SUMMARY
Regulatory T (Treg) cells suppress inflammatory immune responses and autoimmunity caused by self-reactive T cells. The key Treg cell transcription factor Foxp3 is downregulated during inflammation to allow for the acquisition of effector T cell-like functions. Here, we demonstrate that stress signals elicited by proinflammatory cytokines and lipopolysaccharide lead to the degradation of Foxp3 through the action of the E3 ubiquitin ligase Stub1. Stub1 interacted with Foxp3 to promote its K48-linked polyubiquitination in an Hsp70-dependent manner. Knockdown of endogenous Stub1 or Hsp70 prevented Foxp3 degradation. Furthermore, the overexpression of Stub1 in Treg cells abrogated their ability to suppress inflammatory immune responses in vitro and in vivo, and conferred a T helper 1 (Th1) cell-like phenotype. Our results demonstrate the critical role of the stress-activated Stub1-Hsp70 complex in promoting Treg cell inactivation, thus providing a potential therapeutic target for the intervention against autoimmune disease, infection and cancer.
INTRODUCTION
During inflammation, several types of physiological stresses are likely to be encountered within the microenvironment, such as hypoxia, oxidative stress, microbial products (e.g. lipopolysaccharide (LPS)) and inflammatory cytokines. The notion that such stresses or ‘danger signals’ can regulate immune responses has long been proposed (Shi et al., 2003). While intensive studies have demonstrated how such stimuli can activate cells of the innate immune system—mainly through innate receptors such as Toll-like receptors (TLR) and the inflammasome (Schroder and Tschopp, 2010), few studies have addressed how stress signals inactivate the negative regulators of the adaptive immune system, namely regulatory T (Treg) cells, in order to facilitate effective immune responses (Gallucci and Matzinger, 2001; Matzinger, 2007). Understanding the impact of inflammatory stimuli on the Treg cell lineage is crucial given the importance of these cells in mitigating excessive or misdirected immune activation.
Treg cells may lose Foxp3 expression to gain effector T cell function under inflammation, but how the stability of Foxp3 expression and the functional plasticity of the Treg cell lineage are dynamically regulated are currently under debate (Miyao et al., 2012; Rubtsov et al., 2010; Sakaguchi et al., 2013; Zhou et al., 2009). The tissue microenvironment and inflammatory milieu may play a role in the acquisition of T effector-like phenotypes by Foxp3+ T cells (Floess et al., 2007; Fontenot et al., 2005). Furthermore, cytokines such as IL-4, IL-6, and IL-21 have been shown to bestow Foxp3+ T cells with phenotypes of various T effector subsets in vitro (Tsuji et al., 2009; Xu et al., 2007; Yang et al., 2008). However, the mechanism underlying Foxp3 degradation during inflammation remains unclear.
We investigated the impact of inflammatory stress on Treg cell stability and function. Our findings describe a mechanism regulating Foxp3 protein expression that ultimately affects the balance between Treg and T effector cell activity. The downregulation of Foxp3, and subsequent relief from Treg cell-mediated immune suppression in response to inflammatory cues, was dependent on the ubiquitination of Foxp3 by the E3 ligase Stub1. The interaction between Stub1 and Foxp3 was in turn dependent on the stress indicator protein Hsp70. These findings reveal a hitherto unknown pathway for the reduction of Foxp3 protein expression and loss of Treg-mediated immune suppression in the face of inflammatory stimuli, with implications for a variety of diseases resulting from uncontrolled immune responses.
RESULTS
Foxp3 expression is destabilized by inflammation-associated stress signals
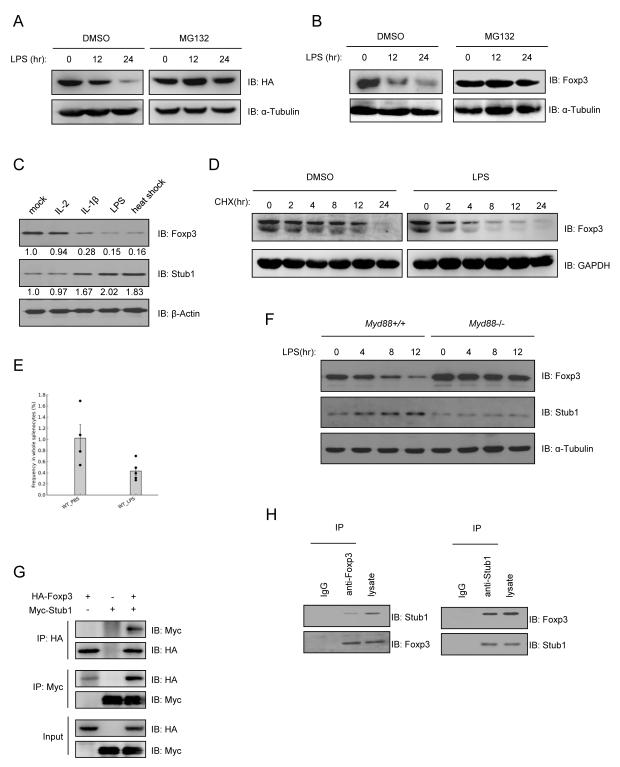
The majority of nTreg cells are relatively stable in a healthy individual (Floess et al., 2007; Gavin et al., 2007). However, 10-15% of these ‘stable’ Treg cells were found to lose Foxp3 expression after their adoptive transfer into lymphopenic hosts, while gaining the capacity to produce IL-2 and IFN-γ. Several groups have observed the loss of Foxp3 expression during autoimmune inflammation through Foxp3 intracellular staining or Foxp3-GFP reporter mice (Fontenot et al., 2005) suggesting that under certain conditions Foxp3 expression and Treg function may be unstable. We set out to determine whether LPS or inflammatory cytokines—the stresses likely encountered as a consequence of infection and inflammation, could negatively affect Foxp3 protein stability at the posttranslational level. To test this, we engineered a Jurkat T cell line stably expressing HA-tagged Foxp3 under control of the constitutive ubiquitin promoter (HAFoxp3 Jurkat T cells), and exposed these cells to several stimuli typical of inflamed tissues. Foxp3 protein expression was noticeably decreased upon exposure to LPS (Figure 1A). The addition of the proteasome inhibitor MG132 prevented Foxp3 loss suggesting that this process was proteasome-dependent. Similar results were observed in CD4+CD25hiCD127lo human primary nTreg cells (Figure 1B); we also found that heat shock, IL-1β and TNFα resulted in the loss of Foxp3 in mouse nTreg cells (Figure 1C), where IL-1β and TNFα-mediated Foxp3 loss was also prevented by the addition of MG132 (Figure S1A). Since exposure to LPS resulted in pronounced loss of Foxp3 protein, we explored further the effects of LPS on the stability of the Foxp3 protein pool. To this end we measured amounts of the transcription factor in cycloheximide (CHX) treated human primary Treg cells activated in the presence or absence of LPS. Foxp3 was reduced by exposure of Treg cells to LPS (Figure 1D). Further calculation revealed that LPS treatment markedly shortened the half-life of Foxp3 compared to that in mock treated cells (Figure S1B). As previously seen, administration of MG132 stabilized Foxp3 levels in these cells (Figure S1C). Further demonstrating the negative impact of inflammatory cues on Foxp3 expression, repeated administration of low dose LPS to C57BL/6 mice resulted in Foxp3 downregulation in vivo. The splenocytes of mice given LPS (i.p.) contained a lower percentage of Foxp3+ CD4+ T cells than control mice injected with PBS (Figure 1E).
