Abstract
Co-signaling molecules are surface glycoproteins that positively or negatively regulate the T cell response to antigen. Co-signaling ligands and receptors crosstalk between the surfaces of antigen-presenting cells (APCs) and T cells, and modulate the ultimate magnitude and quality of T cell receptor (TCR) signaling. In the past 10 years, the field of co-signaling research has been advanced by the understanding of underlying mechanisms of the immune modulation led by newly identified co-signaling molecules and the successful preclinical and clinical trials targeting co-inhibitory molecules called immune checkpoints in the treatment of autoimmune diseases and cancers. In this review, we briefly describe the characteristics of well-known B7 co-signaling family members regarding the expression, functions and therapeutic implications and to introduce newly identified B7 members such as B7-H5, B7-H6, and B7-H7.
Keywords: Co-signaling molecule, B7 family, Co-stimulation, Co-inhibition
INTRODUCTION
The most essential role of the immune system in the human body is to kill invading pathogens by inducing a protective immunity and not to harm the host by inducing tolerance to self-tissues. This is achieved through a fine tuning of T cell activities in initiation, differentiation and effector phase of the immune response (1). T cell response is initiated by specific recognition of cognate peptide presented by MHC proteins on antigen-presenting cell (APC) through T cell receptors (TCRs), which are referred to as a first signal for T cell activation. However, the ultimate magnitude and quality of T cell response is determined by a balance between co-stimulatory and co-inhibitory signals (collectively called co-signals) that are transduced into T cells, which is referred to as a second signal (2,3). Following TCR engagement by cognate peptide-MHC complexes, co-signaling receptors are often mobilized and colocalized with TCRs, forming the immunological synapse between APCs and T cells. This synaptic interface is the place where the crosstalk between co-signaling ligands and receptors synergize or antagonize with TCR signaling, rendering T cells activated or inhibited (4).
The B7 family is a group of surface glycoproteins that share structural features with immunoglobulin (Ig), whose extracellular domains bear homology to IgV and IgC domains of Ig (Table I). A hallmark of the B7 family molecules is their capability to co-stimulate or co-inhibit T cell responses in the presence of peptide/MHC complex-mediated TCR signaling (4). B7 family members primarily bind to the members of CD28 family including CD28, CTLA-4, PD-1, ICOS, and BTLA that transmit co-signals into T cells (Fig. 1). The B7 co-stimulatory ligands are important for full activation of naïve T cells in the lymphoid organs, in which APCs, particularly dendritic cells (DCs), are the primary cellular source providing the ligands including B7.1 and B7.2. In contrast, B7 co-inhibitory ligands are crucial for the termination of overactivated T cell response, maintenance of self-tolerance, and protection of tissues from damage induced by invading pathogens. In addition, co-inhibitory ligands expressed on pathological microenvironment such as tumor microenvironment actively inhibit the effector functions of cytotoxic T lymphocytes (CTLs) or induce the generation of regulatory T cells (2,3), thus, playing as important immune checkpoint proteins that are involved in the immune resistance of cancer cells.
Table I.
Structure, co-signaling nature, and receptors of B7 family members
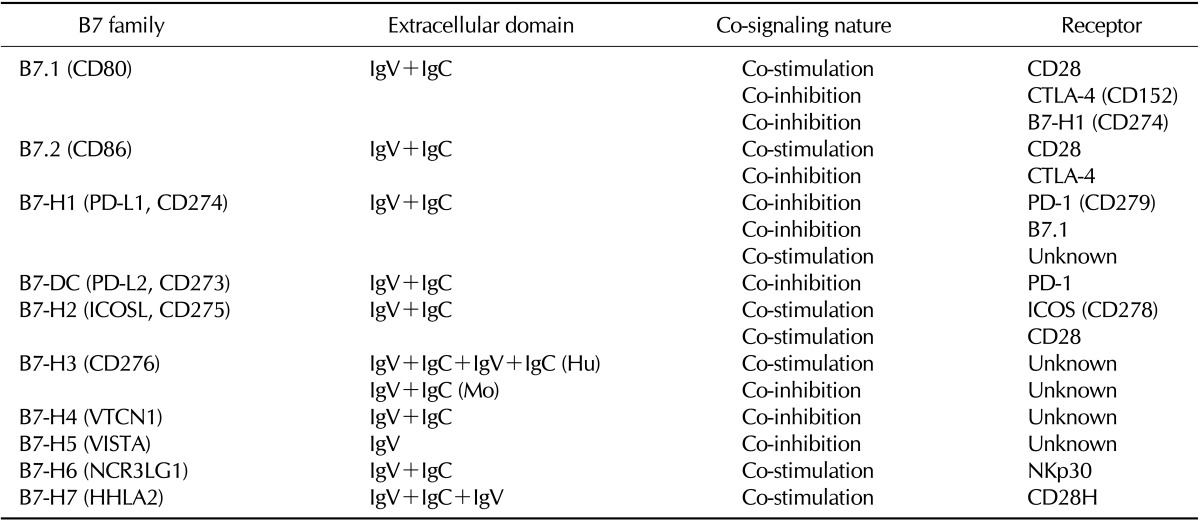
Figure 1.
