρ1 subunit expression, although the remaining inhibitory GABACR-mediated currents differ significantly from WT in both their kinetics and in the total inhibitory influence that they exert on postsynaptic responses.
METHODS
Experimental animals
Three- to 6-wk-old C57Bl/6J (WT) mice and GABAC ρ1 Null mice congenic for the WT strain of either sex were used in these experiments. The targeting strategy and production of GABAC ρ1 Null mice has been described previously (McCall et al. 2002). All experimental procedures were approved by institutional and governmental animal-care and -use committees and were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Brain slice preparation
To obtain acute brain slices, animals were deeply anesthetized with halothane followed by a subcutaneous injection of ketamine (100 mg/kg body wt) and thiazine hydrochloride (1 mg/kg), before they were transcardially perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 123 NaCl, 2.5 KCl, 10 NaH2PO4, 26 NaHCO3, 1.3 MgSO4, 1.8 CaCl2, and 11 glucose, that was continuously gassed with 5% CO2-95% O2. After brain removal, 300-μm-thick coronal slices were cut on a vibratome in ice-cold ACSF. Five to six single SC slices were obtained from each experimental animal. The slices were kept in ACSF at 36°C for ≥1 h to allow recovery from the slicing procedure prior to recording. For recording, they were transferred to a submerged type recording chamber where they were superfused at 3 ml/min with ACSF at room temperature during patch-clamp experiments.
Whole cell patch clamp
Whole cell recordings from SGS neurons were performed under visual guidance, using infrared differential interference video microscopy (Dodt and Zieglgänsberger 1990). For recording, borosilicate glass micropipettes (impedance: 5–8 MΩ) were filled with internal solution composed of (in mM) 130 potassium gluconate, 5 sodium gluconate, 20 HEPES, 4 MgCl2, 4 Na2ATP, 0.4 Na3GTP, and 5 EGTA to which 0.5% biocytin was added for morphological single-cell reconstruction. The pipette solution was also supplemented with the sodium channel blocker QX314 (4 mM) to block the generation of action potentials.
Postsynaptic responses were evoked in the SGS with a concentric bipolar stimulating electrode (SNEX-100X, Rhodes Medical Instruments, Tujunga, CA) placed in the stratum opticum (SO) ventral and lateral to the recording site. The electrical stimulus was optimized for each cell to achieve ∼70% of its maximum response. To meet this criterion, stimuli varied in both amplitude (0.5–2 mA) and duration (100–500 μs) due to differences in the effectiveness of the stimulus altered by factors such as the distance between stimulation and recording sites. The neuronal signals were amplified and filtered using an RK-300 amplifier (BioLogic, Claix, France), digitized at 20 kHz with a CED 1401 laboratory interface (Cambridge Electronic Design, Cambridge, UK), and displayed, stored, and analyzed using WinWCP software (kindly provided by Dr J. Dempster, University of Strathclyde Electrophysiology Software: www.strath.ac.uk/Departments/PhysPharm/ses.htm). Measured membrane potentials were corrected for the junction potential of −10 mV. Unless otherwise stated, postsynaptic current responses evoked by SO stimulation were averaged over five consecutive stimulus applications. Because the resulting excitatory and inhibitory postsynaptic currents overlap in time and cannot be elicited independently, we always defined the negative peak of the current response as the peak excitatory postsynaptic current (EPSC also in the case of multiple EPSC peaks); and the positive peak of the current response as the peak inhibitory PSC (IPSC). All drug effects are presented as means ± SE. Differences were compared using the Student's t-test and were considered statistically significant when the P value was ≤0.05. In the results, P values are given only for statistically significant differences.
Drug delivery
All drugs were bath applied. A 10-min application time proved sufficient to achieve stable responses. Application of 0.2–0.5 μM muscimol (Schmidt et al. 2001) or 50 μM cis-aminocrotonic acid (CACA) was used to selectively activate GABACRs. Muscimol at a concentration of 5 μM was used to activate GABAARs and 10 μM (−)-bicuculline methiodide was used to block them. Other selective antagonists were used to block: GABABRs, [3-[[(3,4-dichlorphenyl) methyl]amino]propyl](diethoxymethyl) phosphinic acid (CGP 52432, 5 μM), and GABACRs, 1,2,5,6-tetrahydropyridine-4-yl phosphinic acid (TPMPA, 50 μM). Because TPMPA also acts as a GABABR agonist, CGP 52432 was always applied before and with TPMPA. Drugs were obtained from Sigma (Deisenhofen, Germany), except for CGP 52432 and TPMPA that were obtained from Tocris (Bristol, UK).
