Abstract
Climate-induced warming of the Arctic tundra is expected to increase nutrient availability to soil microbes, which in turn may accelerate soil organic matter (SOM) decomposition. We increased nutrient availability via fertilization to investigate the microbial response via soil enzyme activities. Specifically, we measured potential activities of seven enzymes at four temperatures in three soil profiles (organic, organic/mineral interface, and mineral) from untreated native soils and from soils which had been fertilized with nitrogen (N) and phosphorus (P) since 1989 (23 years) and 2006 (six years). Fertilized plots within the 1989 site received annual additions of 10 g N⋅m-2⋅year-1 and 5 g P⋅m-2⋅year-1. Within the 2006 site, two fertilizer regimes were established – one in which plots received 5 g N⋅m-2⋅year-1 and 2.5 g P⋅m-2⋅year-1 and one in which plots received 10 g N⋅m-2⋅year-1 and 5 g P⋅m-2⋅year-1. The fertilization treatments increased activities of enzymes hydrolyzing carbon (C)-rich compounds but decreased phosphatase activities, especially in the organic soils. Activities of two enzymes that degrade N-rich compounds were not affected by the fertilization treatments. The fertilization treatments increased ratios of enzyme activities degrading C-rich compounds to those for N-rich compounds or phosphate, which could lead to changes in SOM chemistry over the long term and to losses of soil C. Accelerated SOM decomposition caused by increased nutrient availability could significantly offset predicted increased C fixation via stimulated net primary productivity in Arctic tundra ecosystems.
Introduction
The soils of the northern circumpolar permafrost region, which includes Arctic tundra, contain approximately 50% of the global organic carbon, despite only encompassing 16% of the total land surface area [1]. This large pool of Arctic soil organic carbon (SOC) formed as a result of slow soil organic matter (SOM) decomposition relative to the net primary productivity (NPP) within the biome [2]. Factors contributing to the slow decomposition rates include low temperature [3,4], anoxic conditions caused by a persistently high water table due to underlying permafrost [2], and nutrient limitation for soil microbial activity [5]. However, these constraints may lessen due to rapid climate change in the Arctic [6], with uncertain consequences for net C exchange. Climate warming could accelerate decomposition of the massive SOC pool, turning the Arctic tundra biome into a significant CO2 source [7], resulting in a positive feedback to global warming [8-10].
Accelerated decomposition of SOM and nutrient mineralization due to climate warming can lead to increased nutrient availability [3,4]. Net primary productivity of the Arctic tundra is often limited by nutrients, especially nitrogen (N) [5,11]. Past research has demonstrated that increased nutrient availability leads to shrub domination [5,12] including an increased abundance of Betula nana in ecosystems currently dominated by tussock sedges [13-15]. Such change in dominant forms of vegetation can alter biogeochemistry in the Arctic tundra [16]. For instance, shrubs have higher NPP than tussocks [4], and produce woody tissue and litter that decays more slowly than tussock litter, which could produce more resistant SOM [17] and decrease the efficiency of SOM formation [18]. Snow trapped by shrubs increases soil temperature during winter [19,20], yet shading by shrubs decreases soil temperature during summer [21].
Increased nutrient availability can accelerate mineralization of SOC, a process which is also thought to be nutrient-limited in the Arctic [5,22]. Mack et al. [5] reported that 18 years of N and phosphorus (P) fertilization in a moist acidic tundra ecosystem significantly reduced SOC relative to non-fertilized controls. This was due to increased decomposition of SOM in the lower organic and in mineral soils, which exceeded increased C sequestration associated with stimulated NPP of shrubs [5]. However, Sistla et al. [23] demonstrated that a two-decade-long summer warming experiment in the same Arctic tundra ecosystem increased the depth of soil biological activity and reorganized the soil food web, but did not significantly alter the total quantity of SOC or soil N. Thus, there is uncertainty in how Arctic tundra will respond to predicted future warming, requiring a more mechanistic understanding of the processes that drive biogeochemical cycling in this system.
Extracellular enzymes are primarily produced by soil microbes including bacteria, Archaea and fungi, and regulate SOM decomposition by hydrolyzing polymeric compounds [24-28]. Because enzymes are N-rich proteins, their production can also be regulated by N availability. Given the expected increase in nutrient availability associated with warming in the future [3,4] and its potential roles in SOM decomposition [5], it is critical to assess how increased nutrient availability affects the concentration and activity of extracellular enzymes that degrade various substrates in Arctic tundra soils.
In this study, we examined the effect of long-term field N+P additions on the potential activities of extracellular enzymes in an Arctic tundra ecosystem. We selected total seven extracellular enzymes involved in hydrolysis for C and N products and phosphate (Table 1) in two sites subjected to fertilization since 1989 and 2006 (23 and six years, respectively, as of 2011 when this study was conducted). We hypothesized that increased nutrient availability would increase the abundance of enzymes involved in degrading C-rich substrates, but decrease the abundance of enzymes that degrade N-and P-rich substrates; nutrient-limited soil microbes would reduce their resources to obtain N and P, and reallocate them to obtain C. We also assessed temperature sensitivity of the seven enzymes by conducting enzyme assays at four different temperatures. We hypothesized that increased nutrient availability would alter the temperature sensitivity of enzyme activities (measured as activation energy).
Table 1. List of enzymes assayed in this study.
| Enzyme | Abbreviation | Function |
|---|---|---|
| β-Glucosidase | BG | Releases glucose from cellulose |
| Cellobiohydrolase | CB | Releases disaccharides from cellulose |
| Xylosidase | XYL | Degrades hemi-cellulose |
| α-Glucosidase | AG | Releases glucose from soluble saccharides |
| N-acetyl-glucosaminidase | NAG | Degrades chitin |
| Leucine-amino-peptidase | LAP | Degrades protein into amino acids |
| Phosphatase | PHOS | Releases phosphate ions from phosphate group |
Materials and Methods
Study site and sample collection
Soils were collected from the Arctic Long-Term Ecological Research (LTER) site at Toolik Lake, Alaska, USA (68°38’N, 149°38’W) in late July 2011. The mean annual temperature and precipitation are -7 °C and 400 mm, respectively. Approximately half of the annual precipitation falls as snow. The growing season is limited to between 50 and 70 days in July and August with a mean temperature of approximately 10 °C. The area is dominated by moist acidic tussock tundra with vegetation consisting of graminoids (Eriophorum vaginatum and Carex microchaeta), deciduous shrubs (Betula nana), evergreens (Ledum palustre and Vaccinium vitis-idaea), and mosses (Sphagnum spp., Hylocomium splendens, and Aulacomnium spp.) [12,29-31]. We sampled from two experimental sites subjected to fertilization treatments since 1989 and 2006. The two different sites were located in moist acidic tussock tundra, 175 m apart from each other. At the 1989 site, we sampled from four plots with a high fertilization treatment (10 g N⋅m-2⋅year-1 as NH4NO3 and 5 g P⋅m-2⋅year-1 as P2O5) and four no-fertilization controls. At the 2006 site, we sampled from three plots with a high fertilization treatment, three with a low fertilization treatment (5 g N⋅m-2⋅year-1 and 2.5 g P⋅m-2⋅year-1 in the same forms as the high fertilization treatment), and three no-fertilization controls. Each soil sample were separated into three soil types, organic, organic/mineral interface and mineral soils based on organic matter content and degree of decomposition. Depths of the organic soils varied from 6 to 12 cm, and the interface from 4 to 15 cm. We collected the mineral soils from the top 5 cm of the profile beneath the interface soils. Samples were transported in a cooler on ice to Natural Resource Ecology Laboratory, Colorado State University, Colorado, USA, and stored at 4 °C until analyses were conducted. All the enzyme assays described below were conducted within one month since the collection of the soil samples. We conducted this study under a permit that the Arctic LTER obtained from the United States Bureau of Land Management which owns the land including our sampling locations. We declare that this study did not involve any endangered or protected species.
