Abstract
Background:
Most lung cancer patients are diagnosed at an advanced disease stage and predominantly receive palliative treatment, which increasingly consists of several chemotherapy lines. We report on patients' quality of life (QOL) to gain knowledge on QOL during and across multiple lines of chemotherapy. This includes patients with (neo)adjuvant therapy up to 3rd or above line palliative chemotherapy.
Methods:
Lung cancer patients receiving outpatient chemotherapy at the Kufstein County Hospital completed an electronic version of the EORTC QLQ-C30. Linear mixed models were used for statistical analysis.
Results:
One hundred and eighty seven patients were included in the study. Surprisingly, irrespective of the chemotherapy line patients reported stable QOL scores during treatment. None of the calculated monthly change rates attained clinical significance, referring to established guidelines that classify a small clinical meaningful change as 5 to 10 points. According to treatment line, 3rd or above line palliative chemotherapy was associated with the worst QOL scores, whereas patients undergoing (neo)adjuvant or 1st line palliative chemotherapy reported fairly comparable QOL.
Conclusion:
The essential finding of our study is that all QOL aspects of the EORTC QLQ-C30 questionnaire remained unchanged during each chemotherapy line in an unselected population of lung cancer patients. Between treatment lines pronounced differences were found, indicating that later palliative chemotherapy lines are associated with higher QOL impairments. These changes in QOL may not primarily be related to the treatment, but rather refer to impairments due to disease progression and may be partly due to a consequence of the prior therapies.
Keywords: lung cancer, quality of life, palliative chemotherapy, multiple treatment lines, electronic patient-reported outcome monitoring
Worldwide, lung cancer is one of the most commonly diagnosed oncological diseases and the leading cause of cancer-related death in men. In women, lung cancer ranks number four with regard to incidence but number two in terms of mortality (Ferlay et al, 2010). Most patients are diagnosed at an advanced stage without curative treatment options. In this situation, systemic palliative treatment has only limited effect on survival. Consequently to maintain or improve patients' quality of life (QOL) represents a main treatment goal (Petrosyan et al, 2012).
Owing to enhanced treatment possibilities, a growing percentage of patients nowadays are referred to multiple treatment lines. Several trials provide evidence that chemotherapy (CT) offers a benefit in advanced lung cancer patients with respect to survival and QOL (Shepherd et al, 2000; O'Brien et al, 2006; Ciuleanu et al, 2009). Subjectively reported QOL data favours CT over best supportive care alone, as six out of eight reviewed studies were associated with a more favourable outcome in terms of better QOL (four studies) and improved symptom control (two studies) (Pat et al, 2008). Even studies that primarily did not report a more favourable QOL outcome for CT showed positive effects of CT on specific QOL domains like functional activity (Ranson et al, 2000) and pain (Brown et al, 2005).
Although there is some knowledge about lung cancer patients' QOL undergoing palliative treatment, it has to be kept in mind that most of the studies investigated established treatment options in terms of superiority concerning patients' QOL (Dancey et al, 2004; Gebbia et al, 2010; Thongprasert et al, 2011) in a clinical trial setting with a highly selected patient population. Furthermore, information about patients' QOL is mostly limited to one line of treatment (predominantly 1st (Belani et al, 2006; Gebbia et al, 2010) and 2nd line CT (Dancey et al, 2004; Tassinari et al, 2009)). Although multiple lines of CT are often part of a comprehensive treatment concept, changes in patients' health status from one line to another are rarely investigated (Socinski et al, 2002).
Routine QOL assessments are not yet incorporated in clinical practice, although they are generally recommended (Valderas et al, 2008; Engelen et al, 2012) and known as a more reliable collection method for QOL data, as there is a considerable incongruity between clinicians' ratings and patients' self-report (Pakhomov et al, 2008; Basch, 2010). Hence, as part of the implementation of a computer-based QOL monitoring, our study offers important insights into QOL of lung cancer patients undergoing CT, as it presents longitudinal data with respect to multiple CT lines, assessed within the daily clinical routine of the Kufstein County Hospital. A broad range of patients receiving (neo)adjuvant and/or multiple lines of palliative CT was extensively monitored. Besides the comparison between CT lines, the pattern of changes in functioning and the course of QOL during CT were examined.
Consequently, the aims of this study are as follows:
To compare lung cancer patients' QOL across multiple lines of CT.
To investigate the course of patients' QOL within each line of CT.
To compare the changes of QOL across CT lines in younger (<70 years) and older patients (⩾70 years).
Materials and methods
Sample and procedure
At the Department of Internal Medicine at the Kufstein County Hospital, lung cancer patients were approached at the time of diagnosis to participate in routine QOL monitoring. At each visit, outpatients receiving CT were addressed to complete the QOL questionnaire EORTC QLQ-C30 (Aaronson et al, 1993), using a tablet PC for autonomous data entry. In case of arising questions, a study nurse was available to provide assistance. Quality of life assessment was carried out electronically by means of the Computer-based Health Evaluation System (CHES), which did not only collect and store patient-entered data but also provided the authorised doctor immediately a QOL profile for each patient. Sociodemographic and clinical data were gathered from the hospital record. The study was approved by the ethics committee of Innsbruck Medical University.