Histochemistry
At the end of each recording session, slices were immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C. After ≥24 h in fixative, slices were processed using standard histochemical techniques for visualization of biocytin with 3,3-diaminobenzidine (Sigma-Aldrich). Morphological reconstruction of stained cells was performed with the aid of a camera lucida.
RESULTS
GABAC receptors mediate inhibition in the SGS of WT mice
While we have previously characterized GABACR-mediated responses in the SGS of the rat SC (e.g., Boller and Schmidt 2001, 2003; Schmidt et al. 2001), the characteristics of GABACR-mediated currents have not been reported for the mouse. To this end, we characterized the responses of 79 cells in the SGS of the SC of WT mice. When cells were clamped at or around their resting potential (i.e., to −60 mV), electrical stimulation usually elicited a biphasic PSC that consisted of a short-latency, single peak EPSC followed by a longer-duration IPSC (representative control traces in Fig. 1, A and B). In addition, and similar to the rat SC (Schmidt et al. 2001), responses could consist of multiple EPSC peaks without an obvious IPSC (control traces in Fig. 1, C and D). This probably results from an activation of multiple excitatory inputs either from different sets of afferent fibers or from intrinsic excitatory circuitry. In these cells, additional EPSC peaks appeared in the presence of the inhibitory antagonist, bicuculline, suggesting the presence of inhibitory inputs that are usually masked by excitation (Fig. 1D). While electrical stimulation always elicited an EPSC, the appearance of an IPSC varied, and its presence frequently required depolarization of the cell to holding potentials above −40 mV (Fig. 1), consistent with an ECl close to Vrest.
FIG. 1.

Muscimol at low concentration (0.5 μM) selectively activates GABAC receptors (GABACRs) in wild-type (WT) mouse stratum griseum superficiale (SGS) cells. Inhibitory effect of muscimol on postsynaptic currents in 2 WT mouse SGS cells (A, B and C, D). In both cells, bath application of 0.5 μM muscimol reduced excitatory postsynaptic current (EPSC) amplitudes (A and C). Although 10 μM bicuculline, a GABAAR antagonist, enhanced postsynaptic responses when applied alone, it had no effect on the muscimol induced reduction (B and D), indicating that muscimol at this concentration selectively activates GABACRs. Similar muscimol effects were found in 65% of the recorded WT SGS cells. Current traces in this and in the following figures represent responses averaged from five consecutive stimulus applications. Stimulus artifacts have been truncated for clarity.
As described previously in rat SC (Schmidt et al. 2001), bath-applied muscimol (≤0.5 μM) activation of GABACRs in WT mouse SGS cells induced two effects on electrically evoked PSCs. In 65% of the cells (47/72), all PSCs were reduced, regardless of whether the current consisted of both an EPSC and an IPSC (Fig. 1A) or only an EPSC (not shown). In the case of multi-peak EPSCs, muscimol affected all peaks in a similar way (Fig. 1C). On average, muscimol application reduced EPSC amplitudes 25.5 ± 1.9% and IPSC amplitudes 34.9 ± 3.9% (n = 26) of control. As expected, bicuculline, a selective GABAAR antagonist had a strong effect on the electrically evoked IPSCs (Fig. 1D). However, addition of muscimol in the presence of bicuculline produced similar reductions in EPSC amplitude as in the absence of bicuculline (Fig. 1B and D; 25.5 ± 1.9 vs. 25.1 ± 4.5%; n = 19), which verifies that the muscimol-induced reductions of electrically evoked EPSCs are GABACR mediated.
In the remaining SGS cells, 0.5 μM muscimol produced one of three effects: an increase only in the EPSC amplitude (n = 5; Fig. 2A), a reduction only in the IPSC amplitude (n = 11; Fig. 2B), or an increase in EPSC and decrease in IPSC amplitudes simultaneously (n = 5; Fig. 2C). In these cells, the mean increase in EPSC amplitude was 42.7 ± 15.2% of control, and the mean decrease in IPSC amplitude was 34.6 ± 3.9% of control. The magnitude of these changes is the same as in cells where both the EPSC and IPSC amplitudes were reduced. Again the addition of bicuculline in the presence of muscimol did not further alter the EPSC amplitude (EPSC increase 49.9 ± 15.5 vs. 42.7 ± 15.2%). These results demonstrate the existence of GABACR-mediated currents in WT mouse SGS cells.
FIG. 2.

Postsynaptic excitation is enhanced by muscimol in WT mouse SGS cells. In these 3 cells, bath application of 0.5 μM muscimol either increased EPSC amplitudes (A), decreased IPSC amplitudes (B), or both increased EPSC and decreased inhibitory PSC (IPSC) amplitudes (C). Again, 10 μM bicuculline did not alter the effects induced by 0.5 μM muscimol (A).