Figure 1.
Foxp3 interacts with Stub1 and is degraded in a proteasome-dependent manner upon exposure to inflammatory cues. Either a Jurkat cell line stably expressing HA-Foxp3 (HA-Foxp3 Jurkat T cells) (A), primary human (B, D) or mouse (C) Tregs were stimulated with LPS or other cytokines for the indicated time periods in the absence or presence of 5μM MG132. Cells were harvested and subjected to protein blotting (A, B, C). Panels A-C are representative blots. Numbers under the Foxp3 and Stub1 bands in panel C represent their relative density. (D) LPS decreased Foxp3 half-life in Treg cells. Human Treg cells were treated with 5μg/ml CHX in the absence or presence of LPS. Cells were harvested at the indicated times. Cell lysates were analyzed by Western blotting. (E) Low dose LPS exposure downregulates Foxp3 in vivo. C57BL/6 mice were injected weekly i.p. with either 100μl PBS or 0.5μg/kg LPS (E.coli 0111B4) over a four week period. Total splenocytes were harvested and subjected to flow cytometry analysis of CD4+Foxp3+ cells. The percentages of CD4+Foxp3+ T cells within total splenocytes were quantified and compared. *p<0.05. Error = mean +/−SEM. (F) Myd88 deficiency renders nTregs resistant to LPS-mediated Foxp3 loss. CD4+CD25Hi T cells (nTregs) were purified by flow cytometry from age and sex-matched wild-type and Myd88−/− C57BL/6 mice and activated through CD3/CD28 in the presence or absence of LPS (1μg/ml). Foxp3 and Stub1 protein expression was assessed at the indicated time-points. (G) HA-Foxp3 and Myc-Stub1 expression plasmids were cotransfected into 293T cells. Cells were lysed for co-immunoprecipitation (Co-IP) as indicated. (H) Naïve T cells were isolated and cultured under iTreg skewing conditions for 3 days, followed by heat shock for 45 min before being harvested for endogenous Co-IP and protein blotting, as indicated. Also see Figure S1.
To confirm that LPS destabilizes the Foxp3 protein pool, we tested Treg cells incapable of detecting LPS through the TLR signaling pathway for resistance to LPS-mediated Foxp3 loss. Here we stimulated nTreg cells from wild-type C57BL/6 mice with LPS, which, as expected, resulted in Foxp3 downregulation. In contrast, Foxp3 expression was stabilized in Treg cells isolated from mice genetically deficient in Myd88 (Myd88−/− mice) despite LPS exposure (Figure 1F), suggesting that LPS-mediated TLR signaling negatively impacts the stability of Foxp3. Corroborating the negative effects of LPS on Foxp3 expression, qRT-PCR analysis showed that exposure of nTregs to LPS in vitro elevates the expression of genes normally suppressed by Foxp3 such as IL-2 and IFN-γ. Equally noticeable was the reduced expression of genes activated by Foxp3 and associated with the Treg cell phenotype such as CTLA-4, GITR and CD25 (Figure S1D). These results support a model where Foxp3 expression and Treg cell function may be suppressed in response to an imminent threat or inflammatory microenvironment.
Identification of Hsp70, a recruiter of Stub1, as a subunit of the Foxp3 Complex
To understand the mechanism underlying Foxp3 degradation, we purified Foxp3 and its associated binding partners (Foxp3 complex) from TAP-Foxp3 transfected HEK293T cells using a tandem-affinity purification approach (data not shown). Subsequent mass-spectrometry (MS) sequencing was used to identify individual peptides of any Foxp3 binding partners (Figure S1E). We found that the sequences of nine peptides within the identified Foxp3 protein complex corresponded to heat shock 70kDa protein 1A (also known as Hsp70 or HSPA1A) (UniProtKB: P08107) (Figure S1F). Both Hsp70 and the related Hsc70 are known to recruit a stress-activated E3 ubiquitin ligase named ‘Stub1’ to proteins targeted for degradation (Ballinger et al., 1999). Stub1 was found upregulated in mouse nTreg cells under the same inflammatory stresses shown earlier to result in Foxp3 downmodulation (Figure 1C). Additionally, treatment of human Treg cells with LPS resulted in progressive loss of Foxp3 protein coincident with Stub1 upregulation (Figure S1G). The cytokine IL-18, like IL-1β and TNFα, increased Stub1 expression in Treg cells while the inhibitor SB203580, or exposure to osmotic stress or hypoxic culture conditions all failed to noticeably alter Stub1 expression (Figure S1H). The observation that HIF-1α was not required for LPS-induced Foxp3 downmodulation in Treg cells (Figure S1I) suggested that LPS-Stub1-induced Foxp3 depletion is distinct from the process we previously observed under hypoxic stress (Dang et al. 2011). These results suggest that Stub1 is specifically induced in Treg cells by inflammatory signals that regulate Foxp3 at the protein level.
The selective nature of Stub1 induction was further demonstrated by the observation that while LPS, a potent activator of Toll like receptor signaling, is an effective inducer of both Foxp3 loss and Stub1 mRNA expression, agonists specific to other TLRs failed to significantly upregulate Stub1 in human nTreg cells (Figure S1J). An inability to upregulate Stub1 in response to LPS may be responsible for the stabilization of Foxp3 levels in Myd88−/− Treg cells. Indeed, Stub1 expression in these cells was unchanged by LPS unlike those of wild-type Treg cells (Figure 1F) suggesting Stub1 may be involved in the depletion of Foxp3 from Treg cells under specific inflammatory conditions prompting us to test whether Stub1 could interact with Foxp3 to mediate its degradation. 293T cells were co-transfected with tagged Foxp3 or Stub1 expression constructs for immunoprecipitation (IP) studies. Foxp3 (HA-Foxp3) pull-downed Stub1 (Myc-Stub1), and Foxp3 was reciprocally pull-downed by Stub1 (Figure 1G). The interaction between endogenous Stub1 and Foxp3 was further confirmed in iTreg cells derived in vitro from naïve CD4+ T cells (Figure 1H).
Next, we further characterized the interaction between Foxp3 and Stub1. Through the generation of systematic deletion mutants (Figure S1K) and co-transfection/co-IP experiments, we found that both the leucine zipper and coiled coil sub-domains of Foxp3 were essential for its interaction with Stub1 since neither deletion of the zipper (C1 and N3) or coiled-coil (N2 and N3) regions disrupted their interaction (Figure S1L). Additionally, we found that Stub1 could translocate into the nucleus and interact with Foxp3 under inflammation-associated stimuli (Figure S1M). Without LPS stimulation, Foxp3 mainly localized in the nucleus, with Stub1 in the cytoplasm (Figure S1M, left panels). The addition of the proteosome inhibitor MG132, to prevent the loss of Foxp3, allowed for the visualization of Foxp3 which co-localized with Stub1 after LPS stimulation, supporting the notion that these factors may interact (Figure S1M). The translocation of Stub1 from the cytoplasm to the nucleus after LPS treatment suggests that Stub1 translocation is a physiologically relevant consequence of exposing Treg cells to inflammatory stress, and that Stub1-mediated loss of Foxp3 is both inducible and spatially regulated during inflammation.