Assessment instrument
EORTC QLQ-C30
All patients completed the EORTC QLQ-C30, which is an internationally validated and widely used cancer-targeted QOL questionnaire. It includes five Functioning Scales (Physical, Social, Role, Cognitive, and Emotional Functioning), a scale for Global QOL, and nine Symptom Scales (Fatigue, Pain, Nausea/Vomiting, Dyspnoea, Appetite Loss, Sleep Disturbance, Constipation, Diarrhoea, and Financial Difficulties). Referring to taste alterations, the QLQ-C30 was supplemented with two additional items taken from the EORTC QOL Group item bank. These items were summed up to an already previously used taste alteration subscale (higher values indicating more severe taste alterations) (Zabernigg et al, 2010; Giesinger et al, 2011; Gamper et al, 2012).
Statistical analysis
Patient characteristics are presented as means, standard deviations, and percentages. As linear mixed models allow data modelling with a varying number of assessments per patient and time-varying covariates (such as e.g. CT line), this modelling approach was used to compare the symptom burden and functioning between patients undergoing different CT lines. The following terms were included in the model: a random baseline, a first-order autocorrelation covariance matrix, and a fixed-effect patient group (CT line).
In a secondary analysis, we investigated physical and psychosocial symptom trajectories in terms of monthly change rates within CT lines, further including a focus on differences between younger (<70 years) and older patients (⩾70 years). For interpretation of these change rates, the thresholds for minimal important change should be used. Osoba et al (1998) advice to use the following thresholds for the QLQ-C30: a change of 5–10 score points indicates a small clinical change, 10–20 points can be interpreted as a moderate change, and above 20 points marks a large change.
Results
Patient characteristics
About 220 patients diagnosed with lung cancer and treated at the outpatient unit of the Department of Internal Medicine of the Kufstein County Hospital were approached to participate in routine PRO collection. In total, 187 patients allocated to outpatient CT were included in regular QOL assessments at each hospital visit (inclusion rate of 85%) with a total number of 996 PRO assessments. Reasons for refusal of routine QOL monitoring were severely impaired lung function, treatment only with surgery, rejection of CT by the patient, and in very few instances rejection of PRO assessment or basic language problems.
Mean age at first assessment was 69 years (s.d. 9.9), 68.5% were male, 78.7% of the patients were suffering from NSCLC, and 21.3% from SCLC. At the time of study inclusion, 16.3% of the patients received adjuvant CT, 50.6% received 1st line palliative CT, 21.9% 2nd line palliative CT, and 11.2% 3rd+ line palliative CT.
In Table 1 patient characteristics are given separately for patients during different CT lines including applied treatments. During the study period, 69.5% of the patients received one CT line, 23.3% received two CT lines, and 7.2% received three or more CT lines.
Table 1. Patient characteristics.
| (Neo) adjuvant CT | 1st pall. CT | 2nd pall. CT | 3rd+ pall. CT | |
|---|---|---|---|---|
|
Number of patients |
n=46 |
n=146 |
n=55 |
n=22 |
|
Age | ||||
| Mean (s.d.) |
64.3 (8.7) |
68.3 (10.4) |
67.6 (9.1) |
66.2 (8.7) |
|
Sex | ||||
| Men | 64.1% | 63.9% | 64.1% | 47.9% |
| Women |
35.9% |
36.1% |
35.9% |
52.1% |
|
Diagnosis | ||||
| NSCLC | 78.8% | 84.3% | 90.0% | 90.4% |
| SCLC |
21.2% |
15.7% |
10.0% |
9.6% |
|
Time since diagnosisa | ||||
| Mean (s.d.) |
2.2 (1.3) |
4.6 (7.5) |
17.8 (17.0) |
20.4 (13.5) |
|
Tumour stage | ||||
| I | 19.8% | 3.4% | 3.1% | 0% |
| II | 17.7% | 0% | 4.1% | 0% |
| III | 51.0% | 27.5% | 22.4% | 7.2% |
| IV |
11.5% |
69.1% |
70.4% |
92.8% |
|
Previous surgery | ||||
| Yes |
43.5% |
22.1% |
32.1% |
30.9% |
|
Metastasis | ||||
| Yes |
42.8% |
69.9% |
82.0% |
73.6% |
|
Chemotherapy regimen | ||||
| NSCLC monotherapy Gemcitabine, Docetaxel, Vinorelbine, Palitaxel, Pemetrexed | 12.3% | 20.7% | 82.4% | 84.9% |
| NSCLC platin combination therapy Vinorelbine (+antibodies), Gemcitabine Pemetrexed, Docetaxel, respectively+platines | 64.4% | 63.6% | 7.1% | 7.5% |
| SCLC platin combination therapy Etoposide+platines, Cisplatin/Irinotecan | 23.3% | 12.8% | 0.0% | 3.2% |
| SCLC non-platin therapy CAV, Topotecan | 0.0% | 2.9% | 10.6% | 4.3% |
Abbreviations: CT=chemotherapy; NSCLC=non-small-cell lung carcinoma; pall.=palliative; SCLC=small-cell lung carcinoma.