Soil property analysis
We analyzed soil water content, SOC and total N contents for all samples. Soil water contents were determined by drying soil samples at 105 °C for 48 hours. To measure SOC and total N contents, samples were first dried out at 60 °C, and ground finely using a Brinkmann Retsch mill (Haan, Germany). Organic C and total N contents of the ground samples were quantified using a LECO TruSpec® (Leco Corporation, St. Joseph, Michigan, USA).
Enzyme assays
We quantified potential activities of seven hydrolytic enzymes (Table 1) for each sample using fluorometric techniques [32] modified following Steinweg et al. [33]. We measured the activities of four enzymes hydrolyzing C-rich substrates (BG, CB, XYL and AG), two for N-rich substrates (NAG and LAP) and one for a P-rich substrate (PHOS). Subsamples (1 g for organic and interface soils, and 2.75 g for mineral soils) were homogenized with 91 mL of 50 mM sodium acetate (pH 4.5) using a blender (Waring, New Hartford, CT, USA). Plates with 96 deep-wells were used for the enzyme assay as well as reference standards, in which samples were arranged in columns and different enzymes and standards in rows. Aliquots (800 µL) of each homogenized sample were pipetted into seven wells in one of the 12 columns of a plate using an 8-channel pipetter. When the plate was filled with homogenized samples (up to 12 samples for a plate), 200 µL of each substrate dissolved in DI water was added to each aliquot of sample. Each of the seven substrates (Table 1) was pipetted into wells in a given row (up to 12 wells). The concentration of each substrate was determined based on tests prior to the experiment. We employed 600 µmol·L-1 of CB and PHOS for the organic and interface soils, and 200 µmol·L-1 for the rest of the substrates so that 200 µL of substrate would not be completely degraded by enzymes during an incubation period [34]. After a lid was firmly placed on the plate after substrate addition, the plate was inverted several times to mix soil samples and substrates well, and immediately placed in an incubator. Reference standards were prepared in a similar manner as the soil samples described above using the same apparatus. In the standard plates, we added fluorescent standards, instead of the substrates, in seven concentrations ranging from zero to up to 600 µM. We used two types of fluorescent standards, 7-amino-4-methylcoumarin (MUC) and 4-methylumbelliferone (MUB). MUC standards were used for LAP, and MUB the others (Table 1).
We used four different incubation temperatures (5, 15, 25 and 35°C) to assess the temperature sensitivity of potential enzyme activities in soils. For a set of 12 samples, we set up four plates, each of which was incubated at one of the four temperatures. Four additional plates were prepared as reference standards. Of the four standard plates, two were used for MUC and the other two MUB. One set of MUC and MUB standards were incubated at 5°C and the other at 25 °C along with the soils samples. The standard set incubated at 25°C was used to calculate potential enzyme activities at 15, 25 and 35°C [33]. Incubation periods were 23, 6, 3 and 1.5 hours for 5, 15, 25 and 35°C, respectively.
After incubation, the plates were centrifuged at 350 g for three minutes, the supernatant was removed from each well and pipetted into a corresponding well of a 96-well black plate. Fluorescent activities were immediately measured using an Infinite M500 spectrofluorometer (Tecan, Männedorf, Switzerland) with a set of wavelength at 365 and 450 nm for excitation and emission, respectively. Readings of the fluorescent activities from standards were used to calculate potential enzyme activities for each sample.
Activation energy was calculated using potential enzyme activities assayed at four different temperatures for each enzyme and sample. Temperatures and activity values were fit into the Arrhenius equation [34];
where K is the reaction rate, A is the frequency factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin [35]. This equation can be expressed in the following equation;
Plotting lnK against 1/T, Ea can be calculated from the slope of the linear regression [36]. Activation energy does not have energetic meaning, but rather gauges temperature dependence of enzyme activities [37]. Activation energy describes how enzyme activity increases with temperature [38], thus positively correlates with Q10 calculated from the same data set.
Statistical analyses
We used a mixed-effect ANOVA for statistical analyses with the sites (i.e. the 1989 and 2006 sites) and fertilization levels (i.e. control, low and high) as fixed effects, and blocks as a random effect. All computations were carried out using the package nlme in R [39]. A significance level of p ≤ 0.10 was used to assess statistical significance because of the relatively small sample sizes in this study, and all p-values are for two-sided confidence intervals.
Results
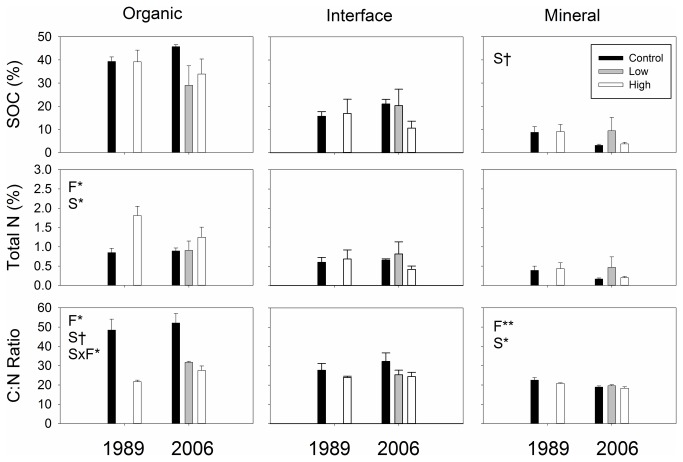
Nutrient addition treatments resulted in increasing the total N content and had no effect on the SOC content in the organic profiles of the soils at the 1989 and 2006 sites (Figure 1). As a result, soil C:N ratios of the organic profiles were significantly reduced with the fertilization treatments (Figure 1). The nutrient addition treatments did not alter either SOC or total N contents in the interface or mineral profiles (Figure 1). C:N ratios of the mineral soil profiles were significantly reduced with the fertilization treatments, though the reduction was small compared to the organic profile (Figure 1).
Figure 1. C and total N contents in organic, interface and mineral soil profiles.
Statistically significant effects found by mixed-effect ANOVA are shown in panels: F; fertilization treatment (control, low and high fertilizations), S; site (i.e. 1989 and 2006 sites), and F×S; interaction between F and S. Symbols indicate: †; p ≤ 0.10, *; p ≤ 0.05, **; p ≤ 0.01.