Stub1 mediates the ubiquitination and degradation of Foxp3
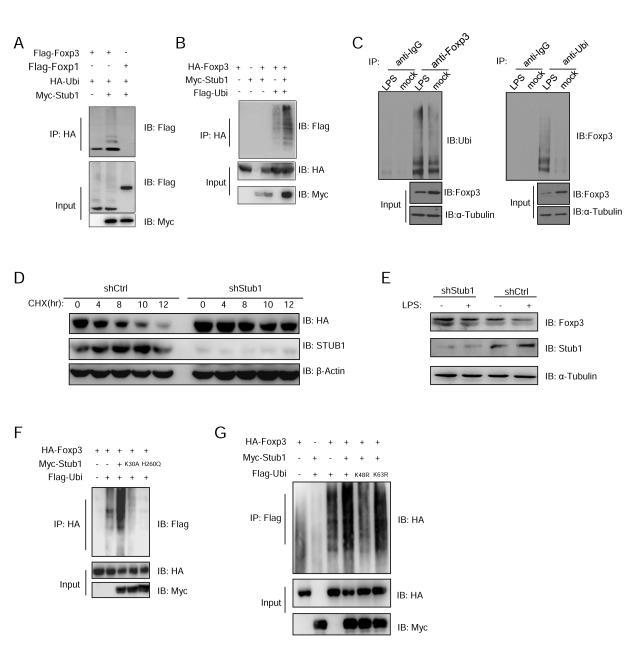
Since Foxp3 interacts with Stub1, which is known to degrade transcription factors such as Runx2 (Li et al., 2008), we asked whether Stub1 could directly ubiquitinate Foxp3 for degradation. To test this, Flag-tagged Foxp3 or Foxp1 expression plasmids were co-transfected with HA-Ubiquitin (HA-Ubi) and Myc-Stub1 into 293T cells, and then the ubiquitinated proteins were immunoprecipitated with anti-HA and immunoblotted with anti-Flag antibody to detect ubiquitinated Foxp proteins. Stub1 promoted the ubiquitination of Foxp3, but not its subfamily member Foxp1 (Figure 2A) that shares high homology at the Zinc-finger-leucine zipper and forkhead domain but not the N-terminal subdomains. Stub1-mediated ubiquitination of Foxp3 was further confirmed in Jurkat T cells (Figure 2B), which were co-transfected with HA-Foxp3, Myc-Stub1 and Flag-Ubi expression plasmids. As expected, Foxp3 polyubiquitination was significantly enhanced upon addition of Stub1 (Figure 2A, B). Also, Foxp3 polyubiquitination was enhanced in iTregs overexpressing Stub1 (Figure S2A) and importantly, Foxp3 ubiquitination was also detected in primary mouse nTreg cells exposed to LPS (Figure 2C).
Figure 2.
Stub1 mediates Foxp3 degradation through a ubiquitination and proteasome dependent pathway. (A) Flag-Foxp3 or Flag-Foxp1 was co-transfected with Myc-Stub1 and HAUbi into 293T cells. Cells were lysed for Co-IP as indicated. (B) HA-Foxp3, Myc-Stub1 and Flag-Ubi were co-transfected into Jurkat T cells. The cells were processed for Co-IP as indicated. (C) Primary Treg cells were stimulated with LPS for 24 hours. Cells were then harvested and lysed, followed by Co-IP, as indicated. (D, E) HA-Foxp3 Jurkat cells were transduced with lentivirus carrying the control shCK or shStub1 constructs, followed by CHX treatment for the indicated periods (D) or human Treg cells transduced with shCK or shStub1 were treated by LPS for 24 hours (E) before harvesting for protein blotting as indicated. (F) HA-Foxp3, Flag-Ubi and Myc-Stub1 or its mutants K30A or H260Q were co-transfected into 293T cells. The cells were lysed for Co-IP as indicated. (G) 293T cells were transfected with HA-Foxp3 and Myc-Stub1 together with Flag-Ubi (WT) or the ubiquitin mutants K48R or K63R. The cells were lysed for Co-IP as indicated. All panels are representative of at least three experiments. Also see Figure S2.
We next determined if Stub1 is indeed required for the LPS-induced loss of Foxp3 protein. A dose dependent effect of Stub1 on Foxp3 expression was observed in 293T cells co-transfected with HA-Foxp3 and various doses of Myc-Stub1 plasmid. When these cells were treated with DMSO, a Stub1 dose dependent, stepwise disappearance of Foxp3 protein was seen. Addition of MG132 resulted in stable Foxp3 protein expression even in the face of abundant Stub1 expression (Figure S2B). We then assessed the stability of Foxp3 expression in the presence or absence of Stub1. To this end we knocked down Stub1 in HA-Foxp3-Jurkat T cells and monitored the half life of HA-Foxp3 in these cells and those carrying a sh-control construct. Halting protein synthesis by CHX treatment of sh-control HA-Foxp3-Jurkat T cells resulted in progressive Foxp3 protein loss (Figure 2D). However, in agreement with earlier observations, inhibiting the proteasome with MG132 preserved Foxp3 protein levels throughout the experiment (data not shown). Importantly, knocking down Stub1 in the presence of CHX similarly extended the half life of Foxp3 (Figure 2D) supporting a role for the E3 ligase in depletion of cellular Foxp3 pools. These results suggest that targeting Stub1 can stabilize Foxp3 expression in the face of inflammatory stimuli. Indeed, shRNA knockdown of Stub1 prevented LPS mediated Foxp3 loss in primary human nTreg confirming its importance in this process (Figure 2E).
To test whether the E3 ligase activity and chaperone binding ability of Stub1 are essential for promoting the ubiquitination of Foxp3, 293T cells were co-transfected with HA-Foxp3, Flag-Ubi and Myc-Stub1 or mutant Stub1 deficient in chaperone-binding (K30A) or enzymatic activity (H260Q). Ubiquitinated proteins were immunoprecipitated with anti-Flag antibody, then immunoblotted with anti-HA antibody to detect ubiquitinated Foxp3. Here, we found that both the H260Q and K30A mutants failed to promote the polyubiquitination of Foxp3 (Figure 2F), which suggests that enzymatic activity and chaperone binding abilities of Stub1 are required for Foxp3 ubiquitination. Moreover, Stub1-mediated Foxp3 ubiquitination occurred via K48 linkage, since the K48R mutant abrogated this process (Figure 2G). These results suggest that Stub1 mediates Foxp3 degradation through ubiquitination and the proteasome in T cells encountering physiological danger signals. To further investigate Stub1-mediated ubiquitination and degradation of Foxp3, we tested whether the elimination of potential ubiquitination sites on Foxp3 could prevent Foxp3 removal by Stub1. Foxp3 constructs with mutated lysine residues were screened for resistance to Stub1-mediated degradation (Figure S3A). Suggesting that ubiquitination is key for Foxp3 downregulation, we found that altering all 20 lysine residues (K to R mutations) prevented the depletion of Foxp3 by overexpressed Stub1 (Figure S3A and S3B). While many of the individual lysine mutations did not stabilize Foxp3 protein levels, a mutant altered at four lysine residues (K227, 250, 263, 268R or ‘4R’) proved highly resistant to degradation (Figure S3B).