To independently confirm the existence of GABACR-mediated currents, we also used the selective GABACR agonist, cis-aminocrotonic acid (CACA). Similar to 0.5 μM muscimol, bath application of 50 μM CACA reduced the EPSC and IPSC mean amplitudes to 30.2 ± 4.2 and 35.1 ± 5.0% of control, respectively, in 9 of 10 additional SGS cells (Fig. 3). Co-application of bicuculline did not alter the CACA-evoked reductions in EPSC amplitudes (Fig. 3), which remained at 28.6 ± 6.5%. Similar to our results in the rat SC, these results strongly suggest that the effects of muscimol (0.5 μM) and CACA application on electrically evoked EPSCs in WT SGS cells are selective to GABACRs and that IPSCs are mediated by both GABAA and GABACRs. These data also suggest that selective activation of the GABACR reduces EPSCs in one group of SGS cells by direct inhibition and reduces IPSCs in another group by disinhibition.
FIG. 3.

cis-Aminocrotonic acid (CACA) selectively activates GABACRs and reduces postsynaptic responses in WT mouse SGS cells. In these 2 cells, 50 μM CACA reduced EPSC amplitudes (A and C) and also IPSC amplitudes (A), which leads to a prolonged EPSC. In both cases, 10 μM bicuculline, which strongly enhanced EPSCs when applied alone, could not block the CACA effects (B and D).
IPSCs in WT SGS are partly mediated by GABAC receptors
Although the results from the activation of GABACRs by muscimol and CACA application indicate the presence of GABACR-mediated currents in SGS neurons, the question remains whether their inhibition modulates synaptic transmission. We examined the effect of the selective GABACR antagonist TPMPA on electrically evoked PSCs (Fig. 4). Because TPMPA also acts as a GABABR agonist (Schmidt et al. 2001), we always applied the GABABR antagonist CGP 52432 before and with TPMPA in these experiments. Similar to our results with GABACR agonists, TPMPA application reduced the IPSC amplitude by 29.2 ± 2.4% of control in 26 of 33 cells tested (79%). In nine cells, EPSC amplitude was enhanced at the same time (Fig. 4A), on average by 10.5 ± 2.6%. As expected, the IPSCs resistant to both CGP52432 and TPMPA were GABAAR-mediated and were always completely eliminated when bicuculline was included in the bath. These results also support our conclusion that in mouse SGS neurons inhibition evoked by electrical stimulation of optic tract fibers is partly mediated by GABACRs.
FIG. 4.

GABACRs contribute to postsynaptic inhibition in WT mouse SGS cells. Postsynaptic responses, at −30 mV holding potential, to stratum opticum (SO) stimulation in 2 SGS cells in the presence of 5 μM [3-[[(3,4-dichlorphenyl)methyl]amino]propyl](diethoxymethyl) phosphinic acid (CGP 52432), CGP +50 μM 1,2,5,6-tetrahydropyridine-4-yl phosphinic acid (TPMPA), and GCP + TPMPA +20 μM bicuculline to block GABAARs, GABABRs and GABACRs, respectively. While TPMPA application reduced the IPSC amplitudes, a complete block of synaptic inhibition only appeared in the presence of all antagonists.
GABACR-mediated function is reduced but not eliminated in SGS cells in GABAC ρ1 Null mice
When excitatory and inhibitory inputs overlap temporally, both the strength of a synaptic input as well as its duration influence the temporal profile of the postsynaptic response. GABACRs exhibit smaller chloride conductances and longer mean open times and do not desensitize compared with GABAARs (Bormann 2000; Enz 2001). When both GABAA and GABACRs mediate an IPSC, GABAARs contribute primarily to the initial phase and to its maximum amplitude, while GABACRs contribute to the more sustained component, primarily governing its decay. These differences have been most thoroughly characterized in retinal bipolar cells (Eggers et al. 2007; Lukasiewicz and Shields 1998; McCall et al. 2002; Palmer 2006) and isolated dLGN cells (Schlicker et al. 2003). In retinal bipolar cells, elimination of GABACρ1 subunit expression leads to a complete loss of GABACR expression and inhibition in the retina (Eggers and Lukasiewicz 2006a,b; Eggers et al. 2007; McCall et al. 2002; Sagdullaev et al. 2006). The remaining GABA- and light-evoked IPSCs in bipolar cells have significantly faster time to peak and decay kinetics, smaller total areas and do not show compensation by other inhibitory inputs (Eggers and Lukasiewicz 2006a,b; Eggers et al. 2007; McCall et al. 2002; Sagdullaev et al., 2006).