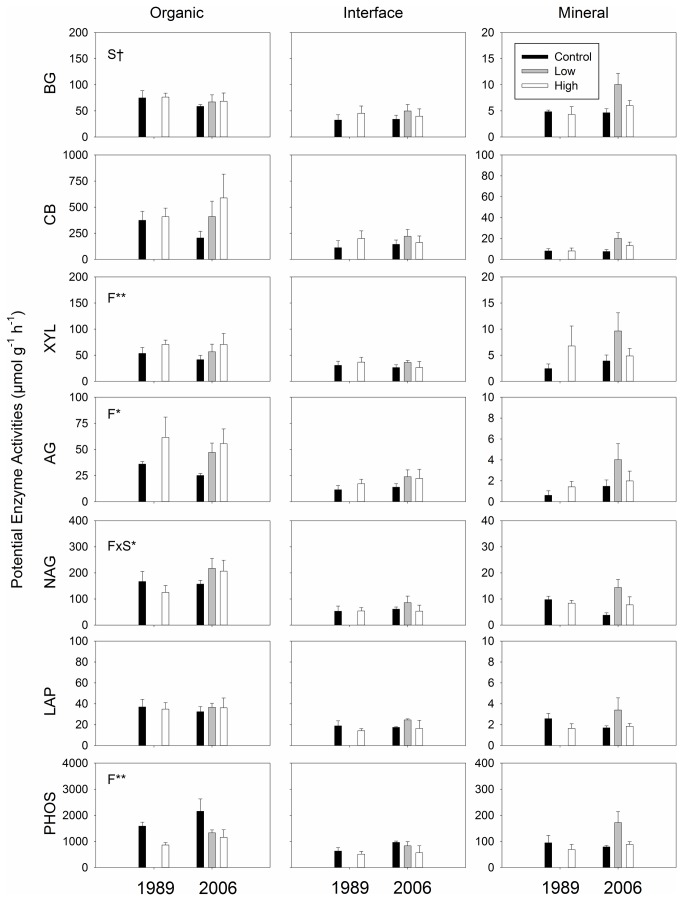
The effects of increased nutrient availability on potential enzyme activities depended on soil profile and varied by enzyme (Table 2, Figure 2, Appendix S1-4). There was a consistent difference in potential activities of all the enzymes among soil profiles with the activities highest in the organic soils, lowest in the mineral soils and intermediate in the interface soils (Table 2, Figure 2, Appendix S1-4). A significant main effect of fertilization was found on potential activities of CB, AG and PHOS (Table 2, Appendix S4) whereby fertilization increased potential activities of CB and AG but decreased those of PHOS (Figure 2, Appendix S1-3). When potential enzyme activities were analyzed for each soil profile, significant effects were only found in the organic soils (Figure 2, Appendix S1-3). Fertilization stimulated potential enzyme activities of XYL and AG for both 1989 and 2006 sites (Figure 2), whereas fertilization significantly decreased potential enzyme activities of PHOS (Figure 2). Fertilization did not significantly change potential enzyme activities of BG, CB or LAP (Figure 2).
Table 2. Summary of p-values resulting from mixed-effect model analyses for potential enzyme activities assayed at 15 °C.
| Independent variables | BG | CB | XYL | AG | NAG | LAP | PHOS |
|---|---|---|---|---|---|---|---|
| Fert | 0.342 | 0.10 | 0.103 | 0.006 | 0.887 | 0.316 | 0.007 |
| Profile | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Site | 0.736 | 0.217 | 0.884 | 0.287 | 0.586 | 0.751 | 0.074 |
| Fert×Profile | 0.277 | 0.613 | 0.572 | 0.482 | 0.898 | 0.664 | 0.376 |
| Fert×Site | 0.008 | 0.571 | 0.613 | 0.920 | 0.511 | 0.758 | 0.839 |
| Profile×Site | 0.859 | 0.341 | 0.364 | 0.217 | 0.209 | 0.856 | 0.824 |
| Fert×Profile×Site | 0.178 | 0.211 | 0.797 | 0.767 | 0.326 | 0.804 | 0.387 |
Fert and Profile represent fertilization and soil profile, respectively.
The p-values equal to or less than 0.10 are shown bold.
Figure 2. Potential enzyme activities in the three soil profiles, incubated at 15 °C.
The scales of y-axes for organic and interface soils are identical. Statistically significant effects found by mixed-effect ANOVA are shown in panels: F; fertilization treatment (control, low and high fertilizations), S; site (i.e., 1989 and 2006 sites), and F×S; interaction between F and S. Symbols indicate: †; p ≤ 0.10, *; p ≤ 0.05, **; p ≤ 0.01.
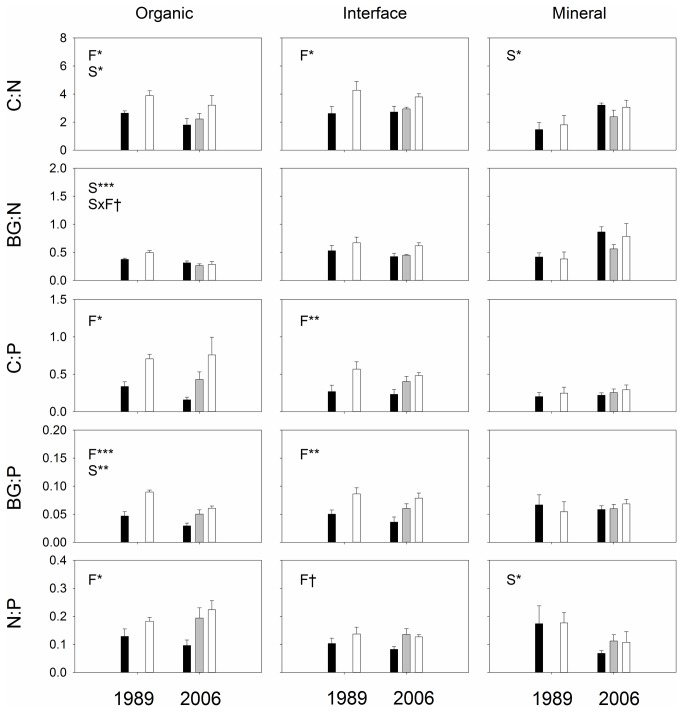
Fertilization significantly changed the stoichiometry of enzyme, also known as enzyme acquisition ratios (Figure 3, Table 3, Appendix S5-8). Increased nutrient availability significantly stimulated potential activities of C-degrading enzymes relative to N-degrading enzymes as well as phosphatase (Figure 3, Table 3, Appendix S5-8). Such alterations were evident in organic and interface soils, but not in mineral soils (Figure 3, Appendix S6-8). Ratios of BG:N, a commonly used enzyme stoichiometric index [40], was not altered by the fertilization treatments, whereas the BG:PHOS ratio significantly increased (Figure 3). There was a significant correlation between soil C:N ratio and corresponding enzyme ratio in the organic profile (Figure 4). The fertilization treatment reduced soil C:N ratio by increasing soil N content (Figure 1) whereas the treatment increased enzyme C:N ration by increasing C-degrading enzyme activities (Figure 2), resulting in a negative, significant correlation between the two ratios in the organic soils (R2=0.44, p < 0.01, Figure 4). Such significant correlation was not found in the interface or mineral soils (Figure 4).
Figure 3. Stoichiometry of potential enzyme activities in the three soil profiles collected assayed at 15 °C.