Total N=187. Sixty three patients were accounted for two, and 14 patients for three or more chemotherapy lines because they passed from one line to another.
Number of months that passed since diagnosis, averaged across all assessments within a CT line.
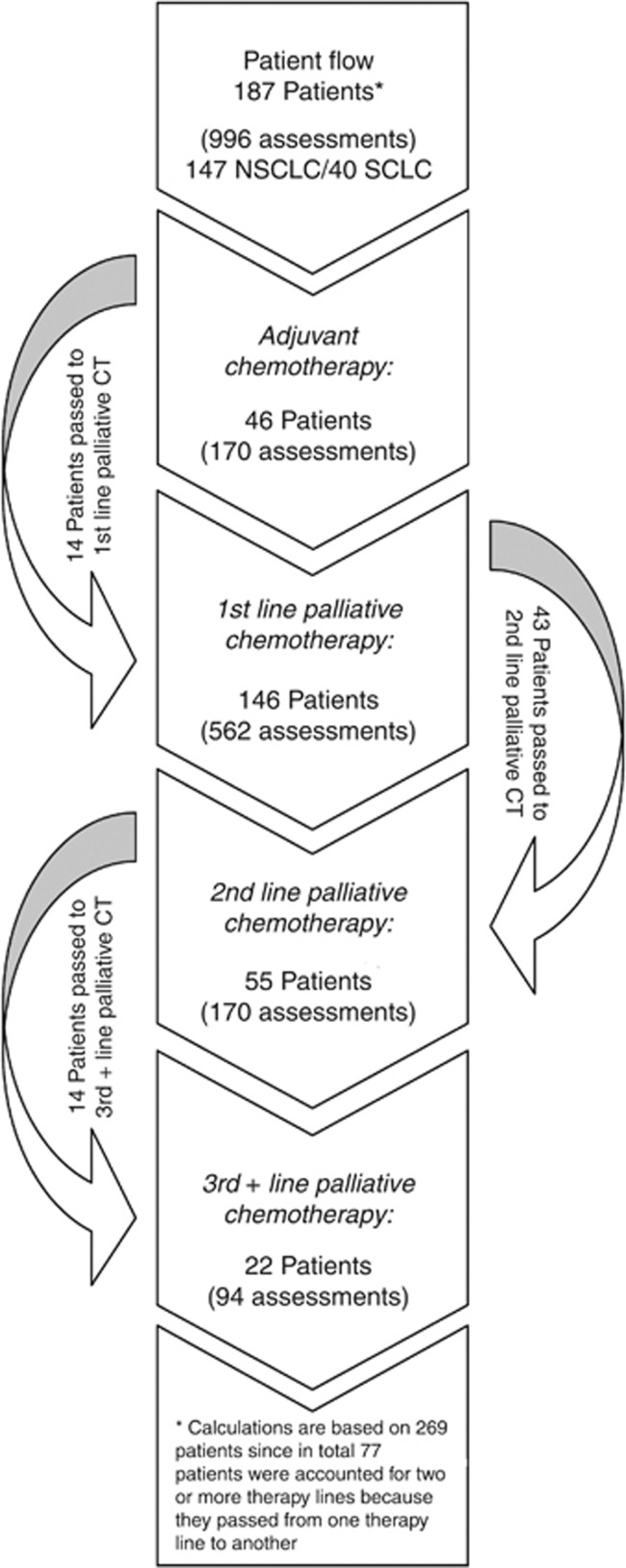
At the time of study inclusion, 78.7% of patients were diagnosed with NSCLC, of which 18.4% received (neo)adjuvant CT, 74.8% 1st, 4.1% 2nd, and 2.7% 3rd line palliative CT. Regarding SCLC patients, 32.5% were undergoing (neo)adjuvant CT, 60.0% 1st, 5.0% 2nd, and 2.5% 3rd line palliative CT. Within the study period, 7.5% of the patients passed from (neo)adjuvant to 1st line palliative CT, 22.9% passed from 1st line to 2nd line palliative CT, and 7.5% from 2nd to 3rd+ line palliative CT (see Figure 1).
Figure 1.
Patient flow. Abbreviation: CT=chemotherapy.