If the subunit composition and control of expression of GABACRs in retina and SC are similar, a comparable functional loss should be evident in the SGS cells of GABAC ρ1 Null mice. To this end, we analyzed and compared the normalized areas under the EPSC and IPSC curves by calculating the ratio of their maximum amplitude and area, similar to our analysis of inhibitory currents in retinal bipolar cells (Eggers et al. 2007; McCall et al. 2002). This approach emphasizes the slow-onset, sustained GABACR contribution and minimizes variability in current amplitude across individual SGS cells, which results from the depth of the cell and its processes in the slice and whether all GABAR bearing processes are intact.
Under control conditions, the amplitude-to-area ratios of the electrically evoked EPSCs in WT cells were significantly larger than those in GABAC ρ1 Null SGS cells (Fig. 5; 0.21 ± 0.02 vs. 0.13 ± 0.01; P < 0.002; n = 6) because EPSCs in WT cells terminated earlier than EPSCs in GABAC ρ1 Null cells (Fig. 5A). These differences were not due to a general difference in excitatory input between WT and GABAC ρ1 Null SGS cells because their EPSC amplitude-to-area ratios were similar (0.054 ± 0.001 vs. 0.055 ± 0.01) when all GABA receptors were blocked with a combination of bicuculline, CGP 52432, and TPMPA (Fig. 5B). Thus the difference in electrically evoked GABAergic inhibition of EPSCs must result from a change in GABAC ρ1 subunit expression. Consistent with this result, IPSCs in WT cells under control conditions had significantly longer durations compared with GABACρ1 Null cells (Fig. 5C), resulting in significantly smaller amplitude-to-area ratios (0.05 ± 0.02 vs. 0.07 ± 0.02; P = 0.02). In contrast to our finding in retinal bipolar cells, when TPMPA was present in the bath, IPSCs in WT cells remained significantly shorter in duration compared with IPSCs in GABAC ρ1 Null cells in control solution (amplitude-to-area ratio: 0.10 ± 0.02 vs. 0.07 ± 0.02, P < 0.035; Fig. 5D). To further quantify and verify this observation, we compared the temporal profiles of the electrically evoked PSCs from WT and ρ1 Null cells in the presence of TPMPA when only GABAARs should contribute to postsynaptic inhibition. The temporal profiles of WT and GABAC ρ1 Null IPSCs were indistinguishable (Fig. 5E) as were their amplitude-to-area ratios (0.10 ± 0.02 vs. 0.09 ± 0.03). These results indicate that the loss of GABAC ρ1 subunit expression alters GABACR-mediated inhibition in SGS cells rather than eliminating its expression. In addition, the results suggest that there is no compensation in GABAAR-mediated input, similar to our finding for retinal bipolar cells. However, our evidence that a TPMPA-sensitive component remains in GABAC ρ1 Null SGS cells is in stark contrast to our results in retina where the IPSCs of ρ1 Null retinal bipolar cells were identical to WT IPSCs in the presence of TPMPA.
FIG. 5.

Synaptic responses to SO stimulation of SGS neurons in WT and GABACR ρ1 Null mice differ. A: at a holding potential of −50 mV, EPSCs from GABACR ρ1 Null cells on average have longer durations than EPSCs from WT cells. B: a complete blockade of GABAergic inhibition (GABAR block), using antagonists to all 3 GABAR types, produces no change in EPSCs durations in WT or GABACR ρ1 Null cells. C: at a holding potential of −30 mV, IPSCs from WT cells have longer durations than IPSCs from GABACR ρ1 Null cells in control solution. D: antagonism of GABACRs in WT cells by application of TPMPA leads to IPSCs that are considerably shorter in duration than IPSCs in GABACR ρ1 Null cells. E: when the GABACR contribution to the IPSC is eliminated by TPMPA in both WT and GABACR ρ1 Null cells, synaptic responses are similar in their temporal profile. Synaptic responses (A–E) were averaged from 6 cells and normalized to equal EPSC or IPSC amplitudes.
Activation of remaining GABACRs in ρ1 Null SGS cells
To characterize the remaining GABACRs in ρ1 Null SGS cells, we examined the effects of low concentrations of muscimol (0.5 μM) on electrically evoked EPSCs and IPSCs. The proportional change induced in PSC amplitude by muscimol in GABAC ρ1 Null SGS cells was not statistically different from that described in the preceding text in WT cells. Namely, bath application of muscimol affected EPSCs and IPSCs in 67% of GABAC ρ1 Null SGS cells (33/49; Fig. 6) and reduced their amplitudes 30.3 ± 3.2 and 45.4 ± 4.6% of control. Co-application of bicuculline did not significantly alter these muscimol-induced reductions (27.7 ± 4.2%), indicating the presence of functional, albeit altered GABACRs in the SC of ρ1 Null mice.