Schematic diagram of B7 co-signaling family network.
In this review, we briefly describe the characteristics of well-known B7 family members regarding the expression, functions and therapeutic implications. We also introduce newly identified B7 members such as B7-H5, B7-H6, and B7-H7.
B7.1 AND B7.2 (CD80 AND CD86)
The B7.1 and B7.2, best-characterized co-signaling ligands, are expressed on APCs including DCs, macrophages, and B cells. The B7.1 and B7.2 deliver a co-stimulatory signal through CD28 constitutively expressed on naïve T cells, whose cytoplasmic tail contains three signaling motif. The first motif containing YMNM sequence serves as a binding site for the SH2-containg proteins, p85, growth factor receptor 2 (Grb2) and GADS. The second motif contains PRRP sequence and interacts with the SH3 domain of IL-2-inducible T cell kinase (Itk) of which involvement in CD28-mediated activation is still controversial. The third motif containing PYAP sequence associates with the SH3 domain of Grb2, GADS, lymphocyte cell-specific protein-tyrosine kinase (Lck), and filamin-A. Particularly, the association of Grb2 to both the YMNM and PYAP motifs is critically involved in the recruitment of protein kinase Cθ (PKCθ) and RAS guanyl nucleotide-releasing protein (RASGRP) to the immunological synapse and its subsequent activation (5-7). Therefore, B7.1/B7.2-CD28 pathway lowers the threshold for optimal T cell activation, subsequently resulting in T cell proliferation, upregulating anti-apoptotic proteins including Bcl-xL, and increasing IL-2 production (8-10). Recently, the B7-CD28 pathway was also shown to play an essential role in both suppressive function and survival of regulatory T (Treg) cells (11).
In addition, B7.1 and B7.2 deliver a co-inhibitory signal into activated T cells through CTLA-4 whose cytoplasmic tail contains the immunoreceptor tyrosine inhibitory motif (ITIM) recruiting Src homology 2 domain-containing phosphatase (SHP)-1 and SHP-2 (12,13). Due to its high affinity to B7.1/B7.2, CTLA-4 competes out CD28 for binding to B7.1 and B7.2, conferring signaling-independent T cell suppression by sequestering B7.1/B7.2 from the APC surface (14). In contrast to the CD28 pathway, the B7-CTLA-4 pathway sets the threshold high enough so that T cell activation can be reduced and finally terminated. Since CTLA-4 is constitutively expressed on Treg cells, CTLA-4 engagement by B7 molecules also enhances their immunosuppressive activity with upregulation of FOXP3 gene transcription (15,16). A great deal of mouse tumor tumor models revealed that blockade of the B7-CTLA-4 pathway could be a potential therapeutic target for cancer immunotherapy. Specifically, ipilimumab, a CTLA-4 blocking antibody, was the first FDA-approved antibody therapeutic for melanoma, adopting the notion that immune checkpoint blockade might enhance endogenous anti-tumor immunity (3).