Differences in symptom burden between CT lines
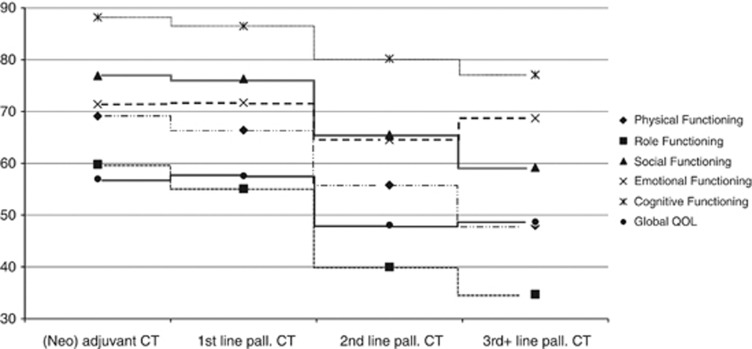
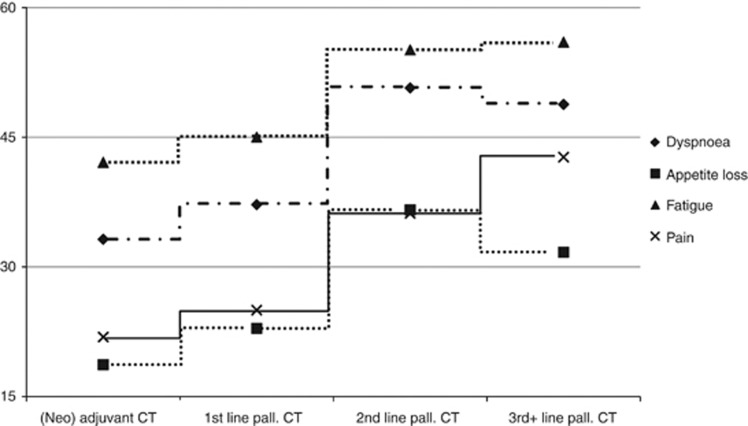
We compared the estimated patient-reported QOL scores across CT lines. As one would expect, we found a significant association between CT line and all Functioning scales of the QLQ-C30 (Physical, Role, Social, Emotional, and Cognitive Functioning), Global QOL, Fatigue, Pain, Dyspnoea, and Appetite Loss with worse outcomes for patients in later treatment lines (see Table 2, Figure 2, and Figure 3). Interestingly, in pairwise comparisons only minor differences were detectable between patients during (neo)adjuvant and 1st line palliative treatments, respectively. Concerning Role, Social, and Cognitive Functioning, Global QOL, Fatigue, Pain, and Dyspnoea, no differences between (neo)adjuvant or 1st line palliative CT were found. In contrast, patients undergoing 2nd or 3rd+ line palliative CT showed significantly worse outcomes. Specifically with regard to Physical Functioning, 3rd+ line CT patients showed the most severe impairments. All in all, patients undergoing 2nd and 3rd+ line palliative CT suffered from high levels of symptom burden in the majority of the QLQ-C30 subscales. However, no significant differences between CT lines were found with regard to Nausea/Vomiting, Sleeping Disturbances, Constipation, Diarrhoea, Financial Impact, and Taste Alteration.
Table 2. Differences between CT lines.
| Adjuvant CT (0) | 1st pall. CT (1) | 2nd pall. CT (2) | 3rd+ pall. CT (3) | P-value | |
|---|---|---|---|---|---|
|
Physical Functioning |
69.12,3 |
66.42,3 |
55.80,1 |
48.00,1,2 |
<0.001 |
|
Role Functioning |
59.82,3 |
55.12,3 |
40.00,1 |
34.70,1 |
<0.001 |
|
Social Functioning |
76.92,3 |
76.32,3 |
65.50,1 |
59.20,1 |
<0.001 |
|
Emotional Functioning |
71.42 |
71.72 |
64.50,1 |
68.7 |
0.030 |
|
Cognitive Functioning |
88.22,3 |
86,52,3 |
80,20,1 |
77.10,1 |
0.001 |
|
Global QOL |
57.02,3 |
57.62,3 |
48.10,1 |
48.70,1 |
<0.001 |
|
Fatigue |
42.12,3 |
45.02,3 |
55.10,1 |
56.00,1 |
<0.001 |
|
Pain |
21.92,3 |
25.02,3 |
36.20,1 |
42.70,1 |
<0.001 |
| Nausea/Vomiting |
8.0 |
10.8 |
12.8 |
10.5 |
0.389 |
|
Dyspnoea |
33.22,3 |
37.22,3 |
50.70,1 |
48.80,1 |
<0.001 |
|
Appetite Loss |
18.72,3 |
22.92 |
36.60,1 |
31.70 |
<0.001 |
| Sleeping Disturbances |
28.7 |
27.2 |
33.3 |
37.8 |
0.098 |
| Constipation |
11.8 |
18.9 |
18.1 |
17.7 |
0.249 |
| Diarrhoea |
4.9 |
5.8 |
10.4 |
8.4 |
0.059 |
| Financial Impact |
17.4 |
14.0 |
19.3 |
17.2 |
0.168 |
| Taste Alterations | 17.8 | 16.4 | 20.2 | 16.7 | 0.570 |
Abbreviations: CT=chemotherapy; pall.=palliative; QOL=quality of life.
Statistically significant (P<0.05) differences between CT lines are marked in bold, and superscript numbers indicate significant differences to CT line.
Figure 2.
Differences in functioning and Global QOL between CT lines. Abbreviations: CT=chemotherapy; pall.=palliative.