FIG. 6.
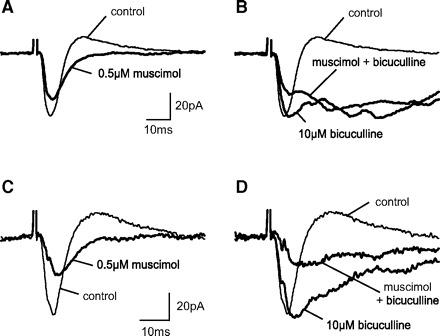
Functional GABACRs are present in GABACR ρ1 Null SGS cells. Inhibitory effect of muscimol on postsynaptic currents in 2 GABACR ρ1 Null SGS cells. As in WT cells, bath application of 0.5 μM muscimol reduced EPSC amplitudes (A and C), and this reduction was not altered by 10 μM bicuculline (B and D). Similar muscimol effects were observed in 67% of GABAC ρ1 Null cells.
Of the remaining 16 SGS cells, 0.5 μM muscimol led to EPSC increases (n = 6; Fig. 7A) or IPSC decreases (n = 4; Fig. 7B) or to both effects at the same time (n = 6; Fig. 7C). On average, EPSC amplitude increased by 32.4 ± 8.0% and IPSC amplitude decreased by 30.2 ± 3.2%. Again these muscimol-induced increases in the EPSCs were insensitive to bicuculline (23.5 ± 2.9%). In nine GABAC ρ1 Null SGS cells, bath application of CACA reduced both EPSC and IPSC amplitudes (36.3 ± 5.4 and 39.4 ± 5.5%, respectively; Fig. 8A). Reductions induced by CACA also were insensitive to bicuculline and decreased by 25.4 ± 5.1% of control (Fig. 8, B and D). Further, the muscimol or CACA-induced reductions in EPSC and IPSC amplitude were similar when GABAC ρ1 Null and WT cells were compared (Fig. 9). Finally, in WT cells (n = 48), bicuculline reduced IPSC amplitudes by 79.9 ± 3.8%, whereas in ρ1 Null cells (n = 39), IPSC reductions were significantly less, 57.0 ± 4.3% (Fig. 9; P < 0.001). This result indicates that the alteration in GABACR-mediated inhibition affects GABAAR-mediated inhibition in the SGS circuit. The only other GABACR subunit expressed in the SC is ρ2. Thus our results suggest that the CACA/muscimol-sensitive current is most likely carried by ρ2 homomeric channels.
FIG. 7.

In GABACR ρ1 Null, as in WT SGS cells, muscimol enhanced postsynaptic excitation. In the 3 cells shown, bath application of 0.5 μM muscimol either increased EPSC amplitudes (A), decreased IPSC amplitudes (B), or both increased EPSC and decreased IPSC amplitudes (C).
FIG. 8.

Postsynaptic responses are reduced by CACA in GABACR ρ1 Null SGS cells. In these 2 cells, 50 μM CACA reduced EPSC amplitudes (A and C) and also IPSC amplitudes (A). In both cases 10 μM bicuculline, which strongly enhances EPSCs when applied alone, could not block the effects of CACA (B and D). This indicates that CACA effects are mediated by GABACRs in SGS cells.
FIG. 9.

Quantitative comparison of muscimol-induced reductions of postsynaptic response amplitudes. Average reductions of EPSC and IPSC amplitudes (indicated as percentage of control amplitude) in the presence of either 0.5 μM muscimol or 50 μM CACA alone or of both agonists in the presence of 10 μM bicuculline, were not significantly different between WT and GABACR ρ1 Null SGS cells. However, a statistically significant difference for IPSC amplitude reductions was observed in response to bicuculline application. Error bars indicate SEs.
Morphological identification of recorded cells
Based on earlier results from rat SGS (Pasternack et al. 1999; Schmidt et al. 2001), we proposed a selective expression of GABACRs by local GABAergic SGS interneurons. We categorized the identified cell types that express GABACRs in mouse SGS (Supplementary Figs. S1 and S2).1 The majority of cells where the electrically evoked EPSC was reduced after GABACR activation had dendritic morphologies of putative local GABAergic interneurons in both WT (20/29) and ρ1 Null (23/28) SC, similar to the rat. Specifically, EPSCs were reduced after either muscimol or CACA application in identified piriform (16/20), horizontal (21/29) and stellate (5/11) cells. Different from our earlier reports on rat SC, we observed enhanced EPSC amplitudes after GABACR activation in only 4 of 16 identified narrow and wide field cells. In the majority of both narrow and wide field cells, EPSC amplitudes instead were reduced after GABACR activation. This suggests that, at least in the mouse, many putative projection neurons also express GABACRs.