Interestingly, B7.1 and B7.2 are believed to deliver bidirectional signalings. Specifically, B7 molecules expressed on T cells act as a counter receptor that receives an inhibitory signal from Treg cells probably through CTLA-4, an important in vivo T-T interaction for T cell homeostasis (17). Another reverse signaling through B7.1 and B7.2 was reported in T cell-APC interaction (18-20). B7 molecules expressed on DCs can transmit a suppressive signal into DCs through CTLA-4 on Treg cells or CTLA-4.Ig by enhancing IFN-γ production of DCs, which, in turn, induces indoleamine 2,3-dioxygenase (IDO), an enzyme metabolizing tryptophan into kynurenine, an immunosuppressive metabolite, in autocrine or paracrine mode (18). Thus, the bidirectional B7-CTLA-4 pathway appears to be critical for the downregulation of T cell response and induction of T cell tolerance.
B7-H1 (CD274, PD-L1) AND B7-DC (CD273, PD-L2)
B7-H1 is constitutively expressed not only on APCs but also in a wide range of normal somatic tissues including the heart, lung, placenta and liver (21,22). In contrast, B7-DC expression is largely restricted to APCs such as DCs and macrophages. Both molecules share the PD-1 receptor that is expressed on T cells, Treg cells, B cells, activated monocytes, DCs, NK cells, and NKT cells (23,24). The cytoplasmic tail of the PD-1 receptor contains ITIM and the immunoreceptor switch motif (ITSM) that binds to SHP-1 and SHP-2, transmitting a co-inhibitory signal into T cells and down-regulating Bcl-xL expression, which leads to T cell functional impairment and apoptosis (21).
Due to its ectopic expression on non-hematopoietic tissues including normal peripheral organs and cancer tissues, B7-H1 is believed to limit T cell activation in peripheral organs, leading to peripheral tolerance. Thus, the B7-H1-PD-1 pathway in tumor microenvironment can be a critical immune escape mechanism (25-27), indicating that B7-H1 and PD-1 can act as key immune checkpoint proteins. Many clinical observations further support the immune checkpoint activities of the B7-H1-PD-1 pathway by demonstrating the correlation between B7-H1 expression levels in cancer tissues and patients' survival. Specifically, high levels of cancer B7-H1 expression is associated with poor prognosis in patients with kidney, lung, pancreas and urothelial cancers (28-32). In addition, chronic exposure to antigens that are derived from cancers and chronic viral infections can upregulate the PD-1 expression on antigen-specific effector T cells, which leads to a state of functional exhaustion or anergy, one of the critical immune evasion mechanisms for cancers and persistently infecting viruses (33-35).
In an effort to discover another possible receptor for B7-H1, recent studies revealed that B7-H1 binds to B7.1 that acts as a counter receptor delivering a co-inhibitory signal. Interestingly, B7-H1 can also act as a counter receptor for B7.1 and transmit a co-inhibitory signal, indicating that the B7-H1-B7.1 pathway regulates immune functions through co-inhibitory bidirectional interaction (22). Furthermore, there are some in vitro and in vivo evidence indicating that both B7-H1 and B7-DC bind to a yet-unknown co-stimulatory receptor that is involved in T cell activation (36,37). Similar to the B7-CTLA-4 pathway, B7-H1-PD-1 pathway is also implicated in expansion and suppressive activity of Treg cells (38).
It has been well known that IFN-γ is a key cytokine to induce B7-H1 in non-hematopoietic cells such as cancer cells. Therefore, B7-H1 induction in tumor microenvironment by PD-1+ CTLs that infiltrated and produced IFN-γ may represent an adaptive immune resistance, leading to immune escape of cancer cells in the presence of anti-tumor responses (3,27,39). Many preclinical and clinical trials have been underway using B-H1 or PD-1 blocking antibodies. Early clinical studies have shown the promise in the treatment of patients with advanced cancers such as colon, renal, and lung cancers (40). Preclinical models also demonstrate a powerful synergy between tumor vaccines and blockade of the B7-H1-PD-1 pathway (41,42).