C, N and P represent potential enzyme activities of (BG+CB+XYL+AG), (NAG+LAP) and PHOS, respectively. Statistically significant effects found by mixed-effect ANOVA are shown in panels: F; fertilization treatment (control, low and high fertilizations), S; site (i.e., 1989 and 2006 sites), and F×S; interaction between F and S. Symbols indicate: †; p ≤ 0.10, *; p ≤ 0.05, **; p ≤ 0.01, ***; p ≤ 0.001.
Table 3. Summary of p-values resulting from mixed-effect model analyses for stoichiometry assayed at 15°C.
| Independent variables | C:N | BG:N | C:P | BG:P | N:P |
|---|---|---|---|---|---|
| Fert | <0.001 | 0.432 | <0.001 | <0.001 | <0.001 |
| Profile | 0.006 | 0.001 | <0.001 | 0.615 | 0.013 |
| Site | 0.897 | 0.747 | 0.711 | 0.119 | 0.411 |
| Fert×Profile | 0.091 | 0.121 | 0.012 | 0.008 | 0.235 |
| Fert×Site | 0.559 | 0.771 | 0.378 | 0.615 | 0.165 |
| Profile×Site | 0.003 | <0.001 | 0.333 | 0.218 | 0.011 |
| Fert×Profile×Site | 0.810 | 0.610 | 0.580 | 0.513 | 0.792 |
C, N and P represent potential enzyme activities of (BG+CB+XYL+AG), (NAG+LAP) and PHOS, respectively.
Fert and Profile represent fertilization and soil profile, respectively.
The p-values equal to or less than 0.10 are shown bold.
Figure 4. Relationship between soil C:N ratio and corresponding ratio of potential enzyme activities.
Enzyme C and N are (BG+CB+XYL+AG) and (NAG+LAP), respectively, assayed at 15 °C.
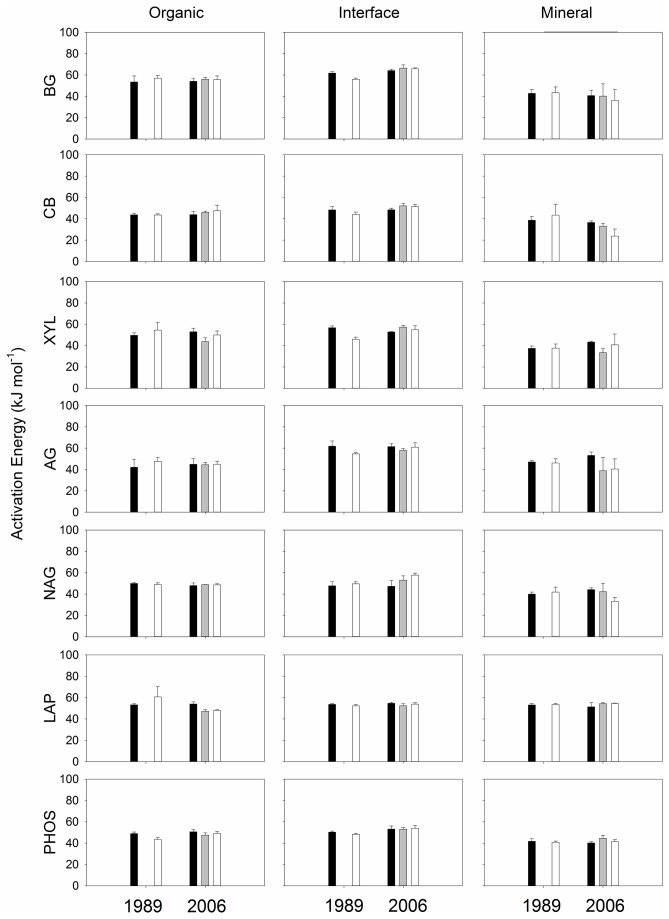
Fertilization did not change activation energy for any of the enzymes assayed in this study (Figure 5, Table 3). There was significant difference in activation energy among the three soil types for all the enzymes except LAP (Table 3), where mineral soils consistently had lower activation energy than organic or interface soils (Figure 5).
Figure 5. Activation energy of potential enzyme activities.
Potential enzyme activities measured at four temperatures (5, 15, 25 and 35 °C) were used to calculate activation energy for the three soil profiles collected from the two sites.
Discussion
Our study demonstrated that increased nutrient availability in an Arctic tundra ecosystem stimulated the activities of extracellular enzymes that degrade C-rich compounds, which are the proximate drivers of SOC decomposition. Furthermore, nutrient additions altered enzyme acquisition stoichiometry, suggesting that microbes reallocated resources towards obtaining C rather than N or P. This accelerated SOC loss caused by increased nutrient availability, which is expected to happen with climate warming, could significantly offset predicted increased C fixation via stimulated NPP in Arctic tundra ecosystems [41].
Soil properties and potential enzyme activities altered by the fertilization treatments
Our results support our first hypothesis that fertilization would increase potential enzyme activities that degrade C-rich substrates (Figure 2, Appendix S1-3). This result suggests that production of these enzymes by soil microbes is nutrient limited, especially by N [19,42]. During the growing season, net N-immobilization is often observed in Arctic tundra soils [3,19,43-46]. Wallenstein et al. [42] observed that most potential enzyme activities did not change over the course of a growing season in the same Arctic tundra ecosystem, despite increasing temperatures, most likely due to N-limitation for production of enzymes by soil microbes. N-limitation for enzyme production is also supported by Hobbie and Gough [47] who showed that litter decomposition rates in acidic tundra ecosystems were twice as fast as those in non-acidic tundra, most likely due to the higher N availability in the acidic tundra. Increases in C-degrading hydrolytic enzyme activities caused by N addition have been observed in other biomes, including boreal forests [48], temperate deciduous forests [32,49-52], and grasslands [51,53-55]. Thus, our results provide additional support for the general hypothesis that enzyme production is N-limited.
We note that potential enzyme activities reflect overall enzyme concentrations in soils [56], which are determined by the balance between enzyme production and degradation rates. We assume that altered potential enzyme activities by nutrient addition found in this study were caused by microbial responses in enzyme production rates rather than enzyme degradation rates. This assumption is based on our finding that potential activities of LAP, which degrades proteins including enzymes themselves, were not altered by increased nutrient availability (Table 2).
Our results also supported our second hypothesis that increased nutrient availability would decrease phosphatase activities (Figure 2). Decreases in phosphatase activities caused by P fertilization have been commonly observed [57-60] and an inverse relationship between phosphatase activity and environmental P availability is generally accepted [61]. These responses are consistent with a resource economics theory for enzyme production by soil microbes [62].
In contrast to C-degrading enzymes and phosphatase, the fertilization treatments did not affect two enzymes that degrade N-rich substrates (i.e., NAG and LAP, Figure 2, Appendix S1-3). Many studies have been conducted to investigate effects of N amendments on these two enzymes across a range of ecosystems, including grasslands [41,54,55,63], temperate forests [49-52] and alpine tundra [64], but there is no consistent trend [61]. One potential reason is that N cycle is more complex compared to those of C or P. For instance, the two N-rich substrates used in this study, chitin (NAG) and protein (LAP), are both C sources as well as N [61]. Thus, even if N-limitation to soil microbes is alleviated, they may still keep producing these two enzymes to acquire C from these substrates. Another potential explanation is that N is still limiting to enzyme production for soil microbes in this system, even after years of chronic N additions.