DISCUSSION
GABAC receptors in the WT mouse SC
Our results indicate that GABACRs mediate inputs to SGS cells in the WT mouse SC. Similar to our observations in the rat (Schmidt et al. 2001), GABACR activation by both muscimol and CACA reduces electrically evoked PSCs in most WT SGS cells that display dendritic morphologies of putative local GABAergic interneurons. In contrast to our results in rat, however, GABACR activation reduced PSCs in most mouse SGS cells with projection cell morphologies (e.g., narrow- and wide-field cells) and enhanced PSCs in only a fraction of the putative projection neurons. In support of this result, GABACR expression has been reported in wide-field rat SGS cells (Kirischuk et al. 2003). In all cases, the changes in the SGS cell responses induced by GABACR agonists are not altered by the addition of bicuculline, verifying that these inputs are indeed GABACR mediated.
When SGS cell responses are evoked electrically, both agonists (CACA or low concentration of muscimol) and antagonists (TPMPA) produce a variety of changes in EPSC and IPSC components that include only increases or decreases or a combination of the two. Agonist activation of WT GABACRs could directly reduce interneuron (IN) activity and, presumably, would reduce GABA release, leading to increased excitation in postsynaptic target cells (disinhibition) (Fig. 10). In SGS projection neurons (PN) that express GABACRs, agonist activation will instead mediate a direct inhibition reducing EPSC amplitude. Further, if both pre- and postsynaptic partners express GABACRs, both activation and blockade of GABACRs can influence postsynaptic inhibition reducing inhibition or increasing excitation. Finally, because effects mediated by pre- and postsynaptic GABACRs might overlap temporally, the resulting response will depend on their relative contributions and timing at pre- and postsynaptic sites, and this may depend on their location in the SC circuitry. Thus our results indicate that GABACRs mediate multiple functions in the mouse SGS circuit, serving to either increase or decrease the strength of the output of SGS projection cells.
FIG. 10.

Schematic diagram of the proposed synaptic arrangement in SGS. In SGS, both projection neurons (PN) and local interneurons (IN) receive excitatory input from retinal afferents mediated by ionotropic GluRs. Interneurons release GABA onto projection neurons where it acts mainly through GABAARs (red) but also through GABACRs (yellow). GABACRs also serve as autoreceptors in GABAergic interneurons. In addition to excitatory input, interneurons also receive inhibitory input through GABAARs and GABACRs.
GABACR-mediated responses in GABAC ρ1 Null SGS cells are altered but not eliminated
In the absence of ρ1 expression, the temporal profiles of WT and GABAC ρ1 Null IPSCs differ. WT IPSCs had longer durations than those in GABAC ρ1 Null SGS cells, consistent with the absence of an inhibitory receptor with long channel open times and little desensitization, like the GABAC receptor (Bormann 2000; Bormann and Feigenspan 1995; Chebib and Johnston 1999). A similar change in the time course of GABA-mediated currents has already been reported for rod bipolar cells in the GABAC ρ1 Null retina where the majority of the GABAergic current is mediated by GABACRs (Euler and Wässle 1998; Frech and Backus 2004; McGillem et al. 2000).
When we compared the IPSCs in WT SGS cells in the presence of TPMPA with GABAC ρ1 Null cells in control solution, we found an unexpected result inconsistent with the effects that we observed in the retina. In the presence of TPMPA, the IPSCs in WT SGS cells were not similar to those of GABAC ρ1 Null cells (Eggers and Lukasiewicz 2006b). Further, a TPMPA-sensitive component was evident in GABAC ρ1 Null SGS cells. Although a TPMPA-sensitive current (and with it GABACR function) persists in GABAC ρ1 Null SGS cells, the difference in the time course of the WT and ρ1 Null IPSCs is consistent with the absence of receptors that are dominated by the kinetics of the ρ1 subunit. This result argues that GABAC ρ1 subunits are more critical to GABACR assembly and/or function in retinal bipolar cells than they are in SGS cells in the SC.
Differences in inhibitory circuitry in the absence of GABAC ρ1 subunit expression
In addition to the difference in GABACR IPSC kinetics between ρ1 Null and WT SGS cells, we also observed two surprising results: that the effects of activating the GABACRs in ρ1 Null SGS cells are similar to WT and that there was a statistically significant reduction in the sensitivity of GABAC ρ1 Null SGS cells to the specific GABAAR antagonist, bicuculline. Neither were consistent to our findings in the retina, where all GABACR-mediated currents were eliminated and no compensatory changes in GABA or glycine currents were found (Eggers and Lukasiewicz 2006a,b; Eggers et al. 2007;McCall et al. 2002; Sagdullaev et al. 2006).