B7-H2 (ICOSL, CD275)
B7-H2 is a co-stimulatory ligand that binds only to ICOS, a co-stimulating receptor. B7-H2 is detected on the surface of APCs including B cells, DCs, and macrophages and a subset of CD3+ T cells. It is also expressed on the surface of non-lymphoid cells such as endothelial cells and some epithelial cells (43-48). B7-H2 mRNA is constitutively expressed in non-hematopoietic tissues including the kidney, liver, lung and testes (43-45,49,50). Anatomically, B7-H2 is expressed in B cell areas and follicles of lymphoid organs and ICOS is detected on T cells in the germinal centers and T cell areas (51-53). B7-H2 delivers a co-stimulatory signal through ICOS, whose cytoplasmic tail contains the YMFM motif that binds to the p85 subunit of PI3K, but not Grb2 (54). The B7-H2-ICOS pathway augments T cell effector function, but not proliferation of naïve T cells, which leads to enhancement of Th1 and Th2 cytokine production (55-57). The B7-H2-ICOS pathway also regulates humoral immune responses by enabling the germinal center T cells to provide critical helper signals to B cells, leading to the formation of germinal centers and antibody maturation. The fact that the B7-H2-ICOS pathway stimulates IL-10 production suggests that this signaling pathway may contribute to the regulation of Treg cell function, T cell tolerance, and autoimmunity (58-60). It was recently discovered that only human B7-H2, but not mouse B7-H2, binds to CD28 and CTLA-4, and that the B7-H2-CD28 pathway delivers co-stimulatory signals into T cells. Thus, ICOS, CD28 and CTLA-4 may compete for a similar binding site on human B7-H2 (61). However, the questions about the physiologic role of the B7-H2-CD28 and B7-H2-CTLA-4 pathway in vivo still remain unanswered.
B7-H3 (CD276)
B7-H3 differs in extracellular domain composition between humans and mice; human B7-H3 has a tandem repeat of IgV and IgC domains giving rise to IgV-IgC-IgV-IgC, but mouse B7-H3 contain set of IgV and IgC domains (62,63). However, the functional significance remains unclear between the 2 structures. Although human and mouse B7-H3 mRNA are detectable in lymphoid and non-lymphoid organs, human B7-H3 protein is not expressed on resting monocytes, B cells, T cells, or NK cells, but it can be induced on these cell types in response to GM-CSF, LPS, or phorbol myristate acetate (PMA) and ionomycin (62-64). Aberrant expression of B7-H3 has been reported in a wide range of solid cancer tissues including brain, lung, and pancreatic cancers (65-67). In some diseases including cancers, sepsis, and meningitis, B7-H3 can be secreted from the cells, and soluble B7-H3 levels are positively correlated with disease states (68-70).
The receptor for B7-H3 has not yet been identified, although a previous study suggested the triggering receptor expressed on myeloid cell (TREM)-like transcript 2 (TLT-2) as a possible receptor for mouse B7-H3, which is not confirmed in human B7-H3 (71). Initial studies indicated that B7-H3 is a co-stimulatory molecule as evidenced by the facts that human B7-H3.Ig fusion protein co-stimulates T cell proliferation in an in vitro assay and the B7-H3-overexpressing tumor cells are completely regressed in the tumor-bearing mouse. However, some in vivo experiments using B7-H3 knockout (KO) mice suggested that B7-H3 is a co-inhibitory player, which is supported by the observation that B7-H3 KO mice showed an increased alloreactive T cell expansion in mixed lymphocyte reaction (MLR), and developed more severe airway inflammation, experimental allergic encephalomyelitis (EAE) (5). In addition, tumor B7-H3 expression is often correlated with increased tumor size, the decreased number of tumor-infiltrating lymphocytes, and suppression of anti-tumor immunity (72-74). Thus, dual activity of B7-H3 may depend on the presence of putative co-stimulatory or co-inhibitory receptors on T cells in tissue microenvironment.