Figure 3.
Differences in symptoms between CT lines. Abbreviations: CT=chemotherapy; pall.=palliative.
Symptom trajectories within CT lines
According to the minimal important difference thresholds for the QLQ-C30 reported by Osoba et al (1998), all estimated changes for a period of on average 7.6 months (s.d. 7.3, minimum 0 and maximum 42) that reached statistical significance did not come within the category of small clinical changes (difference between 5 and 10 points) in terms of clinical significance. Social Functioning and Taste significantly worsened during adjuvant CT and 2nd line palliative CT, respectively. During adjuvant, 1st, and 2nd line palliative CT, Cognitive Functioning improved, whereas during 3rd+ line palliative CT, this function area showed deterioration (for detailed numbers please see Table 3).
Table 3. Monthly change rates during chemotherapy.
| Adjuvant CT | 1st pall. CT | 2nd pall. CT | 3rd+ pall. CT | |
|---|---|---|---|---|
| Physical Functioning |
−0.5 |
−0.1 |
−0.3 |
−0.3 |
| Role Functioning |
−1.8 |
0.0 |
−0.3 |
0.1 |
|
Social Functioning |
−1.7 |
0.2 |
−1.1 |
−0.2 |
| Emotional Functioning |
1.3 |
0.3 |
−0.2 |
0.1 |
|
Cognitive Functioning |
0.3 |
0.1 |
0.1 |
−0.6 |
| Global QOL |
−0.4 |
0.1 |
−0.1 |
−0.1 |
| Fatigue |
2.8 |
−0.1 |
0.3 |
0.2 |
| Pain |
−0.1 |
−0.3 |
0.1 |
−0.2 |
| Nausea/Vomiting |
2.0 |
0.0 |
0.1 |
0.0 |
| Dyspnoea |
2.6 |
0.6 |
0.3 |
0.3 |
| Appetite Loss |
0.3 |
−0.3 |
−0.2 |
−0.3 |
| Sleeping Disturbances |
−1.8 |
0.1 |
0.0 |
−0.1 |
| Constipation |
3.0 |
0.1 |
−0.1 |
−0.3 |
| Diarrhoea |
0.0 |
0.2 |
−0.1 |
0.0 |
| Financial Impact |
0.1 |
0.0 |
0.4 |
0.2 |
| Taste Alterations | 1.1 | −0.1 | 0.4 | −0.4 |
Abbreviations: CT=chemotherapy; pall.=palliative; QOL=quality of life.
Statistically significant (P<0.05) change rates within CT lines are marked in bold.
A further analysis considering age groups (younger patients <70 years and older patients ⩾70 years) and CT line yielded only one statistically significant change that was associated with an calculated difference approximating the lower limit of clinical significant changes of five points (Osoba et al, 1998). The monthly change rate of self-reported Constipation in older patients receiving adjuvant CT was about 3.4 points higher than that of younger patients (data not shown).
Altogether, only minor changes were detectable, which, although statistically significant, were below the usually accepted threshold of clinical significance.
Pattern of change in Functioning and Symptom Scales within age groups across CT lines
Further analyses taking age groups (younger patients <70 years and older patients ⩾70 years) into account showed differences in the trend of Physical Functioning and Appetite Loss between CT groups according to their age (see Table 4). In younger patients, Physical Functioning is quite the same during (neo)adjuvant and 1st line palliative CT (mean 71.8 and 72.8 points, respectively). During 2nd and 3rd line CT, it is significantly lower than during previous lines (mean 56.3 and 53.4 points, respectively). Older patients seem to have a relatively stable Physical Functioning until a drop at the time of 3rd+ line CT. A comparable age-related difference in symptom trajectory could also be true for Pain, as PRO scores show a similar pattern (P=0.053, narrowly missing statistical significance). Concerning Appetite Loss, we observed a substantial deterioration in younger patients between 1st and 2nd line CT with similar levels in previous (mean 16.6 points during (neo)adjuvant and 17.8 points during 1st line palliative CT) and following lines (mean 36.0 points during 2nd and 40.3 points during 3rd+ line palliative CT). However in older patients, Appetite Loss decreased from 2nd to 3rd line CT, again being stable in previous lines. This particular finding may be explained by the fact that only a few patients received 3rd+ line CT and therefore single ratings unduly biased the overall results.
Table 4. Differences in change patterns of QLQ-C30 subscales according to age.
| P-value | Age | (Neo) adjuvant CT | 1st pall. CT | 2nd pall. CT | 3rd+ pall. CT | |
|---|---|---|---|---|---|---|
| Physical Functioning |
0.008 |
<70 |
71.8 |
72.8 |
56.3 |
53.4 |
| |
|
⩾70 |
65.2 |
58.7 |
57.5 |
42.4 |
| Pain |
0.053 |
<70 |
22.5 |
26.5 |
43.8 |
42.9 |
| |
|
⩾70 |
19.9 |
23.6 |
25.8 |
43.1 |
| Appetite Loss |
0.027 |
<70 |
16.6 |
17.8 |
36.0 |
40.3 |
| ⩾70 | 24.0 | 29.1 | 36.8 | 22.3 |
Abbreviations: CT=chemotherapy; pall.=palliative; QOL=quality of life.