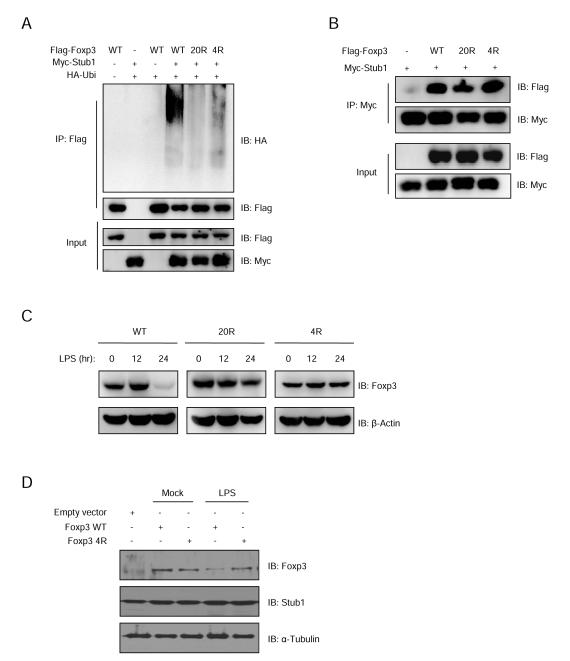
These Stub1-resistant mutants were expected to be less readily ubiquitinated than wild type Foxp3. To test this, 293T cells were transfected with Flag-Foxp3, the 20R mutant or the 4R mutant, together with Myc-Stub1 and HA-Ubi. 48 hours later, cell lysates were immunoprecipitated by anti-Flag antibody and analyzed by blotting for HA, Flag or Myc. We found that while wild type Foxp3 was highly ubiquitinated in the presence of Stub1, both the 20R and 4R mutants were not (Figure 3A). Interestingly, these Foxp3 mutants retained their ability to interact with Stub1 despite resisting ubiquitination (Figure 3B). These findings support the notion that Stub1 mediates Foxp3 degradation through ubiquitination at key lysine residues.
Figure 3.
Two Foxp3 mutants lacking distinct lysine residues interact with Stub1, but are resistant to Stub1-mediated ubiquitination. (A) 293T cells were transfected with Flag-Foxp3 or the 20R mutant (20 lysine residues lost) or the K227/250/263/268R (4R) mutant, together with Myc-Stub1 and HA-Ubi. 48 hours post-transfection, the cells were harvested and the cell lysates were immunoprecipitated by anti-Flag antibody prior to analysis by protein blotting for HA, Flag or Myc. (B) 293T cells were transfected with Flag-Foxp3 or 20R mutant or K227/250/263/268R (4R) mutant, together with Myc-Stub1. The cells lysates were immunoprecipitated with anti-Myc antibody and analyzed by protein blotting. (C) Jurkat T cells expressing Foxp3 WT or its mutant 20R or 4R were stimulated with 1μg/ml LPS for the indicated time periods. The Foxp3 levels in cell lysates were analyzed by protein blotting. (D) nTregs were transduced with lentivirus carrying Stub1-IRES-dsRed2 cassette; 48 hours post-transduction, dsRed2+ cells were sorted out and re-transduced with lentivirus carrying either empty vector, WT Foxp3 or the 4R mutant (all harboring GFP marker as an internal control) and cultured for another 2 days. The GFP+ cells were sorted out and stimulated for 24 hours before being harvested for immunoblotting as indicated. Also see Figure S3.
To investigate whether these Foxp3 mutants were also resistant to degradation mediated by inflammatory stress, Jurkat T cells expressing wild type Foxp3, or its 20R or 4R mutants were stimulated with 1μg/ml LPS as indicated. Protein blotting analysis of the cell lysates revealed that unlike wild type Foxp3, which was progressively lost, the 20R and 4R mutants were stably expressed (Figure 3C). Furthermore, introducing the 4R Stub1-resistant mutant into nTreg cells confirmed its enhanced stability in the face of LPS treatment. Here, nTregs cells were doubly transduced with lentivirus carrying a Stub1-IRES-dsRed2 cassette containing either empty vector, wild type Foxp3 or the 4R mutant (marked by GFP) prior to stimulation (Figure 3D). Collectively, these results indicate a mechanism for Foxp3 loss by Treg cells receiving inflammatory danger signals, which hinges on ubiquitin conjugation by Stub1.
Hsp70 is a recruiter of Stub1 for the ubiquitination and degradation of Foxp3
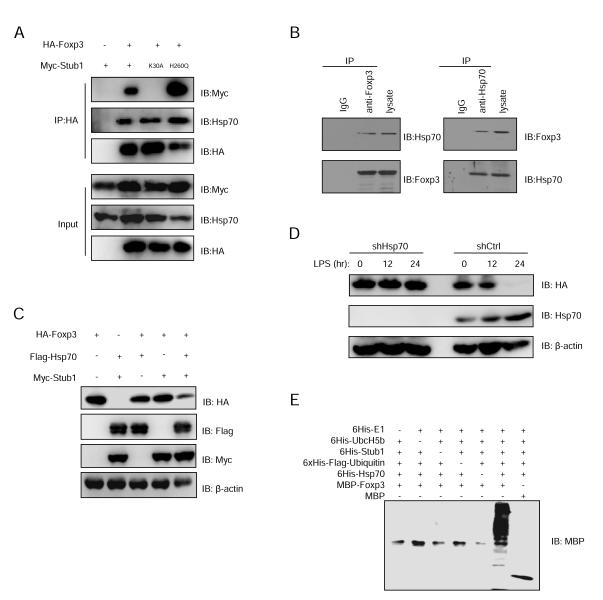
Since the biochemical stress indicator Hsp70 is required for Stub1-mediated degradation of many proteins (Luo et al., 2010), and given that Hsp70 co-purified with TAP-Foxp3 from ectopically expressed cell lines (Figure S1E, F), we asked whether the Foxp3-Stub1 interaction was dependent on Hsp70. To address this we co-transfected HA-Foxp3 with either Myc-Stub1, its K30A or H260Q mutants into 293T cells and immunoprecipitated Foxp3 with anti-HA antibody followed by immunoblotting with anti-Myc antibody, and found both wild type and the H260Q Stub1 mutant interact with Foxp3; however, the chaperone-binding deficient mutant K30A could not (Figure 4A). The interaction between Hsp70 and Foxp3 was also demonstrated by endogenous co-IP in nTreg cells (Figure 4B). These results suggest that Hsp70 may mediate the interaction between Foxp3 and Stub1. Moreover, we found that overexpression of both Stub1 and Hsp70 had a synergistic effect on mediating Foxp3 degradation (Figure 4C). We also found that the knockdown of Hsp70 in Foxp3-Jurkat T cells mitigated Stub1-mediated degradation of Foxp3 (Figure 4D). To further verify the involvement of Hsp70 in Foxp3 ubiquitination and degradation by Stub1, we purified MBP, MBP-Foxp3, His-E1, His-Ubc5Hb (E2), His-Stub1 (E3), His-Flag-Ubi and His-Hsp70 expressed in Escherichia coli for an in vitro ubiquitination assay (Figure 4E). Hsp70 was essential for Stub1-mediated ubiquitination of Foxp3 in this cell-free system (Figure 4E). Interestingly, although both Hsp70 and the closely related, but non-inflammation associated Hsc70 interact with Foxp3 as assessed by co-IP (Figure S4A), we found overexpression of Hsp70 but not Hsc70 could promote Foxp3 ubiquitination and degradation (Figure S4B). These results suggest the stress indicator Hsp70, but not constitutively expressed Hsc70, is an essential mediator of the Stub1-Foxp3 interaction.
Figure 4.