Enzyme acquisition ratios, which provide insights into metabolism of soil microbial communities for energy and nutrient acquisitions, were significantly altered by the fertilization treatments (Figure 3, Appendix S6, 7) indicating that soil microbes reallocated their resources to obtain more C. This result emphasizes our finding that enzyme production by soil microbes is nutrient limited. Enzyme expression is determined by microbial cellular metabolism in response to environmental signals, including nutrient availability [40,61]. Thus, shifts in enzyme stoichiometry indicate relative demand of nutrients that soil microbes need for growth and maintenance. One notable finding is that there were significant fertilization effects on enzyme stoichiometry for the interface soils (Figure 3, Appendix S7) even though we did not find any significant fertilization effect when assessing potential activities of individual enzymes (Figure 2, Appendix S2), most likely because of small sample sizes in this study and heterogeneity of the soil samples.
We found a significant negative correlation between soil C:N ratio and the corresponding ratio of potential enzyme activities in the organic soils (Figure 4) due to contrasting responses of soil and potential enzyme activities to the fertilization treatments. The treatments reduced soil C:N ratio by increasing total N content with SOC content unchanged (Figure 1). The fertilization treatments increased enzyme C:N ratio by increasing activities of enzymes degrading C-rich substrates with no change in N-degrading enzymes (Figure 2). Soil C:N ratios in the controls, approximately 50 in average (Figure 1), are among the highest for organic soils reported in Arctic tundra in the region [65], reflecting high C:N ratios of litter, ranging from 40 to over 100, produced by various plants in the ecosystem [47]. The fertilization treatments reduce such high C:N ratio of organic soils at an early stage of decomposition to ones of highly decomposed soils found in the interface and mineral soils of the controls (Figure 1 and 4) [66]. We did not find such significant, negative correlation in interface or mineral soils (Figure 4) most likely because the C:N ratios of those highly decomposed soils were so low that the fertilization treatments could not lower them further (Figure 1).
We note a limitation of this study due to the one time measurement. Potential enzyme activities in Arctic soils can be dynamic over a course of a growing season [42]. Thus, our findings of potential enzyme activities could have been different if measured in other time of the growing season. However, our measurement was conducted around the time when soil temperature peaked, thus SOC decomposition rates were most likely highest [67,68].
Activation Energy
We did not find significant fertilization effects on activation energy in any of the seven enzymes assayed (Figure 5, Table 4). Our finding of no change in activation energy indicates that compositions of isoenzymes were not significantly altered by the fertilization treatments. A pool of enzymes that breaks down a polymer in a natural environment consists of a number of isoforms [69,70]. These isoenzymes can have different activation energy [71], thus a change in the composition of isoenzymes would lead to change in activation energy. Our finding is surprising given that fertilization treatments often change community compositions of soil microbes (e.g. [63,72-76] but see 77,78) which produce different isoenzymes [79,80]. Wallenstein et al. [42] reported temperature sensitivity measured as Q10 changed along a growing season for six enzymes in soils collected from the same ecosystem as this study, suggesting compositions of isoenzymes changed over the sampling time. Stone et al. [52] observed no change in temperature sensitivity of potential enzyme activities in temperate forest soils when N was amended.
Table 4. Summary of p-values resulting from mixed-effect model analyses for activation energy as independent variable.
| Independent variables | BG | CB | XYL | AG | NAG | LAP | PHOS |
|---|---|---|---|---|---|---|---|
| Fert | 0.929 | 0.632 | 0.424 | 0.439 | 0.562 | 0.972 | 0.148 |
| Profile | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.634 | <0.001 |
| Site | 0.431 | 0.704 | 0.856 | 0.986 | 0.642 | 0.269 | 0.044 |
| Fert×Profile | 0.577 | 0.750 | 0.509 | 0.361 | 0.208 | 0.616 | 0.307 |
| Fert×Site | 0.731 | 0.641 | 0.813 | 0.663 | 0.982 | 0.490 | 0.181 |
| Profile×Site | 0.091 | 0.015 | 0.425 | 0.762 | 0.416 | 0.086 | 0.366 |
| Fert×Profile×Site | 0.490 | 0.082 | 0.192 | 0.418 | 0.099 | 0.129 | 0.935 |
Fert and Profile represent fertilization and soil profile, respectively.
The p-values equal to or less than 0.10 are shown bold.
Activation energies for most of the enzymes in the mineral soils were lower than those in the organic or interface soils (Figure 5, Table 4). One potential explanation for the difference is compositions of isoenzymes produced in different soil profiles. For instance, organic soils were subject to temperature increase during a growing season, whereas the mineral soils we collected were kept relatively cold in the deeper profiles above permafrost. Such temperature difference in soil profiles where enzymes were produced could contribute to kinetic characteristics of enzymes; the activation energy of psychrophilic enzymes is lower than that of mesophilic enzymes [38]. Another potential explanation is stabilization of enzymes. Enzymes can be stabilized with humic acids [81-83], tannic acids [83,84], and clay minerals [85-87] increasing activation energy compared to free enzymes [88]. Humic and tannic acids in organic and interface soils might contribute to higher activation energy than the mineral soils. Psychrophilic microbes produce more exopolysaccharides than mesophilic microbes [89]. Exopolysaccharides can also affect activation energy as exopolysaccharides stabilize extracellular enzymes and prevent enzyme diffusion [90].
Conclusions
Our results suggest that nutrient availability limits enzyme production, and thus constrains SOM decomposition in this Arctic tundra ecosystem. Our results indicate that SOM could decompose more rapidly in response to climate change; SOM decomposition stimulated by higher temperature increases nutrient availability, which may act in a synergetic manner. Such accelerated SOM decomposition could stimulate NPP and result in an increase in shrub abundance, which would feedback to alter SOM quantity and quality. A new balance between C sequestration by vegetation and SOC mineralization will determine whether Arctic tundra ecosystems become a C sink, source or vary across space and time. Recently, enzymes have been incorporated as a factor regulating SOM decomposition in several process models to simulate biogeochemical dynamics (e.g. [91,92]). Responses of enzyme activities to increased nutrient availability could provide a mechanistic basis for predicting long-term feedbacks between climate change, microbial activity, and soil C sequestration.
Supporting Information
Potential enzyme activities in the organic soils assayed at 5, 25 and 35 °C. Statistically significant effects found by mixed-effect ANOVA are shown in panels: F; fertilization treatment (control, low and high fertilizations), S; site (i.e. long- and short-term fertilization sites), and F×S; interaction between F and S. Symbols indicate: †; p ≤ 0.10, *; p ≤ 0.05, **; p ≤ 0.01.
(TIF)
Potential enzyme activities in the interfacesoils assayed at 5, 25 and 35 °C. Statistically significant effects found by mixed-effect ANOVA are shown in panels: S; site (i.e. long- and short-term fertilization sites). Symbols indicate: †; p ≤ 0.10.
(TIF)
Potential enzyme activities in the mineralsoils assayed at 5, 25 and 35 °C.