B7-H4 (B7-S1, B7x, VTCN1)
Although the protein structure of B7-H4 is predicted as a type 1 transmembrane protein, partial sensitivity to cleavage by phosphatidylinositol-phospholipase C (PI-PLC) suggested that B7-H4 is a GPI-anchored molecule, which makes B7-H4 different from other members of the B7-family in terms of topology (75,76). B7-H4 mRNA is expressed in lymphoid and non-lymphoid organs, but B7-H4 protein is not detectable in normal lymphoid and non-lymphoid organs except for activated APCs and breast ductal and lobular epithelia (75,77). Like B7-H1 and B7-H3, B7-H4 is ectopically expressed in a wide range of solid cancers including lung, thyroid, breast, ovary, esophageal cancers, and its expression in cancer tissues is correlated with poor prognosis (78-81). It is believed that immunosuppressive cytokines in tumor microenvironment such as IL-6 and IL-10 induce B7-H4 expression on tumor infiltrating macrophages, but not on cancer cells (82). There is still no report on which factors are responsible for B7-H4 induction in cancer cells. Although the receptor for B7-H4 is still unknown, it functions as a co-inhibitory factor whose engagement suppresses T cell expansion, cytokine production, and arrest cell cycle at the G0/G1 phase (75). A recent study using B7-H4 KO mice demonstrated that B7-H4 exerts an inhibitory effect on neutrophil expansion by inhibiting the growth of bone marrow-derived neutrophil progenitor (83), indicating that B7-H4 can also regulate innate immunity.
Recently, there have been several reports on an unexpected activity of B7-H4 in cancer progression in addition to its involvement in immune escape mechanisms. Unlike B7-H1, B7-H4 is expressed not only on the cell surface but also the inside of cancer cells which is an observation that attracts attention to its role in cancer cell biology. Specifically, many in vitro studies using siRNA for B7-H4 knockdown showed that downregulation of cancer B7-H4 inhibits proliferation, colony formation, and migration of cancer cells (84-86) and that intracellular B7-H4 may act as a cellular regulator promoting cancer cell proliferation and metastasis. However, molecular mechanisms behind B7-H4-mediated cancer progression remain unclear.
B7-H5 (PD-1H, VISTA, GI24, DIES1)
B7-H5, also known as VISTA, Gi24, or Dies1 has recently been identified as a co-inhibitory ligand bearing homology to B7-H1. Unlike B7-H1, B7-H5 contains a single IgV domain which has 3 additional cysteine residues, a unique structural feature different from other B7 family members (87,88). The cytoplasmic tail of B7-H5 does not contain any signaling motifs. B7-H5 is preferentially expressed on mature myeloid APCs including peritoneal macrophages, mature BMDC, neutrophils and CD11c+ DCs, and to a less extent on T cells and activated Treg cells. B7-H5 expression is inducible on T cells in response to PMA/ionomycin and on the myeloid cell population in response to OVA immunization with adjuvants such as complete Freund's adjuvant (CFA), indicating that B7-H5 expression is inducible during inflammatory response (87).
B7-H5 functions as a co-inhibitory ligand through an unknown receptor by inhibiting T cell proliferation and cytokine production and by arresting cell cycle (87). The co-inhibitory activity of B7-H5 is further supported by the finding that B7-H5-expressing MCA105 tumor cells grow vigorously in vaccinated hosts, whereas the control tumors do not. This result suggests that B7-H5 may inhibit a protective antitumor immunity in the host (87). Interestingly, consistent with cancer-associated B7- H4 showing non-immune activity, B7-H5 expressed on cancer cells enhances tumor-invasive growth by augmenting membrane type 1 matrix metalloproteinase (MMP) (89). In addition, B7-H5 is also required for proper differentiation of mouse embryonic cells via the bone morphogenetic protein 4 (BMP4) pathway (90).
B7-H6 (NCR3LG1)
B7-H6, also known as NCR3LG1, is a newly identified B7 family member, acting as a co-stimulatory ligand that delivers a stimulatory signal to NK cells through the receptor NKp30 (91). B7-H6 consists of an IgV-IgC extracellular domain, a transmembrane region and a long cytoplasmic tail that contains signal transducing motifs such as an ITIM, a Src homology 2 (SH2)-binding domain, and an SH3-binding motif. However, there have been few reports on the receptor activity of B7-H6. B7-H6 mRNA and protein are not detected in normal tissues. In contrast, B7-H6 is expressed on primary blood and bone marrow cells from hematological malignancies, including acute lymphoblastic leukemia, acute nonlymphoblastic leukemia, and non-Hodgkin's lymphoma (91). B7-H6 expression is regulated by class I histone deacetylases (HDACs) 2 and 3, so that knockdown of HDAC3 down-regulates B7-H6 surface expression (92).