Hsp70 is required for Foxp3 degradation and synergizes with Stub1 in this process. (A) HA-Foxp3 and Myc tagged wild type Stub1 or the K30A or H260Q mutants were co-expressed in 293T cells. Foxp3 protein in the cell lysates was immunoprecipitated by anti-HA antibody and pulled down Stub1 was detected by protein blotting. (B) Primary Tregs were sorted from IRES-GFP reporter mice and stimulated with LPS for 24 hours before harvesting for Co-IP and immunoblotting as indicated. (C) HA-Foxp3, Myc-Stub1 and Flag-Hsp70 were cotransfected into 293T cells. Cell lysates were analyzed by protein blotting. (D) HA-Foxp3 Jurkat T cells were transduced with lentivirus carrying the shCK control or shHsp70 constructs carrying a puromycin selection marker. Selected cells were treated with LPS for the indicated time periods before harvesting for protein blotting with the indicated antibodies. (E) Purified 6His-E1, 6His-UbcH5b, 6His-Stub1, 6His-Flag-Ubi, 6His-Hsp70, and MBP-Foxp3 or MBP were mixed together in a cell-free system and incubated at 30oC for 2 hours. Foxp3 ubiquitination was detected by protein blotting with an anti-MBP antibody. Depicted are the representative results of at least three independent experiments. Also see Figure S4.
Stub1 negatively regulates Foxp3 protein expression, transcriptional repression and Treg function in mouse primary T cells
To further test the effects of overexpressing Hsp70 and Stub1 on Foxp3-mediated suppressive activity, Jurkat T cells were transfected with a luciferase reporter construct under the control of the IL-2 promoter and plasmids encoding either Stub1, Foxp3, Hsp70 in various combinations prior to stimulation and measurement of luciferase activity. As expected, Foxp3 expression suppressed IL-2 promoter activity. However, co-expression of Foxp3 with Stub1 resulted in a near complete reversal of IL-2 promoter suppression, a trend made more pronounced by simultaneous expression of Hsp70 (Figure S5A). Supporting results were also obtained using a luciferase-based transcriptional repression assay (Li et al., 2007) in 293T cells (Figure S5B). Western blotting of the cells confirmed that Foxp3 protein was nearly completely lost when cells received both Stub1 and Hsp70 (Figure S5C) suggesting that the loss of transcriptional suppression was indeed due to Foxp3 loss. These findings also reinforce the need for cooperation between Hsp70 and Stub1 for Foxp3 degradation.
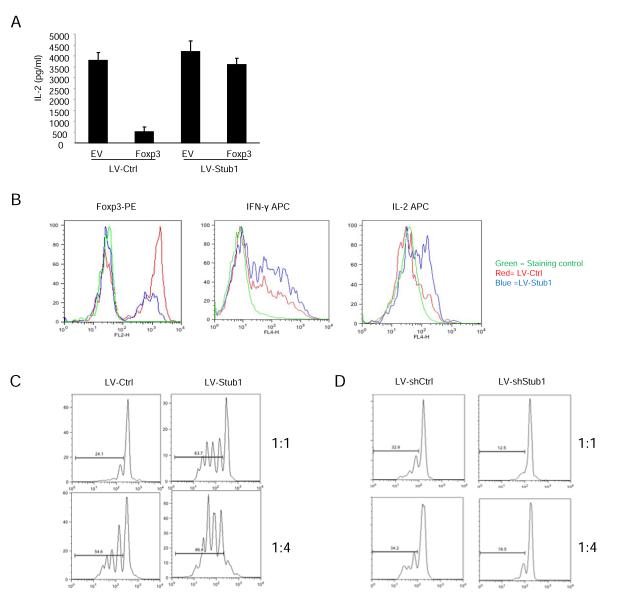
As Foxp3 is responsible for the suppression of IL-2 expression in primary T cells (Lopes et al., 2006; Zheng et al., 2007), we tested whether Stub1 was able to mitigate IL-2 suppression by Foxp3 in T cells by using lentivirus-based delivery of Stub1 into primary CD4+ T cells that were then transduced with a bicistronic retroviral vector encoding full-length Foxp3. As in Jurkat cells, the expression of Foxp3 almost completely blocked IL-2 production in primary T cells. However, the co-expression of Stub1 reversed Foxp3-mediated suppression of IL-2 secretion (Figure 5A). Supporting the notion that Stub1 removes Foxp3 from the transcriptional regulatory landscape by promoting its degradation, Western blotting of these double transduced cells confirmed that Stub1 overexpression coincided with the reduction of Foxp3 protein levels (Figure S5D). These results suggest that within T cells, Stub1 negatively regulates Foxp3-mediated transcriptional repression.
Figure 5.
Effects of Stub1 over expression or knocking down on Foxp3-mediated gene suppression in vitro. (A) Primary mouse T cells were transduced with either lentivirus-based control vector (LV-ctrl) or LV-Stub1 expression plasmids carrying an IRES-GFP marker for monitoring transduction efficiency. GFP+ cells were sorted, and then transduced with either a biscistronic retroviral empty vector (carrying an IRES-dsRed2 marker) or the vector expressing full-length Foxp3. GFP+ dsRed2+ cells were sorted from each group, and cultured in the presence of anti-CD3 plus anti-CD28 antibodies overnight. The supernatants were collected, and IL-2 production was determined by ELISA with samples run in triplicate. Results are represented as mean +/− SEM. (B) Mouse primary nTreg cells (CD4+CD25High) were transduced with either lentivirus-based control vector (LV-ctrl) or LV-Stub1 expression plasmid carrying an IRES-GFP marker for monitoring transduction efficiency. Cells were treated as in (A) prior to intracellular staining of Foxp3, IFN-γ and IL-2 then analysed by flow cytometry. Shown are representative histogram overlays from one of at least three trials depicting staining intensity of the indicated marker in transduced cells (GFP+ cells). Red lines indicate LV control; Blue lines represent Stub1 overexpressing cells and isotype controls are depicted in green. (C, D) Thy1.1 marked naïve CD4+ T cells were stained with CFSE and co-incubated with irradiated APCs (CD3− splenocytes) with normal or Stub1 overexpressing (C) or Stub1 silenced (D) Thy1.2+ Treg cells (obtained by retroviral transduction as mentioned above) at the indicated ratios. CFSE dilution was determined for Thy1.1+ responder cells by flow cytometry. Data are representative of at least three independent experiments. Also see Figure S5.
We next asked whether Stub1 could negatively regulate Foxp3 in primary mouse nTreg cells. CD4+CD25High T cells isolated from C57BL/6 mice were transduced with retrovirus containing empty vector (carrying GFP as a marker) or a Stub1 expression construct followed by intracellular staining for Foxp3, IFN-γ and IL-2. We observed that nTreg cells overexpressing Stub1 displayed reduced Foxp3 expression with increased production of IL-2 and IFN-γ (Figure 5B, S5E, S5F), suggesting a loss of characteristic Treg gene silencing in these cells. Furthermore, we measured expression of several Treg and T effector (Teff) cell-associated genes. In agreement with our earlier results, transcript levels of IL-2 and IFN-γ were elevated by Stub1 overexpression in nTregs. Additionally, expression of CD25, CTLA-4, GITR and IL-10 were reduced compared to control vector recipients (Figure S5G).