When we observed that a TPMPA-sensitive current remained in GABAC ρ1 Null SGS cells, our first hypothesis was that it was mediated by homooligomeric ρ2 receptors. However, this is inconsistent with published data showing that homooligomeric ρ2 receptors have lower channel conductance than homooligomeric ρ1 or heteromeric ρ1/ρ2 receptors (Enz and Cutting 1998, 1999; Wang et al. 1994). In this scenario, we also would have to postulate that the remaining GABACRs in GABAC ρ1 Null SGS cells are upregulated to compensate for the loss of ρ1-mediated current. An additional consequence of this argument is that wherever the receptors are located they will mediate IPSCs that are shorter in duration. Because our results, both from mouse and rat SGS, indicate that the majority of local GABAergic interneurons express GABACRs (Boller and Schmidt 2001, 2003; Pasternack et al. 1999; Schmidt et al. 2001), this would be consistent with a role as autoinhibitors that regulate GABA release (Fig. 10). In the absence of receptors that contain ρ1 subunits on these interneurons, autoinhibition would be decreased, GABA release would be enhanced, and the sensitivity of the postsynaptic receptors to the competitive antagonist, bicuculline, could be reduced. An additional hypothesis that could explain some of our results is a difference in the combination and/or location of heteromeric versus homomeric GABACRs in the SGS projection cells. For example, it is possible that homomeric GABAC ρ2Rs are always present but are located extrasynaptically, whereas homomeric GABAC ρ1Rs are synaptic. In this scenario, homomeric GABACρ2R inputs would never be evident as both the amount of GABA available for their activation, the total charge that they carry and their faster kinetics would be swamped by the inputs of the homomeric GABAC ρ1Rs. In the absence of GABAC ρ1Rs, the influence of the homomeric GABAC ρ2Rs would be revealed. If this occurs in conjunction with increased GABA release, then there could be compensation for their reduced total charge, although we would not expect this compensation to fully restore GABAC receptor function.
Alternatively, or in conjunction (again in contrast to the retina), the increased duration of GABA release could cause a downregulation of postsynaptic GABAA receptors, particularly during development. In this case, the proportion of receptors available to bicuculline would be smaller and paired with an increase in GABA in the synapse could underlie the reduced sensitivity because bicuculline is a competitive antagonist. There remain too many inconsistencies in this issue of reduced bicuculline sensitivity in the GABAC ρ1 Null SC and the resolution of this interesting finding requires further investigation.
Taken together our results strongly suggest a fundamental difference in the function of the GABAC ρ1 subunit in the trafficking, the composition and/or function of GABACRs between the SC and the retina.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 509, “Neurovision”, TP A8 to M. Schmidt and National Eye Institute Grant EY-014701 to M. A. McCall and an unrestricted grant from the Research to Prevent Blindness to University of Louisville Department of Ophthalmology and Visual Sciences.
Supplementary Material
Acknowledgments
We thank M. Möllmann for excellent technical assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Boller and Schmidt 2001.BollerMBoller M, Schmidt M. Postnatal maturation of GABAA and GABAC receptor function in the mammalian superior colliculus. Eur J Neurosci 14: 1185–1193, 2001. [DOI] [PubMed] [Google Scholar]
- Boller and Schmidt 2003.BollerMBoller M, Schmidt M. GABAC receptors in the rat superior colliculus and pretectum participate in synaptic neurotransmission. J Neurophysiol 89: 2035–2045, 2003. [DOI] [PubMed] [Google Scholar]
- Bormann 2000.BormannJBormann J. The “ABC” of GABA receptors. Trends Pharmacol Sci 21: 16–19, 2000. [DOI] [PubMed] [Google Scholar]
- Bormann and Feigenspan 1995.BormannJBormann J, Feigenspan A. GABAC receptors. Trends Neurosci 18: 515–519, 1995. [DOI] [PubMed] [Google Scholar]
- Boué-Grabot et al. 1998.Boué-GrabotEBoué-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J Neurochem 70: 899–907, 1998. [DOI] [PubMed] [Google Scholar]
- Chebib and Johnston 1999.ChebibMChebib M, Johnston GA. The “ABC” of GABA receptors: a brief review. Clin Exp Pharmacol Physiol 26: 937–940, 1999. [DOI] [PubMed] [Google Scholar]
- Dodt and Zieglgänsberger 1990.DodtHUDodt HU, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res 537: 333–336, 1990. [DOI] [PubMed] [Google Scholar]
- Dong and Werblin 1998.DongCJDong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol 79: 2171–2180, 1998. [DOI] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz 2006a.EggersEDEggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz 2006b.EggersEDEggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers et al. 2007.EggersEDEggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz 2001.EnzREnz R. GABAC receptors: a molecular view. Biol Chem 382: 1111–1122, 2001. [DOI] [PubMed] [Google Scholar]