NKp30 is a NK cell surface receptor that delivers an activating signal into NK cells. Since NKp30 consists of a single IgV domain in its extracellular region, a unique structural feature of the CD28 family, it belongs to CD28 family. NKp30 also has another ligand, BAT3 which is a nuclear protein involved in the interaction with P53 and apoptosis induction after DNA damage-induced cellular stress (93,94). NKp30 has been shown to mediate anti-tumor effects in gastrointestinal stromal tumors and lymphoid leukemia (95,96). Many studies indicate that the B7-H6-NKp30 pathway is implicated in anti-tumor activity by stimulating primary NK cells to produce IL-2 and IFN-γ and to enhance the cytotoxic activity (91,92). Further studies are needed to investigate the physiological role of B7-H6 in T cell immunity.
B7-H7
B7-H7 was previously known as human endogenous retrovirus-H long terminal repeat associating 2 (HHLA2 (97)) with unidentified function. Recently, B7-H7 has been identified as a specific ligand for human CD28H. Notably, mice and rats do not have the ortholog for human B7-H7 that consists of 3 signature of extracellular domains (IgV-IgC-IgV) (98). Interestingly, the first IgV domain of B7-H7, which presumably binds to a putative receptor, shows the highest homology to other B7 family members. B7-H7 mRNA is expressed in APCs, and non-lymphoid organs including the testis, colon, lung, kidney and pancreas while its transcript was not detected in T cells and a little in the small intestine, liver and skeletal muscle. Unlike the transcript expression pattern, cell surface B7-H7 expression is not detected on T cells, B cells, NK cells or monocytes freshly isolated from human PBMCs, but induced by poly (I:C), suggesting that B7-H7 is largely an inducible molecule on APCs in response to proinflammatory stimuli (99).
The B7-H7-CD28H pathway strongly promoted CD4+ T-cell proliferation and cytokine production (IL-2, IFN-γ, TNF-α and IL-10) via an AKT-dependent signaling cascade in the presence of TCR signaling, suggesting B7-H7 comprises a new co-stimulatory pathway (99). Co-stimulation via the B7-H7-CD28H pathway appears to be dependent on endogenous B7- CD28 interaction because addition of CTLA-4.Ig blocking B7-CD28 interactions greatly inhibited T-cell proliferation and cytokine production, even in the presence of agonistic CD28H mAb. The potential involvement of B7-H7 in human diseases has not yet been reported. However, it is worth while to further investigate the B7-H7-CD28H pathway in patients with autoimmune diseases or cancer, which will shed insight on a new potential therapeutic target in immunotherapy.
CONCLUSION
Co-signaling molecules comprise a complex molecular network that positively or negatively modulates T cell responses. Thanks to the improvement of in silico cloning technology and the development of high throughput screening method, more co-signaling ligands and receptors have been discovered, which establishes much more complicated co-signaling interactions. Due to the nature of suppressive activity, co-inhibitory molecules act as immune checkpoints that maintain self-tolerance and regulate the ultimate magnitude and quality of normal physiological immune response. Recently, promising clinical outcomes has come from several clinical trials that have used immune checkpoint blockade strategies for the treatment of autoimmune diseases and cancers. It is quite clear that successful clinical trials targeting immune checkpoints must be preceded by understanding fundamental mechanisms of immune regulations led by co-signaling molecules in normal and pathological microenvironment.
ACKNOWLEDGEMENTS
This study was supported by the 2005 Inje University research grant.
Abbreviations
- MHC
major histocompatibility
- CTLA-4
cytotoxic T lymphocyte antigen-4
- PD-1
programmed death-1
- ICOS
inducible T-cell costimulator
- BTLA
B- and T-lymphocyte attenuator
- IL-2
interleukin-2
- IFN-γ
interferon-γ
- GM-CSF
granulocycte-monocyte colony-stimulating factor
- LPS
lipopolysaccharide
- VSITA
V-domain Ig suppressor of T cell activation
- BMDC
bone marrow-derived dendritic cell
- OVA
ovalbumin
Footnotes
The authors have no financial conflict of interest.