Stub1 also negatively impacts Treg function in vitro. Unlike nTreg cells receiving the control vector, those overexpressing Stub1 were markedly less capable of suppressing the proliferation of naïve CD4+ T cells in a CFSE dilution assay (Figure 5C). On the other hand, shRNA knockdown of Stub1 improved the suppression of naïve T cell proliferation (Figure 5D). These findings suggest that Stub1 in primary Treg cells results in polyubiquitination-mediated degradation of Foxp3, loss of effector gene silencing, acquisition of effector function and a loss of Treg suppressive function.
Stub1 overexpression in Treg cells eliminates their ability to suppress pathologic immune responses
To verify our in vitro findings, we determined the impact of Stub1 overexpression on the function of Treg cells in an in vivo colitis model where severe Th1 cell-mediated inflammation within the colon is induced after the transfer of naïve CD4+CD25−CD62Lhi T cells in the absence of Treg cells into Rag2−/− recipient mice (Powrie et al., 1993). In this model, co-transfer of CD4+CD25+ nTregs with naïve T cells is sufficient to prevent the development of disease (Izcue et al., 2006). Wild-type or lentiviral (LV)-control modified Treg cells effectively suppressed the development of disease as judged by both body weight loss (Figure 6A) and histological analysis of tissue isolated from the colon (Figure 6B). However, transfer of LV-Stub1 expressing nTreg cells in the presence of CD4+CD25−CD62Lhi effector T cells failed to prevent disease (Figure 6A-C).
Figure 6.
Stub1 overexpression renders Treg cells incapable of suppressing colitis in Rag2−/− mice. 1×106 naïve CD4+ T cells (CD62LhiCD25−) were injected i.v. into Rag2−/− mice alone, or mixed with the indicated Treg cells (2×105). (A) Mice were monitored weekly for weight loss. Shown are mean percentages of initial body weight (+/− SEM) for each group observed over at least three experiments (n=10 per group). (B) Representative photomicrography of the distal colon of Rag2−/− mice after T cell transfer. Eight weeks post-transfer, colons were harvested and processed for standard H/E staining and histological analysis. i-iv present bright field micrographs (100X). (C) H/E slides were scored in a blinded fashion and colon pathology was scored as described previously. Shown are mean scores for each treatment group +/− s.d. from at least three independent experiments. (D) Foxp3 expression by transferred Treg cells carrying control vector (blue) or Stub1-expression vector (green) was determined after isolating the cells from the indicated tissues and staining for surface markers and intracellular Foxp3. Staining controls are in red. Depicted are representative histograms gated on CD4+Thy1.1+ events (original Treg cells). (E, F) Intracellular IL-2 and IFN-γ levels in co-transferred CD4+Thy1.2+ cells were assessed as above. Shown are represented dot plots from at least two experiments. (G) and (H) As above colitis was induced but lower ratios of Treg:Tnaive were used (0.5×105:1×106). (G) Rag2−/− mice received naïve T cells alone or in combination with WT Tregs, or Tregs transduced with either control vector or sh-Stub1 constructs and the weight of the mice was monitored. (H) HE stained sections were scored as mentioned previously. Shown are the mean percentage of starting mouse weights and histology scores +/− SEM from at least two experiments. Also see Figure S6.
Additionally, we tracked the fate of injected Treg and naïve T cells (marked by congenic markers Thy1.1 and Thy1.2, respectively). Lymph node (LN), spleen (SPL), and gut lamina propria (LP) infiltrating cells were recovered and stained for the indicated surface markers as well as Foxp3 and IL-2 and IFN-γ as described previously (Dang et al., 2011). In all tissues sampled, the nTreg cells overexpressing Stub1 displayed a marked reduction of Foxp3 staining (Figure 6D, green line) compared to Treg cells harboring the control vector (Figure 6D, blue line). Commensurate with Foxp3 loss in Stub1 overexpressing Treg cells, the co-transferred naïve T cells recovered from these mice produced elevated levels of IL-2 relative to those of the control group (Figure 6E) indicative of a more robust and less restrained T cell response. This was accompanied by severe disease evident in the histological data (Figure 6A-C). A similar trend was observed when staining the naïve T cell population for the proinflammatory cytokine IFN-γ (Figure 6F), which further suggests that the loss of Treg-mediated suppression was a consequence of forced Stub1 expression in these cells. Also, analysis of the cytokine produced by the original Treg cells revealed that Stub1 overexpressing cells not only lost Foxp3 and the ability to suppress colitis, but as seen in our in vitro experiments they took on a Th1 cell-like phenotype producing considerable levels of IFN-γ unlike empty vector carrying Treg cells (Figure S6A).
Analysis of the frequency of transduced Tregs (Thy1.1+) among the cells infiltrating relevant tissues and their absolute numbers was also informative concerning the impact of Stub1 on in vivo Treg function. Despite the fact that Stub1 overexpressing Treg cells typically comprised a smaller percentage of LN, SPL and LP cells compared to empty vector controls (a potential indication of inferior suppression of naïve T cell proliferation), the numbers of Stub1 overexpressing Treg cells were either comparable to (LN, SPL) or greater (LP) than controls (Figure S6B and S6C). These results suggest that forced Stub1 expression in Treg cells does not hinder their survival during adoptive transfer during this experimental period but rather it renders them less capable of restraining the colitogenic response.
Silencing Stub1 expression enhances Treg suppressive function in vitro and in vivo
Because silencing Stub1 resulted in stabilized Foxp3 protein levels in cell lines and primary nTregs, we suspected that knocking down Stub1 in T cells might cause aberrant Foxp3 accumulation even under non-Treg favoring conditions. As in experiments described earlier, we introduced an sh-Stub1 construct or an sh-control into primary T cells by lentiviral transduction. As expected, shRNA knockdown of Stub1 in naïve T cells under Th1 or Th17-skewing culture conditions increased Foxp3 expression relative to controls (modestly in the former, but markedly in the latter). Additionally, upregulation of IFN-γ and IL-17 in these Th1 and Th17 skewed T cells were suppressed in the absence of Stub1 (Figure S6D and S6E). Despite this dramatic accumulation of Foxp3 protein seen upon Stub1 knockdown, the expression of Foxp3 transcript measured by qRT-PCR did not change significantly (Figure S6F). These results suggest that Stub1 is required to prevent Foxp3 upregulation during T helper development and therefore may also play a relevant role in non-Treg cells. Activation of naïve T cells in the presence of IL-6 and other STAT3 signaling cytokines will drive Th17 cell differentiation and Treg cells activated in the presence of IL-6 down-regulate Foxp3 and take on effector cell characteristics. In order to determine if these conditions involve Stub1-mediated Foxp3 protein removal, we activated human Treg cells in the presence of IL-6 and measured both Foxp3 and Stub1 protein. We found that IL-6 in conjunction with TCR stimulation resulted in progressive Foxp3 downregulation and Stub1 accumulation (Figure S6G).
In addition to an accumulation of Foxp3 protein, we hypothesized that cells lacking Stub1 would possess enhanced expression of Treg cell-associated genes. In support of this, and in contrast to the effects of Stub1 overexpression, when Stub1 is knocked down in mouse nTregs, transcripts for CD25, CTLA-4, GITR and IL-10 were increased over that seen in sh-controls (Figure S6H). These results confirm a role for Stub1 in controlling Foxp3 expression at the posttranscriptional level and they also offer potential mechanistic insights into the enhanced in vitro suppression of Stub1 deficient Tregs.