(TIF)
Summary of p-values resulting from mixed-effect model analyses for potential enzyme activities assessed at 5, 25 and 35 °C. Fert and Profile represent fertilization and soil profile, respectively. p-values equal to or less than 0.10 are shown bold.
(PDF)
Summary of p-values resulting from mixed-effect model analyses for stoichiometry assessed at 5, 25 and 35°C. Fert and Profile represent fertilization and soil profile, respectively. p-values equal to or less than 0.10 are shown bold.
(PDF)
Stoichiometry of potential enzyme activities in the organicsoils assayed at 5, 25 and 35 °C.
(TIF)
Stoichiometry of potential enzyme activities in the interfacesoils assayed at 5, 25 and 35 °C.
(TIF)
Stoichiometry of potential enzyme activities in the mineralsoils assayed 5, 25 and 35°C.
(TIF)
Acknowledgments
We thank Gaius R. Shaver, Laura Gough and Jennie R. McLaren for coordinating field work, and Greg Selby and Jason Leverton for sampling soils. Logistic support was provided by Toolik Field Station, University of Alaska, Fairbanks, USA.
Funding Statement
Funding for this research was provided by the National Science Foundation (OPP-0909507 to J.C.M), and grants to the Marine Biological Laboratory at Woods Hole, MA in support of the Arctic LTER (DEB-0423385). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G et al. (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycles 23: GB2023 [Google Scholar]
- 2. Oechel WC, Billings W (1992) Effects of global change on the carbon balance of Arctic plants and ecosystems. Arctic ecosystems in a changing climate: An ecophysiological perspective. San Diego: Academic Press; pp. 139-168. [Google Scholar]
- 3. Nadelhoffer KJ, Giblin AE, Shaver GR, Laundre JA (1991) Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecology 72: 242-253. doi: 10.2307/1938918. [DOI] [Google Scholar]
- 4. Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66: 503-522. doi: 10.2307/2963492. [DOI] [Google Scholar]
- 5. Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS (2004) Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 431: 440-443. doi: 10.1038/nature02887. PubMed: 15386009. [DOI] [PubMed] [Google Scholar]
- 6. Hansen J, Sato M, Ruedy R, Lo K, Lea DW et al. (2006) Global temperature change. Proc Natl Acad Sci U_S_A 103: 14288-14293. doi: 10.1073/pnas.0606291103. PubMed: 17001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuur EAG, Vogel JG, Crummer KG, Lee H, Sickman JO et al. (2009) The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 459: 556-559. doi: 10.1038/nature08031. PubMed: 19478781. [DOI] [PubMed] [Google Scholar]
- 8. Oechel WC, Hastings SJ, Vourlrtis G, Jenkins M, Riechers G et al. (1993) Recent change of Arctic tundra ecosystems from a net carbon dioxide sink to a source. Nature 361: 520-523. doi: 10.1038/361520a0. [DOI] [Google Scholar]
- 9. Kirschbaum MUF (2000) Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry 48: 21-51. doi: 10.1023/A:1006238902976. [DOI] [Google Scholar]
- 10. Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433: 298–301. doi: 10.1038/nature03226. PubMed: 15662420. [DOI] [PubMed] [Google Scholar]
- 11. Hobbie SE, Nadelhoffer KJ, Högberg P (2002) A synthesis: The role of nutrients as constraints on carbon balances in boreal and Arctic regions. Plant Soil 242: 163-170. doi: 10.1023/A:1019670731128. [DOI] [Google Scholar]
- 12. Chapin FS III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76: 694-711. doi: 10.2307/1939337. [DOI] [Google Scholar]
- 13. Sturm M, Holmgren J, McFadden JP, Liston GE, Chapin FS et al. (2001) Snow–shrub interactions in Arctic tundra: A hypothesis with climatic implications. J Climatol 14: 336-344. doi: 10.1175/1520-0442(2001)014. [DOI] [Google Scholar]
- 14. Sturm M, Racine C, Tape K (2001) Climate change: Increasing shrub abundance in the Arctic. Nature 411: 546-547. doi: 10.1038/35079180. PubMed: 11385559. [DOI] [PubMed] [Google Scholar]
- 15. Sturm M, Schimel J, Michaelson G, Welker JM, Oberbauer SF et al. (2005) Winter biological processes could help convert Arctic tundra to shrubland. BioScience 55: 17-26. [Google Scholar]
- 16. Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T et al. (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6: 045509. doi: 10.1088/1748-9326/6/4/045509. [DOI] [Google Scholar]
- 17. Cornelissen JHC, Van Bodegom PM, Aerts R, Callaghan TV, Van Logtestijn RSP et al. (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10: 619-627. doi: 10.1111/j.1461-0248.2007.01051.x. PubMed: 17542940. [DOI] [PubMed] [Google Scholar]
- 18. Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19: 988-995. doi: 10.1111/gcb.12113. PubMed: 23504877. [DOI] [PubMed] [Google Scholar]
- 19. Schimel JP, Bilbrough C, Welker JM (2004) Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol Biochem 36: 217-227. doi: 10.1016/j.soilbio.2003.09.008. [DOI] [Google Scholar]
- 20. Wahren CHA, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan Arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Chang Biol 11: 537-552. doi: 10.1111/j.1365-2486.2005.00927.x. [DOI] [Google Scholar]
- 21. Blok D, Heijmans MMPD, Schaepman-Strub G, Kononov AV, Maximov TC et al. (2010) Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Glob Chang Biol 16: 1296-1305. doi: 10.1111/j.1365-2486.2009.02110.x. [DOI] [Google Scholar]
- 22. Nowinski N, Trumbore S, Schuur E, Mack M, Shaver G (2008) Nutrient addition prompts rapid destabilization of organic matter in an Arctic tundra ecosystem. Ecosystems 11: 16-25. doi: 10.1007/s10021-007-9104-1. [DOI] [Google Scholar]
- 23. Sistla SA, Moore JC, Simpson RT, Gough L, Shaver GR et al. (2013) Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497: 615-618. doi: 10.1038/nature12129. PubMed: 23676669. [DOI] [PubMed] [Google Scholar]
- 24. Burns RG (1982) Enzyme activity in soil: Location and a possible role in microbial ecology. Soil Biol Biochem 14: 423-427. doi: 10.1016/0038-0717(82)90099-2. [DOI] [Google Scholar]
- 25. Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17: 69-74. doi: 10.1007/BF00418675. [DOI] [Google Scholar]
- 26. Wallenstein MD, Burns RG (2011) Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process; Dick RP. Madison, WI, USA: Soil Science Society of America; pp. 35-55. [Google Scholar]
- 27. Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47: 53-59. doi: 10.1016/j.soilbio.2011.12.026. [DOI] [Google Scholar]
- 28. Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME et al. (2013) Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol Biochem 58: 216-234. doi: 10.1016/j.soilbio.2012.11.009. [DOI] [Google Scholar]
- 29. Shaver GR, Chapin FS III (1991) Production: biomass relationships and element cycling in contrasting Arctic vegetation types. Ecol Monogr 61: 1-31. doi: 10.2307/1942997. [DOI] [Google Scholar]