References
- 1.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito T, Yokosuka T, Hashimoto-Tane A. Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett. 2010;584:4865–4871. doi: 10.1016/j.febslet.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 6.Isakov N, Altman A. PKC-theta-mediated signal delivery from the TCR/CD28 surface receptors. Front Immunol. 2012;3:273. doi: 10.3389/fimmu.2012.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 8.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 9.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 10.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 11.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 13.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622–4628. doi: 10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 19.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 21.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 24.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 25.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 26.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 27.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 29.Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, Aubry MC. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14:157–163. doi: 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 34.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 35.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 38.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 40.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 43.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 44.Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, Miyashiro JS, Jacobs KA, Collins M. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- 45.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 46.Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 47.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, Mayer L. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Wiendl H, Mitsdoerffer M, Schneider D, Melms A, Lochmuller H, Hohlfeld R, Weller M. Muscle fibres and cultured muscle cells express the B7.1/2-related inducible co-stimulatory molecule, ICOSL: implications for the pathogenesis of inflammatory myopathies. Brain. 2003;126:1026–1035. doi: 10.1093/brain/awg114. [DOI] [PubMed] [Google Scholar]
- 49.Brodie D, Collins AV, Iaboni A, Fennelly JA, Sparks LM, Xu XN, van der Merwe PA, Davis SJ. LICOS, a primordial costimulatory ligand? Curr Biol. 2000;10:333–336. doi: 10.1016/s0960-9822(00)00383-3. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni V, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- 51.Beier KC, Hutloff A, Dittrich AM, Heuck C, Rauch A, Buchner K, Ludewig B, Ochs HD, Mages HW, Kroczek RA. Induction, binding specificity and function of human ICOS. Eur J Immunol. 2000;30:3707–3717. doi: 10.1002/1521-4141(200012)30:12<3707::AID-IMMU3707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Mages HW, Hutloff A, Heuck C, Buchner K, Himmelbauer H, Oliveri F, Kroczek RA. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur J Immunol. 2000;30:1040–1047. doi: 10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Yoshinaga SK, Zhang M, Pistillo J, Horan T, Khare SD, Miner K, Sonnenberg M, Boone T, Brankow D, Dai T, Delaney J, Han H, Hui A, Kohno T, Manoukian R, Whoriskey JS, Coccia MA. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int Immunol. 2000;12:1439–1447. doi: 10.1093/intimm/12.10.1439. [DOI] [PubMed] [Google Scholar]
- 54.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 55.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 56.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sperling AI. ICOS costimulation: is it the key to selective immunotherapy? Clin Immunol. 2001;100:261–262. doi: 10.1006/clim.2001.5084. [DOI] [PubMed] [Google Scholar]
- 58.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 59.Riella LV, Dada S, Chabtini L, Smith B, Huang L, Dakle P, Mfarrej B, D'Addio F, Adams LT, Kochupurakkal N, Vergani A, Fiorina P, Mellor AL, Sharpe AH, Yagita H, Guleria I. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol. 2013;182:2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu YL, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 61.Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, Flies D, Luo L, Wang S, Chen L. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34:729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 63.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 64.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG, Huang JA. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2013;319:96–102. doi: 10.1016/j.yexcr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong F, Zhang ZX, Zhang GB, Zhang XG, Zhao H. B7-H3 overexpression in pancreatic cancer promotes tumor progression. Int J Mol Med. 2013;31:283–291. doi: 10.3892/ijmm.