Discussion
In patients with advanced stage lung cancer, a main goal of systemic treatment is to maintain or improve QOL. Palliative CT offers the possibility to control or decrease cancer-associated symptoms (Hickish et al, 1998). Maintaining or improving QOL is also one of the patients' major concerns, as in burdened patients the wish for symptom relief even exceeds the wish for survival (Silvestri et al, 1998). This general preference in patients' treatment wishes remains basically unchanged, even though the therapeutic options considerably advanced within the past decades (e.g. emergence of maintenance CT) (Gerber et al, 2012). Current knowledge on lung cancer patients' QOL during CT is generally based on data provided by clinical trials including selected patient populations. Such kind of studies is mostly investigating only a single line of CT (Claassens et al, 2011; Damm et al, 2013).
In contrast, the data reported here was collected in an outpatient unit of the Kufstein County Hospital including unselected patients receiving CT for lung cancer, for what reason a broad spectrum of patients is mirrored. The most important result of our analyses is that systemic therapies on average are mainly associated with a stable QOL over time irrespective of treatment line and extent of already experienced QOL impairments. Consequently, CT itself seems not to deteriorate patients' QOL as a drop in scores mostly occurs between treatment lines. These differences between CT lines may primarily be related to impairments because of comorbidities, longer time since diagnosis, worse tumour stage (Lee et al, 2011), and especially disease progression.
Our data offer additional evidence that adjuvant CT has only limited negative impact on QOL, which is in line with data from a previous randomised trial (Bezjak et al, 2008). Only the monthly change rate of Constipation in older patients undergoing adjuvant CT approximated the lower range for a clinical significant change (Osoba et al, 1998) (data not shown), which is consistent with recent findings of Park et al (2013). As a consequence, the assumption that adjuvant CT would negatively impact the QOL in elderly patients with lung cancer needs to be questioned.
For patients receiving different lines of palliative treatment, it is necessary to keep in mind that they might suffer from an inadequate QOL deterioration in the long run. Unrecognised burden may especially occur if symptoms and QOL are not continuously monitored. Besides the encouragement of the need for close-meshed routine QOL monitoring, the data of our study also shows that according to age patients may have different needs. In patients aged 70 and younger, there seems to occur a pronounced aggravation of impairment of Physical Functioning, Pain, and Appetite Loss from adjuvant or 1st line palliative treatment to 2nd and 3rd+ line palliative CT. On the other hand, patients older than 70 years appear to experience quite similar Physical Functioning and Pain during adjuvant, 1st, and 2nd line palliative CT until an increasing deterioration at 3rd+ line palliative CT.
Thus, close-meshed, longitudinal QOL assessment across CT treatment lines should enable early recognition of arising symptom aggravation and consequently facilitates timely intervention in terms of drug dose modifications, offering of additional medication or psychosocial intervention. Regular QOL monitoring even outside the hospital setting has also been suggested by the results of different studies investigating the concept of maintenance treatment in 1st line therapy of patients with metastatic NSCLC. This goal of patients' QOL monitoring at home may be reached by using specialised software, as we did within our study, that can also be applied via web on several devices (computers, smart phones, tablets). Previous studies support the feasibility of web-based PROs as well as their potential to facilitate a more comprehensive medical care at hospital-free intervals at home. Web-based PRO assessments enhance patient–clinician communication in terms of a numerically increased and more targeted discussion of symptoms (Basch et al, 2007a, 2007b; Berry et al, 2011; Snyder et al, 2013).
The longitudinal assessment of QOL provides insight into patients' perception of their medical condition and associated treatment over time. Certainly, many components influence patients' reports on their QOL, as they experience a broad range of distressing symptoms depending not only on the type of cancer and its treatment but also on patients' age and sex (Deshields et al, 2011). Furthermore, conceptual changes due to response shift, the psychosocial situation of the patient and other factors not obvious to others but nevertheless relevant for the individual have an important role on how patients appraise their health condition and well-being. The given medical condition of a patient before the start of cytotoxic therapy influences the efficacy and patients' perception of treatment as well. Small-cell lung cancer patients who suffered from weight loss, extensive disease, and low performance status before the start of CT reported significantly worse QOL, whereas they experienced a relatively higher gain in QOL, although they did not reach QOL levels of patients with limited disease, without weight loss, and better performance status (Bernhard et al, 1996). Nevertheless, how valuable achieved QOL improvements are can only be determined by the patient him-/herself, and this also indicates as to why QOL needs to be assessed and discussed in clinical routine for treatment evaluation.
Our study suggests that regular QOL assessments can be effectively conducted within the busy routine of an outpatient setting. Also, patients at an advanced disease stage and with higher age contributed to the data and proved the feasibility of the used approach.