- 30. McKane RB, Rastetter EB, Shaver GR, Nadelhoffer KJ, Giblin AE et al. (1997) Climatic effects on tundra carbon storage inferred from experimental data and a model. Ecology 78: 1170-1187. doi:10.1890/0012-9658(1997)078[1170:CEOTCS]2.0.CO;2. [Google Scholar]
- 31. Gough L, Ramsey EA, Johnson DR (2007) Plant–herbivore interactions in Alaskan Arctic tundra change with soil nutrient availability. Oikos 116: 407-418. doi: 10.1111/j.0030-1299.2007.15449.x. [DOI] [Google Scholar]
- 32. Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34: 1309-1315. doi: 10.1016/S0038-0717(02)00074-3. [DOI] [Google Scholar]
- 33. Steinweg JM, Dukes JS, Wallenstein MD (2012) Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol Biochem 55: 85-92. doi: 10.1016/j.soilbio.2012.06.015. [DOI] [Google Scholar]
- 34. Arrhenius S (1889) Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z Physikalische Chem 4: 226-248. [Google Scholar]
- 35. Rej R, Vanderlinde RE (1981) Effects of temperature on the steady-state kinetics and measurement of aspartate aminotransferases. Clin Chem 27: 213-219. PubMed: 7460269. [PubMed] [Google Scholar]
- 36. Parham JA, Deng SP (2000) Detection, quantification and characterization of [beta]-glucosaminidase activity in soil. Soil Biol Biochem 32: 1183-1190. doi: 10.1016/S0038-0717(00)00034-1. [DOI] [Google Scholar]
- 37. Mayer LM (1989) Extracellular proteolytic enzyme activity in sediments of an intertidal mudflat. Limnol Oceanogr 34: 973-981. doi: 10.4319/lo.1989.34.6.0973. [DOI] [Google Scholar]
- 38. Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh R (2011) Controls on the temperature sensitivity of soil enzymes: A key driver of in situ enzyme activity rates. Soil Enzymology. Springer Verlag; pp. 245-258. [Google Scholar]
- 39. Developement R Core Team (2011) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0 Available: http://www.R-project.org/. [Google Scholar]
- 40. Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462: 795-798. doi: 10.1038/nature08632. PubMed: 20010687. [DOI] [PubMed] [Google Scholar]
- 41. Chapin FS III, Shaver GR (1985) Individualistic growth response of tundra plant species to environmental manipulations in the field. Ecology 66: 564-576. doi: 10.2307/1940405. [DOI] [Google Scholar]
- 42. Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15: 1631-1639. doi: 10.1111/j.1365-2486.2008.01819.x. [DOI] [Google Scholar]
- 43. Chapin FS III, Fetcher N, Kielland K, Everett KR, Linkins AE (1988) Productivity and nutrient cycling of Alaskan tundra: Enhancement by flowing soil water. Ecology 69: 693-702. doi: 10.2307/1941017. [DOI] [Google Scholar]
- 44. Giblin AE, Nadelhoffer KJ, Shaver GR, Laundre JA, McKerrow AJ (1991) Biogeochemical diversity along a riverside toposequence in Arctic Alaska. Ecol Monogr 61: 415-435. doi: 10.2307/2937049. [DOI] [Google Scholar]
- 45. Jonasson S, Michelsen A, Schmidt IK (1999) Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl Soil Ecol 11: 135-146. doi: 10.1016/S0929-1393(98)00145-0. [DOI] [Google Scholar]
- 46. Schmidt IK, Jonasson S, Michelsen A (1999) Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Appl Soil Ecol 11: 147-160. doi: 10.1016/S0929-1393(98)00147-4. [DOI] [Google Scholar]
- 47. Hobbie SE, Gough L (2004) Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140: 113-124. doi: 10.1007/s00442-004-1556-9. PubMed: 15164284. [DOI] [PubMed] [Google Scholar]
- 48. Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14: 1156-1168. doi: 10.1111/j.1365-2486.2008.01549.x. [DOI] [Google Scholar]
- 49. DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil. Sci Soc Am: J 68: 132-138 [Google Scholar]
- 50. Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14: 1172-1177. doi: 10.1890/03-5120. [DOI] [Google Scholar]
- 51. Keeler B, Hobbie S, Kellogg L (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 12: 1-15. doi: 10.1007/s11252-008-0079-2. [DOI] [Google Scholar]
- 52. Stone MM, Weiss MS, Goodale CL, Adams MB, Fernandez IJ et al. (2012) Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob Chang Biol 18: 1173-1184. doi: 10.1111/j.1365-2486.2011.02545.x. [DOI] [Google Scholar]
- 53. Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31: 769-777. doi: 10.1016/S0038-0717(98)00177-1. [DOI] [Google Scholar]
- 54. Henry HAL, Juarez JD, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Chang Biol 11: 1808-1815. doi: 10.1111/j.1365-2486.2005.001007.x. [DOI] [Google Scholar]
- 55. Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51: 90-98. doi: 10.1007/s00248-005-5156-y. PubMed: 16389463. [DOI] [PubMed] [Google Scholar]
- 56. Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40: 2098-2106. doi: 10.1016/j.soilbio.2008.01.024. [DOI] [Google Scholar]
- 57. Clarholm M (1993) Microbial biomass P, labile P, and acid phosphatase activity in the humus layer of a spruce forest, after repeated additions of fertilizers. Biol Fertil Soils 16: 287-292. doi: 10.1007/BF00369306. [DOI] [Google Scholar]
- 58. Olander L, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49: 175-191. doi: 10.1023/A:1006316117817. [DOI] [Google Scholar]
- 59. Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37: 937-944. doi: 10.1016/j.soilbio.2004.09.014. [DOI] [Google Scholar]
- 60. Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193: 696-704. doi: 10.1111/j.1469-8137.2011.03967.x. PubMed: 22122515. [DOI] [PubMed] [Google Scholar]
- 61. Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43: 313-343. doi: 10.1146/annurev-ecolsys-071112-124414. [DOI] [Google Scholar]
- 62. Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2011) Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. Soil Enzymology. Springer Verlag; pp. 229-243. [Google Scholar]
- 63. Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154: 349-359. doi: 10.1007/s00442-007-0836-6. PubMed: 17724617. [DOI] [PubMed] [Google Scholar]
- 64. Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Fierer N et al. (2008) The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ Microbiol 10: 3093-3105. doi: 10.1111/j.1462-2920.2008.01735.x. PubMed: 18764871. [DOI] [PubMed] [Google Scholar]
- 65. Ping CL, Bockheim JG, Kimble JM, Michaelson GJ, Walker DA (1998) Characteristics of cryogenic soils along a latitudinal transect in Arctic Alaska. JGR Atmos 103: 28917-28928. [Google Scholar]
- 66. Tan KH (2011) Principles of soil chemistry. CRC Press. [Google Scholar]
- 67. Grogan P, Chapin FS III (1999) Arctic soil respiration: Effects of climate and vegetation depend on season. Ecosystems 2: 451-459. doi: 10.1007/s100219900093. [DOI] [Google Scholar]