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Zhang G, Li Y, Feng X, Wan F, Zhang L, Wang J, Zhang X. Circulating B7-H3(CD276) elevations in cerebrospinal fluid and plasma of children with bacterial meningitis. J Mol Neurosci. 2009;37:86–94. doi: 10.1007/s12031-008-9133-z. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, Redmond HP, Wang JH, Zhang X. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185:3677–3684. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- 71.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124:105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katayama A, Takahara M, Kishibe K, Nagato T, Kunibe I, Katada A, Hayashi T, Harabuchi Y. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol. 2011;38:1219–1226. doi: 10.3892/ijo.2011.949. [DOI] [PubMed] [Google Scholar]
- 75.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 76.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 77.Mugler KC, Singh M, Tringler B, Torkko KC, Liu W, Papkoff J, Shroyer KR. B7-h4 expression in a range of breast pathology: correlation with tumor T-cell infiltration. Appl Immunohistochem Mol Morphol. 2007;15:363–370. doi: 10.1097/01.pai.0000213159.79557.71. [DOI] [PubMed] [Google Scholar]
- 78.Li ZY, Zhang XH, Chen Y, Guo JG, Sai K, Yang QY, Chen ZP, Mou YG. Clinical significance of B7-H4 expression in matched non-small cell lung cancer brain metastases and primary tumors. Onco Targets Ther. 2013;6:869–875. doi: 10.2147/OTT.S48085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu J, Chu BF, Yang YP, Zhang SL, Zhuang M, Lu WJ, Liu YB. B7-H4 expression is associated with cancer progression and predicts patient survival in human thyroid cancer. Asian Pac J Cancer Prev. 2013;14:3011–3015. doi: 10.7314/apjcp.2013.14.5.3011. [DOI] [PubMed] [Google Scholar]
- 80.Fauci JM, Straughn JM, Jr, Ferrone S, Buchsbaum DJ. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127:420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 81.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, Rietz AC, Huang J, Xu H, Flies AS, Flies SJ, Tamada K, Colonna M, van Deursen JM, Chen L. B7-H4-deficient mice display augmented neutrophilmediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian Y, Hong B, Shen L, Wu Z, Yao H, Zhang L. B7-H4 enhances oncogenicity and inhibits apoptosis in pancreatic cancer cells. Cell Tissue Res. 2013;353:139–151. doi: 10.1007/s00441-013-1640-8. [DOI] [PubMed] [Google Scholar]
- 85.Cheng L, Jiang J, Gao R, Wei S, Nan F, Li S, Kong B. B7-H4 expression promotes tumorigenesis in ovarian cancer. Int J Gynecol Cancer. 2009;19:1481–1486. doi: 10.1111/IGC.0b013e3181ad0fa2. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Wu H, Lu D, Li G, Sun C, Song H, Li J, Zhai T, Huang L, Hou C, Wang W, Zhou B, Chen S, Lu B, Zhang X. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene. doi: 10.1038/onc.2012.600. In press: http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. doi: 10.1016/j.it.2013.07.003. In press: http://dx.doi.org/10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakr MA, Takino T, Domoto T, Nakano H, Wong RW, Sasaki M, Nakanuma Y, Sato H. GI24 enhances tumor invasiveness by regulating cell surface membrane-type 1 matrix metalloproteinase. Cancer Sci. 2010;101:2368–2374. doi: 10.1111/j.1349-7006.2010.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aloia L, Parisi S, Fusco L, Pastore L, Russo T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J Biol Chem. 2010;285:7776–7783. doi: 10.1074/jbc.M109.077156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fiegler N, Textor S, Arnold A, Rolle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684–693. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 93.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H. HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev. 2007;21:848–861. doi: 10.1101/gad.1534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vdelta1XMLLink_XYZ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 96.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, Minard-Colin V, Poirier-Colame V, Chaba K, Flament C, Baud V, Authier H, Kerdine-Romer S, Pallardy M, Cremer I, Peaudecerf L, Rocha B, Valteau-Couanet D, Gutierrez JC, Nunes JA, Commo F, Bonvalot S, Ibrahim N, Terrier P, Opolon P, Bottino C, Moretta A, Tavernier J, Rihet P, Coindre JM, Blay JY, Isambert N, Emile JF, Vivier E, Lecesne A, Kroemer G, Zitvogel L. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 97.Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3) Genomics. 1999;59:255–263. doi: 10.1006/geno.1999.5877. [DOI] [PubMed] [Google Scholar]
- 98.Flajnik MF, Tlapakova T, Criscitiello MF, Krylov V, Ohta Y. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7s historical relationship with the MHC. Immunogenetics. 2012;64:571–590. doi: 10.1007/s00251-012-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W, Taube JM, Zheng L, Luo L, Zhu G, Chen J, Chen L. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]