There are some limitations of the study that should be noted: related to the unselected patient group including NSCLC and SCLC patients, the shrinking sample size across CT lines, and the variety of administered cytotoxic agents, especially the reported results concerning 3rd+ line CT need to be interpreted with caution. Owing to smaller groups of later treatment lines and the fact that several patients passed from one line to another, especially from 2nd to 3rd line and above, the PROs of some few patients may be over-represented and bias the analysis (e.g. as discussed within the Results section for changes in symptom pattern according to age). The decrease in the number of patients across treatment lines, however, reflects the dwindling of patients in a real-world setting, as patients receiving more than three CT lines generally are an exception. Furthermore, as it is not clear how and if the progression of patients is linked to specific treatments, the variety of CT regimen may be an additional confounder. As this study reports longitudinal QOL data across several CT lines, these constraints take a backseat, but should be considered in future studies to refine the reported results.
Conclusion
As there were no strict exclusion criteria, this sample represents the daily clinical practice in lung cancer patients receiving outpatient CT at the Kufstein county hospital. Although QOL significantly deteriorated between treatment lines, most QOL aspects remained unchanged during CT, irrespective of CT line. This means that palliative treatment per se did not negatively impact QOL. This information could support patients and their physician to better understand benefits and harms of the treatment. Close-meshed QOL monitoring using computer-based assessment methods offer the possibility to recognise changes in QOL at an early stage, enabling the treating physician to improve the therapeutic management.
Acknowledgments
We cordially thank Barbara Trixl for her efforts in the context of data collection. The work of LMW and MS was financially supported by the Austrian National Bank (project nos. 14324 and 14492, respectively). The work of JMG was funded by the FWF Austrian Science Fund (project nos. J3353 and L502). The study was supported by the Society of Tumour Research (Verein für Tumorforschung).
The authors declare no conflict of interest.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85 (5:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362 (10:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch E, Artz D, Iasonos A, Speakman J, Shannon K, Lin K, Pun C, Yong H, Fearn P, Barz A, Scher HI, McCabe M, Schrag D. Evaluation of an online platform for cancer patient self-reporting of chemotherapy toxicities. J Am Med Inform Assoc. 2007a;14 (3:264–268. doi: 10.1197/jamia.M2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch E, Iasonos A, Barz A, Culkin A, Kris MG, Artz D, Fearn P, Speakman J, Farquhar R, Scher HI, McCabe M, Schrag D. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007b;25 (34:5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- Belani CP, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna A, Fidias P, Millward M, Fossella F. Effect of chemotherapy for advanced non-small cell lung cancer on patients' quality of life. A randomized controlled trial. Lung Cancer. 2006;53 (2:231–239. doi: 10.1016/j.lungcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bernhard J, Hurny C, Bacchi M, Joss RA, Cavalli F, Senn HJ, Leyvraz S, Stahel R, Ludwig C, Alberto P. Initial prognostic factors in small-cell lung cancer patients predicting quality of life during chemotherapy. Swiss Group for Clinical Cancer Research (SAKK) Br J Cancer. 1996;74 (10:1660–1667. doi: 10.1038/bjc.1996.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Blumenstein BA, Halpenny B, Wolpin S, Fann JR, Austin-Seymour M, Bush N, Karras BT, Lober WB, McCorkle R. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011;29 (8:1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezjak A, Lee CW, Ding K, Brundage M, Winton T, Graham B, Whitehead M, Johnson DH, Livingston RB, Seymour L, Shepherd FA. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J Clin Oncol. 2008;26 (31:5052–5059. doi: 10.1200/JCO.2007.12.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Thorpe H, Napp V, Fairlamb DJ, Gower NH, Milroy R, Parmar MK, Rudd RM, Spiro SG, Stephens RJ, Waller D, West P, Peake MD. Assessment of quality of life in the supportive care setting of the big lung trial in non-small-cell lung cancer. J Clin Oncol. 2005;23 (30:7417–7427. doi: 10.1200/JCO.2005.09.158. [DOI] [PubMed] [Google Scholar]
- Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374 (9699:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- Claassens L, van Meerbeeck J, Coens C, Quinten C, Ghislain I, Sloan EK, Wang XS, Velikova G, Bottomley A. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011;29 (15:2104–2120. doi: 10.1200/JCO.2010.32.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev. 2013;3 (1:15. doi: 10.1186/2191-1991-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer. 2004;43 (2:183–194. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Deshields TL, Potter P, Olsen S, Liu J, Dye L. Documenting the symptom experience of cancer patients. J Support Oncol. 2011;9 (6:216–223. doi: 10.1016/j.suponc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Engelen V, Detmar S, Koopman H, Maurice-Stam H, Caron H, Hoogerbrugge P, Egeler RM, Kaspers G, Grootenhuis M. Reporting health-related quality of life scores to physicians during routine follow-up visits of pediatric oncology patients: is it effective. Pediatr Blood Cancer. 2012;58 (5:766–774. doi: 10.1002/pbc.23158. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127 (12:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gamper EM, Giesinger JM, Oberguggenberger A, Kemmler G, Wintner LM, Gattringer K, Sperner-Unterweger B, Holzner B, Zabernigg A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: prevalence, course of severity, and quality of life correlates. Acta Oncol. 2012;51 (4:490–496. doi: 10.3109/0284186X.2011.633554. [DOI] [PubMed] [Google Scholar]