The impact of Stub1 knockdown on in vivo Treg function was also studied in the colitis model mentioned earlier. As before, Treg cells were co-transferred along with naïve CD4+ T cells into lymphopenic recipient mice. However the ratio of Treg cells to colitogenic naïve T cells in the adoptive transfer was lower (0.5×105:1×106 as opposed to 2×105:1×106). In this approach, co-transfer of normal Tregs will only partially rescue recipient mice from colitis, and importantly, enhancement of Treg suppression should be readily apparent. We hypothesized that under these conditions recipients of sh-Stub1 treated nTregs will be more protected than sh-control treated Tregs recipients. Indeed, Treg cells carrying Stub1 targeting shRNA largely protected recipients from colitis yielding weight change curves resembling those of mice receiving no colitogenic naïve T cells. Co-transfer of wild type or sh-control carrying nTregs with naïve T cells on the other hand afforded only a partial reduction in disease severity (Figure 6G). Histological scoring of stained tissue sections echoed these results (Figure 6H). In all, these results reinforce the conclusions drawn from our in vitro assays and support the notion that Stub1, an E3 ligase capable of interacting with the chaperone Hsp70 and its target Foxp3, is a key regulator of Treg identity and function during inflammation.
DISCUSSION
Tight control over the intensity and timing of an immune response is necessary to defend the host from invading pathogens while sparing host tissues from unnecessary immunopathology. Suppressor cells such as Foxp3 expressing Tregs provide a level of immune regulation by promoting tolerance and dampening immune activation. However, during acute infection, the function of Foxp3+ Treg cells in an inflammatory site may be temporarily neutralized permitting immune activation adequate to fight infection. In this scenario, a rapid mechanism for down-modulating Treg cell function is important for host defense. While many recent studies have focused heavily on how Foxp3 is induced in Treg cells, much less attention has been paid as to how Foxp3 protein level is negatively regulated and the consequences for suppressive function. In the present work we explored how posttranslational modification of Foxp3 may serve as a Treg ‘off switch’ negatively regulating Foxp3 stability and activity during inflammation. Here, we report that the E3 ligase Stub1, which is known to be expressed in response to danger signals during inflammation (Connell et al., 2001; Dai et al., 2003) is responsible for ubiquitinating Foxp3 with the help of the chaperone Hsp70 chaperone--a process that leads to the degradation of the chief Treg cell transcription factor.
There existed reasons to suspect an immunomodulatory role for elements of the inflamed microenvironment. We have recently found a role for elements of the hypoxia response (HIF-1α) in tipping the balance between opposing T cell subsets (Dang et al., 2011), by specifically promoting Th17 differentiation at the expense of Foxp3 expression and Treg cell generation. However, hypoxia is not the only aspect of inflamed or infected tissues that affect immune responses. The impact of microbial products and proinflammatory cytokines on the immune response have been studied in great detail (Kawai and Akira, 2007) as have those of oxidative stresses (Mougiakakos et al., 2011). It is not surprising that Treg cell behavior responds to these stimuli as well. The HSPs act as important molecular chaperones but in addition to regulating protein homeostasis during the heat shock response, they are also involved in bolstering immune responses under various stress conditions, including fever, oxidative stress and inflammatory cytokine signaling during viral infection. In response to stress signals such as these, HSPs are thought to mediate both constitutive and inducible danger signals delivered to activate immune responses. In the immune system, HSPs, such as Hsp60 and Hsp70, have been recently reported to activate the innate immune system (Gallucci and Matzinger, 2001; Matzinger, 2007). It is possible that Hsp70, by recruiting Stub1 under stress conditions, also allows rapid unleashing of the immune system by undermining the Foxp3 protein pool.
In this study we found that Foxp3 can be downregulated in Tregs by danger signals such as LPS and proinflammatory cytokines. It should be noted however that a prior study reported LPS mediated enhancement of the Treg population and its suppressive function (Caramalho et al., 2003). However it did not assess Foxp3 expression and it is difficult to discern the expected tolerogenic effects of LPS mediated through antigen presentation cells (APCs) from its direct results on Foxp3 protein. Interestingly, however, LPS was shown to increase effector cytokine production by Tregs (Milkova et al., 2010)–an observation compatible with ours. While purified Tregs have shown an increase in Foxp3 mRNA upon treatment with both LPS and IL-2, a sizable increase in Foxp3 protein level was not seen (Milkova et al., 2010). These results could reflect opposing processes regulating transcript and protein pools that may be responsive to distinct inputs. Here we provide evidence that the direct effects of LPS, and potentially other inflammation cues on Tregs have a unique outcome–namely the downregulation of Foxp3 at the posttranslational level through the E3 ubiquitin ligase activity of Stub1. The resulting suspension of Treg function is likely to enhance the amplitude of an immune system in order to overcome an infectious threat. It is tempting to speculate that the well documented, tolerogenic effects of LPS on APCs (Bryn et al., 2008; Maldonado and von Andrian, 2010; Tattevin et al., 2010) and their ability to expand or induce Tregs represent a compensatory mechanism to restore the Foxp3+ cell pool and prevent widespread loss of immune regulation.
Our previous study has shown that Foxp3 is subject to ubiquitination (Dang et al., 2011). However, the enzyme responsible for the modification of Foxp3 remains unknown. Our findings reveal a mechanism for Foxp3 regulation at the posttranslational level. They also further the notion forwarded by several groups of a plastic Treg cell lineage that is capable of suppressing or permitting (or perhaps even contributing to) immune responses. Previously, potential mechanisms for a loss of Foxp3 expression involved the transcription level. Specifically the methylation state of distinct regions of the Foxp3 gene contributes to the stability or relative instability of Treg cells. For instance, repeated in vitro activation of human Treg cells result in CpG island methylation within a conserved region of the Foxp3 gene in CD4+CD25+CD127lo T cells that result in the loss of Foxp3 expression and acquisition of effector cytokine production (Hoffmann et al., 2009). Conversely, inhibition of DNA methylation results in Foxp3 expression even in CD25− conventional T cells and the cytokine TGF-β enforces a non-methylated state of this conserved regulatory region (Nagar et al., 2008). Additionally, inhibition of DNA methylation in vivo was noted to increase the number of Treg cells and enhanced suppression of diabetes in mice (Zheng et al., 2009) suggesting that modulating the stability of Treg cells has physiological consequences in the disease setting.
These findings have expanded our understanding of Treg cells and the dynamic nature of their participation in the immune response. Adding another layer of complexity to the subject, we show a key and novel molecular mechanism for the regulation of Foxp3 at the protein level that integrates physiological cues from the microenvironment. This ubiquitination-dependent pathway likely plays a role in the newly appreciated potential for Treg cells to regulate the immune response in ways other than suppression (i.e. by swapping inhibitory function for effector function). Importantly, as we have shown ubiquitination-mediated removal of Foxp3 protein modulates Treg cell function it stands to reason that the enzymatic inhibition of such a process should have opposing effects. Since this indeed appears to be the case (van Loosdregt et al. 2013), ubiquitination disrupting therapies demand vetting as interventions for autoimmune disease.