- Enz et al. 1996.EnzREnz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAC receptor rho subunits in the mammalian retina. J Neurosci 16: 4479–4490, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz and Cutting 1998.EnzREnz R, Cutting GR. Molecular composition of GABAC receptors. Vision Res 38: 1431–1441, 1998. [DOI] [PubMed] [Google Scholar]
- Enz and Cutting 1999.EnzREnz R, Cutting GR. GABAC receptor rho subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur J Neurosci 11: 41–50, 1999. [DOI] [PubMed] [Google Scholar]
- Euler and Wässle 1998.EulerTEuler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 79: 1384–1395, 1998. [DOI] [PubMed] [Google Scholar]
- Feigenspan et al. 1993.FeigenspanAFeigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature 361: 159–162, 1993. [DOI] [PubMed] [Google Scholar]
- Flores-Herr et al. 2001.Flores-HerrNFlores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21: 4852–4863, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazao et al. 2007.FrazaoRFrazao R, Noguiera MI, Wässle H. Colocalization of synaptic GABAC-receptors with GABAA-receptors and glycine-receptors in the rodent central nervous system. Cell Tissue Res 330: 1–15, 2007. [DOI] [PubMed] [Google Scholar]
- Frech and Backus 2004.FrechMJFrech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci 21: 645–652, 2004. [DOI] [PubMed] [Google Scholar]
- Greka et al. 2000.GrekaAGreka A, Lipton SA, Zhang D. Expression of GABAC receptor rho1 and rho2 subunits during development of the mouse retina. Eur J Neurosci 12: 3575–3582, 2000. [DOI] [PubMed] [Google Scholar]
- Kirischuk et al. 2003.KirischukSKirischuk S, Akyeli J, Iosub R, Grantyn R. Pre- and postsynaptic contribution of GABAC receptors to GABAergic synaptic transmission in rat collicular slices and cultures. Eur J Neurosci 18: 752–758, 2003. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz 1996.LukasiewiczPDLukasiewicz PD. GABAC receptors in the vertebrate retina. Mol Neurobiol 12: 181–194, 1996. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz and Shields 1998.LukasiewiczPDLukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol 79: 3157–3167, 1998. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz and Werblin 1994.LukasiewiczPDLukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci 14: 1213–1223, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall et al. 2002.McCallMAMcCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the ρ1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J Neurosci 22: 4163–4174, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillem et al. 2000.McGillemGSMcGillem GS, Rotolo TC, Dacheux RF. GABA responses of rod bipolar cells in rabbit retinal slices. Vis Neurosci 17: 381–389, 2000. [DOI] [PubMed] [Google Scholar]
- Palmer 2006.PalmerMJPalmer MJ. Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. J Physiol 577: 45–53, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan and Lipton 1995.PanZHPan ZH, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci 15: 2668–2679, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack et al. 1999.PasternackMPasternack M, Boller M, Pau B, Schmidt M. GABAA and GABAC receptors have contrasting effects on excitability in superior colliculus. J Neurophysiol 82: 2020–2023, 1999. [DOI] [PubMed] [Google Scholar]
- Rozzo et al. 2002.RozzoARozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur J Neurosci 15: 1747–1758, 2002. [DOI] [PubMed] [Google Scholar]
- Sagdullaev et al. 2006.SagdullaevBTSagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006. [DOI] [PubMed] [Google Scholar]
- Schlicker et al. 2003.SchlickerKSchlicker K, Boller M, Schmidt M. GABAC receptor mediated inhibition in acutely isolated neurons of the rat dorsal lateral geniculate nucleus. Brain Res Bull 63: 91–97, 2003. [DOI] [PubMed] [Google Scholar]
- Schmidt et al. 2001.SchmidtMSchmidt M, Boller M, Özen G, Hall WC. Disinhibition in rat superior colliculus mediated by GABAC receptors. J Neurosci 21: 691–699, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle et al. 1998.WässleHWässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res 38: 1411–1430, 1998. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1994.WangTLWang TL, Guggino WB, Cutting GR. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J Neurosci 14: 6524–6531, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegelius et al. 1998.WegeliusKWegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saarma M, Reeben M. Distribution of GABA receptor rho subunit transcripts in the rat brain. Eur J Neurosci 10: 350–357, 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