- Gebbia V, Lorusso V, Galetta D, Caruso MM, Palomba G, Riccardi F, Borsellino N, Carrozza F, Leo S, Ferrau F, Cinieri S, Mancuso G, Mancarella S, Colucci G. First-line cisplatin with docetaxel or vinorelbine in patients with advanced non-small-cell lung cancer: a quality of life directed phase II randomized trial of Gruppo Oncologico Italia Meridionale. Lung Cancer. 2010;69 (2:218–224. doi: 10.1016/j.lungcan.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Gerber DE, Hamann HA, Rasco DW, Woodruff S, Lee SJ. Patient comprehension and attitudes toward maintenance chemotherapy for lung cancer. Patient Educ Counsel. 2012;89 (1:102–108. doi: 10.1016/j.pec.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesinger JM, Wintner LM, Oberguggenberger AS, Gamper EM, Fiegl M, Denz H, Kemmler G, Zabernigg A, Holzner B. Quality of life trajectory in patients with advanced cancer during the last year of life. J Palliat Med. 2011;14 (8:904–912. doi: 10.1089/jpm.2011.0086. [DOI] [PubMed] [Google Scholar]
- Hickish TF, Smith IE, O'Brien ME, Ashley S, Middleton G. Clinical benefit from palliative chemotherapy in non-small-cell lung cancer extends to the elderly and those with poor prognostic factors. Br J Cancer. 1998;78 (1:28–33. doi: 10.1038/bjc.1998.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Chung CW, Chang YY, Lee YC, Yang CH, Liou SH, Liu PH, Wang JD. Comparison of the quality of life between patients with non-small-cell lung cancer and healthy controls. Qual Life Res. 2011;20 (3:415–423. doi: 10.1007/s11136-010-9761-y. [DOI] [PubMed] [Google Scholar]
- O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, Thatcher N, Ross GA, Dane GC, Crofts T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24 (34:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16 (1:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- Pakhomov SV, Jacobsen SJ, Chute CG, Roger VL. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manage Care. 2008;14 (8:530–539. [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim IR, Baek KK, Lee SJ, Chang WJ, Maeng CH, Hong JY, Choi MK, Kim YS, Sun JM, Ahn JS, Park K, Jo J, Jung SH, Ahn MJ. Prospective analysis of quality of life in elderly patients treated with adjuvant chemotherapy for non-small-cell lung cancer. Ann Oncol. 2013;24 (6:1630–1639. doi: 10.1093/annonc/mds649. [DOI] [PubMed] [Google Scholar]
- Pat K, Dooms C, Vansteenkiste J. Systematic review of symptom control and quality of life in studies on chemotherapy for advanced non-small cell lung cancer: how CONSORTed are the data. Lung Cancer. 2008;62 (1:126–138. doi: 10.1016/j.lungcan.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Petrosyan F, Daw H, Haddad A, Spiro T. Targeted therapy for lung cancer. Anticancer Drugs. 2012;23 (10:1016–1021. doi: 10.1097/CAD.0b013e3283585149. [DOI] [PubMed] [Google Scholar]
- Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, Lopez P, Anderson H, Gustafson N, Jeynes A, Gallant G, Washington T, Thatcher N. Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92 (13:1074–1080. doi: 10.1093/jnci/92.13.1074. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18 (10:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ. 1998;317 (7161:771–775. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CF, Blackford AL, Wolff AC, Carducci MA, Herman JM, Wu AW. Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology. 2013;22 (4:895–901. doi: 10.1002/pon.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, Unger P, Lee J, Lee JH, Tynan M, Moore M, Kies MS. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002;20 (5:1335–1343. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- Tassinari D, Scarpi E, Sartori S, Tamburini E, Santelmo C, Tombesi P, Lazzari-Agli L. Second-line treatments in non-small cell lung cancer. A systematic review of literature and metaanalysis of randomized clinical trials. Chest. 2009;135 (6:1596–1609. doi: 10.1378/chest.08-1503. [DOI] [PubMed] [Google Scholar]
- Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, Liao M, Chen YM, Kuo HP, Negoro S, Lam KC, Armour A, Magill P, Fukuoka M. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS) J Thorac Oncol. 2011;6 (11:1872–1880. doi: 10.1097/JTO.0b013e31822adaf7. [DOI] [PubMed] [Google Scholar]
- Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, Revicki DA, Symonds T, Parada A, Alonso J. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17 (2:179–193. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- Zabernigg A, Gamper EM, Giesinger JM, Rumpold G, Kemmler G, Gattringer K, Sperner-Unterweger B, Holzner B. Taste alterations in cancer patients receiving chemotherapy: a neglected side effect. Oncologist. 2010;15 (8:913–920. doi: 10.1634/theoncologist.2009-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]