Here we have revealed the degradation of Foxp3 by Hsp70-mediated recruitment of Stub1 as a mechanism responsible for the observed instability of Foxp3+ Treg cells. Besides the inducible expression of Hsp70 as an adaptor between Foxp3 and Stub1, the spatial and temporal regulation of Stub1 level and activity during T cell differentiation deserves further attention. Additionally, the long-term fate of Stub1 expressing Treg cells should be studied further in physiological settings. These cells may regain Foxp3 and Treg function upon removal of Stub1-inducing stresses or continue to display characteristics of effector cells. Since, Foxp3+ Treg cells capable of IFN-γ expression and having reduced immune suppressive activity can be found in human MS patients (Dominguez-Villar et al., 2011), it is possible that factors like Stub1 that drive acquisition of Teffector-like phenotypes in Tregs are at play in human autoimmune diseases. While many aspects of Treg cell instability remain unclear, our findings contribute to the understanding of Foxp3 expression and function.
The importance of transcriptional, and specifically epigenetic, control of Foxp3 has been demonstrated by numerous groups of late. This level of regulation is now known to be important for determining the relative stability or instability of Treg cells (Ohkura et al., 2013). We show in this work, for the first time to our knowledge, a post-translational mechanism for negatively regulating Foxp3 by Stub1 influencing Treg cell function. Our findings could have exciting therapeutic implications for human diseases. In chronic infection, Treg cells have been shown to prevent sterile clearance resulting in small numbers of organisms latently present and capable of reactivation (Belkaid et al., 2002). Treg cells are known to limit anti-tumor responses and allow the persistence and growth of cancer (Nishikawa and Sakaguchi, 2010). Temporary release from the suppressive influence of Treg cells or conversion of these cells into active participants in the inflammatory immune response may prove beneficial to individuals suffering from such diseases. Conversely, many autoimmune diseases arise from Treg cell deficits and thus targeting the machinery responsible for Foxp3 loss, such as the Hsp70/Stub1 degradation axis, could yield successful therapies for numerous diseases stemming from inadequate immune restraint.
Experimental Procedures
Mice
All animal experiments were performed in the specific-pathogen-free facilities of the Johns Hopkins Animal Resource Center in accordance with national, state and institutional guidelines and with the approval of the Johns Hopkins Animal Care and Use Committee.
Human primary cells
Human primary cells were purified from PBMCs of healthy donors at the Shanghai Blood Center using a FACS ARIA II cell sorter (BD). The assay for cell stimulation was performed as described in Supplementary Information.
Quantitative real-time PCR
Total RNA was isolated using the Qiagen miniRNA extraction kit following the manufacturer’s instructions. RNA was quantified and complementary DNA was reverse-transcribed using the cDNA archive kit (Applied Biosystem) following the manufacturer’s instruction. PCR reactions were run in an ABI Prism 7500 Sequence Detection System. Quantification of relative mRNA expression was determined by the comparative CT method normalizing to endogenous β-actin or 18s rRNA expression.
Cells, virus, and transfection
Virus containing supernatants were obtained from human HEK 293T cells transfected with the indicated plasmids using lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
For Stub1 overexpression or knockdown experiments, primary Treg cells (CD25High/CD4+) were FACS purified and transduced with the pLV-Stub1 or pLV-shStub1 vector, or a control vector encoding either GFP alone or a scrambled shRNA control (where appropriate). GFP signal was used as an indicator of successful transfection or transduction.
Luciferase assay
Luciferase promoter assays were performed as described previously (Li et al., 2007).
Immunoblotting and Immunoprecipitation
Immunoblotting and immunoprecipitation were performed as described in the Supplemental Experimental Procedures.
Colitis induction and histological assessment
Naïve CD4+CD25−CD62Lhigh T cells were isolated from BALB/c mice and injected via the tail vein (i.v.) into BALB/c RAG2−/− immunodeficient recipients (1×106/mouse). BALB/c wild-type CD4+CD25+ Treg or Stub1 transduced Treg cells or those receiving sh-Stub1 or a sh-Control constructs or an empty vector were co-injected i.v. as indicated. In all experiments, mouse weights were monitored weekly and tissues were removed 8 weeks after transfer and processed and scored as described previously (Pan et al 2009; and Supplementary Experimental Procedures). Leukocytes recovered from recipient lymph node, spleen and lamina propria were isolated and restimulated as previously described before surface staining for CD4 and Thy1.1 and Thy1.2 and intracellular staining for Foxp3, IL-2 and IFN-γ.
Statistical Analysis
The significance of differences, unless otherwise indicated was determined by an unpaired student’s t test with a p value less than 0.02 being considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Coffer and J. van Loosdregt for helpful discussions. Our research is supported by NSFC 30972702, 31170825, 31200647, 31200646, 81271835, 81270083, 31150110337, 81161120417; National Science and Technology Major Project 2012ZX10002007-003, 2013ZX10003009-002; NN-CAS Foundation. B.L. is supported by Shanghai Science and Technology ‘Rising Star’ Program 10QA1407900 and CAS “100-talent” program. S.Z. is a Tina C Foundation/Lupus Research Award winner. We gratefully acknowledge the support of the SASIBS scholarship program and the Knowledge Innovation Program of SIBS, CAS 2012KIP204. We thank X. Cao and Y-J Liu for their critical reading for the manuscript. We thank the members of the Pan and Pardoll Labs for helpful discussions. F.P. is a Stewart Trust Scholar, Melanoma Research Alliance Young Investigator Award winner. F.P.’s laboratory is partially supported by grants from the NIH (RO1AI099300), “Kelly’s Dream” Foundation, the Janey Fund and Seraph Foundation, and gifts from Bill and Betty Topecer and Dorothy Needle. J.B. is supported by a Crohn’s and Colitis Foundation of America Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental Information includes seven figures and Supplemental Experimental Procedures can be found with this article online.
REFERENCES
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Bryn T, Yaqub S, Mahic M, Henjum K, Aandahl EM, Tasken K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. International immunology. 2008;20:235–245. doi: 10.1093/intimm/dxm134. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L, et al. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003;22:5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang M, Zheng H, Wang Y, Ren F, Shang Y, Zhai Y, Irwin DM, Shi Y, Chen D, Chang Z. CHIP promotes Runx2 degradation and negatively regulates osteoblast differentiation. J Cell Biol. 2008;181:959–972. doi: 10.1083/jcb.200711044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, Ziegler SF. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol. 2006;177:3133–3142. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- Luo W, Zhong J, Chang R, Hu H, Pandey A, Semenza GL. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha. J Biol Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Advances in immunology. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- Milkova L, Voelcker V, Forstreuter I, Sack U, Anderegg U, Simon JC, Maier-Simon C. The NF-kappaB signalling pathway is involved in the LPS/IL-2-induced upregulation of FoxP3 expression in human CD4+CD25high regulatory T cells. Experimental dermatology. 2010;19:29–37. doi: 10.1111/j.1600-0625.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- Nagar M, Vernitsky H, Cohen Y, Dominissini D, Berkun Y, Rechavi G, Amariglio N, Goldstein I. Epigenetic inheritance of DNA methylation limits activation-induced expression of FOXP3 in conventional human CD25-CD4+ T cells. International immunology. 2008;20:1041–1055. doi: 10.1093/intimm/dxn062. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. International immunology. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Tattevin P, Monnier D, Tribut O, Dulong J, Bescher N, Mourcin F, Uhel F, Le Tulzo Y, Tarte K. Enhanced indoleamine 2,3-dioxygenase activity in patients with severe sepsis and septic shock. The Journal of infectious diseases. 2010;201:956–966. doi: 10.1086/650996. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Xu Y, Liu Y, Zhang B, Li X, Guo F, Zhao Y. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J Mol Med (Berl) 2009;87:1191–1205. doi: 10.1007/s00109-009-0530-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.