- 68. Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in Arctic tundra soils above and below freezing. Soil Biol Biochem 34: 1785-1795. doi: 10.1016/S0038-0717(02)00168-2. [DOI] [Google Scholar]
- 69. Glazer AN, Nikaido H (2007) Microbial biotechnology: Fundamentals of applied microbiology. Cambridge University Press. [Google Scholar]
- 70. Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: Distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol Biochem 37: 35-48. doi: 10.1016/j.soilbio.2004.05.024. [DOI] [Google Scholar]
- 71. Di Nardo C, Cinquegrana A, Papa S, Fuggi A, Fioretto A (2004) Laccase and peroxidase isoenzymes during leaf litter decomposition of Quercus ilex in a Mediterranean ecosystem. Soil Biol Biochem 36: 1539-1544. doi: 10.1016/j.soilbio.2004.07.013. [DOI] [Google Scholar]
- 72. Tiquia SM, Lloyd J, Herms DA, Hoitink HAJ, Michel FC Jr (2002) Effects of mulching and fertilization on soil nutrients, microbial activity and rhizosphere bacterial community structure determined by analysis of TRFLPs of PCR-amplified 16S rRNA genes. Appl Soil Ecol 21: 31-48. doi: 10.1016/S0929-1393(02)00040-9. [DOI] [Google Scholar]
- 73. Enwall K, Nyberg K, Bertilsson S, Cederlund H, Stenström J et al. (2007) Long-term impact of fertilization on activity and composition of bacterial communities and metabolic guilds in agricultural soil. Soil Biol Biochem 39: 106-115. doi: 10.1016/j.soilbio.2006.06.015. [DOI] [Google Scholar]
- 74. Jangid K, Williams MA, Franzluebbers AJ, Sanderlin JS, Reeves JH et al. (2008) Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol Biochem 40: 2843-2853. doi: 10.1016/j.soilbio.2008.07.030. [DOI] [Google Scholar]
- 75. Allison SD, Gartner TB, Mack MC, McGuire K, Treseder K (2010) Nitrogen alters carbon dynamics during early succession in boreal forest. Soil Biol Biochem 42: 1157-1164. doi: 10.1016/j.soilbio.2010.03.026. [DOI] [Google Scholar]
- 76. Weber CF, Vilgalys R, Kuske CR (2013) Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Front Microbiol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33: 1437-1445. doi: 10.1016/S0038-0717(01)00052-9. [DOI] [Google Scholar]
- 78. Chu H, Lin X, Fujii T, Morimoto S, Yagi K et al. (2007) Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol Biochem 39: 2971-2976. doi: 10.1016/j.soilbio.2007.05.031. [DOI] [Google Scholar]
- 79. Baldrian P (2009) Laccases of fungi in nature and biotechnology. Advances in fungal biotechnology: IK International Pvt Ltd.. p. 109.
- 80. Lang E, Gonser A, Zadrazil F (2000) Influence of incubation temperature on activity of ligninolytic enzymes in sterile soil by Pleurotus sp. and Dichomitus squalens . J Basic Microbiol 40: 33-39. doi: 10.1002/(SICI)1521-4028(200002)40:1. PubMed: 10746197. [DOI] [PubMed] [Google Scholar]
- 81. Nanmpieri P, Ceccanti B, Bianchi D (1988) Characterization of humus-phosphatase complexes extracted from soil. Soil Biol Biochem 20: 683-691. doi: 10.1016/0038-0717(88)90153-8. [DOI] [Google Scholar]
- 82. Nannipieri P, Sequi P, Fusi P (1996) Humus and enzyme activity. Humic substances in terrestrial ecosystems. Amsterdam: Elsevier; pp. 293-328. [Google Scholar]
- 83. Gianfreda L, De Cristofaro A, Rao MA, Violante A (1995) Kinetic behavior of synthetic organo- and organo-mineral-urease complexes. Soil. Sci Soc Am: J 59: 811-815 [Google Scholar]
- 84. Joanisse GD, Bradley RL, Preston CM, Munson AD (2007) Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia . New Phytol 175: 535-546. doi: 10.1111/j.1469-8137.2007.02113.x. PubMed: 17635228. [DOI] [PubMed] [Google Scholar]
- 85. Boyd SA, Mortland M (1990) Enzyme interactions with clays and clay-organic matter complexes. In: Bollag J-MaGS, editor. Soil biochemistry; Dekker Marcel New York. pp. 1-28 [Google Scholar]
- 86. Naidja A, Huang PM, Bollag J-M (2000) Enzyme-clay interactions and their impact on transformations of natural and anthropogenic organic compounds in soil. J Environ Qual 29: 677-691. doi: 10.2134/jeq2000.00472425002900030002x. [DOI] [Google Scholar]
- 87. Zimmerman A, Ahn M-Y (2011) Organo-mineral–enzyme interaction and soil enzyme activity. In: Shukla G, Varma A. Soil Enzymology. Berlin Heidelberg: Springer Verlag; pp. 271-292. [Google Scholar]
- 88. Gianfreda L, Rao MA, Violante A (1995) Formation and activity of urease-tannate complexes affected by aluminum, iron, and manganese. Soil. Sci Soc Am: J 59: 805-810 [Google Scholar]
- 89. Nicolaus B, Kambourova M, Oner ET (2010) Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environ Technol 31: 1145-1158. doi: 10.1080/09593330903552094. PubMed: 20718297. [DOI] [PubMed] [Google Scholar]
- 90. Qin G, Zhu L, Chen X, Wang PG, Zhang Y (2007) Structural characterization and ecological roles of a novel exopolysaccharide from the deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913. Microbiology 153: 1566-1572. doi: 10.1099/mic.0.2006/003327-0. PubMed: 17464071. [DOI] [PubMed] [Google Scholar]
- 91. Schimel J, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35: 549-563. doi: 10.1016/S0038-0717(03)00015-4. [DOI] [Google Scholar]
- 92. Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3: 336-340. doi: 10.1038/ngeo846. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential enzyme activities in the organic soils assayed at 5, 25 and 35 °C. Statistically significant effects found by mixed-effect ANOVA are shown in panels: F; fertilization treatment (control, low and high fertilizations), S; site (i.e. long- and short-term fertilization sites), and F×S; interaction between F and S. Symbols indicate: †; p ≤ 0.10, *; p ≤ 0.05, **; p ≤ 0.01.
(TIF)
Potential enzyme activities in the interfacesoils assayed at 5, 25 and 35 °C. Statistically significant effects found by mixed-effect ANOVA are shown in panels: S; site (i.e. long- and short-term fertilization sites). Symbols indicate: †; p ≤ 0.10.
(TIF)
Potential enzyme activities in the mineralsoils assayed at 5, 25 and 35 °C.
(TIF)
Summary of p-values resulting from mixed-effect model analyses for potential enzyme activities assessed at 5, 25 and 35 °C. Fert and Profile represent fertilization and soil profile, respectively. p-values equal to or less than 0.10 are shown bold.
(PDF)
Summary of p-values resulting from mixed-effect model analyses for stoichiometry assessed at 5, 25 and 35°C. Fert and Profile represent fertilization and soil profile, respectively. p-values equal to or less than 0.10 are shown bold.
(PDF)
Stoichiometry of potential enzyme activities in the organicsoils assayed at 5, 25 and 35 °C.
(TIF)
Stoichiometry of potential enzyme activities in the interfacesoils assayed at 5, 25 and 35 °C.
(TIF)
Stoichiometry of potential enzyme activities in the mineralsoils assayed 5, 25 and 35°C.
(TIF)