Abstract
OBJECTIVE:
In First Nations communities of northwestern Ontario, where rates of type 2 diabetes mellitus are some of the highest in the world, ascertaining wild food dietary practices is extremely challenging owing to seasonal availability, environmental factors, life circumstances and language/cultural barriers. The purpose of this study was to determine whether analysis of isotopic and fatty acid (FA) profiles could provide more comprehensive information to discriminate between three categories of wild food consumption (that is, plants and animals) in two isolated First Nations communities of northwestern Ontario. In addition, this analysis also highlights whether wild food consumption as practiced in these two communities can increase circulating levels of polyunsaturated FAs (PUFAs), which provide a number of important metabolic benefits that could impact the prevention/treatment of T2DM.
RESULTS:
13C enrichment (in expired CO2, plasma and hair), 15N enrichment (in hair) and FA profiles in plasma phospholipids (phospholipid fatty acid (PL-FA)) were quantified in men and in women consuming various amounts of wild food. 13C/12C ratios were lower and 15N/14N ratios were higher in participants consuming wild food at least once a week. In addition, FA results indicated that the relative contributions of 20:4 Ω-6 and 22:6 Ω-3 to total PL-FAs were higher and 18:2 Ω-6 lower in wild food consumers.
CONCLUSION:
Together, these findings confirm that isotopic and lipid markers discriminate between the different wild food categories in these two First Nations communities. Knowing the close relationship between dietary intake and the potential role of PUFA in the prevention/treatment of obesity and obesity-related diseases, it is critical to accurately measure the composition of diet for individuals in their specific environments.
Keywords: aboriginal, lipids, isotopic tracers, dietary practices
Introduction
In research and clinical perspectives, adequate assessments of dietary practices are essential for the prevention/treatment of type 2 diabetes mellitus. This holds particularly true in Canada's First Nations communities of northwestern Ontario where rates of type 2 diabetes mellitus are some of the highest in the world.1, 2, 3, 4, 5 Although store-bought foods are available in these isolated communities, they are generally of poor quality and expensive.6 Some nutritious choices are available for purchase but they are generally five to six times more expensive than in southern Canadian cities. In this context, locally harvested foods remain a key source of nourishment for these communities, making its incorporation into diet an important strategy for reducing high rates of obesity and type 2 diabetes mellitus. Locally harvested plants and animals are significant sources of dietary protein, essential minerals, vitamins and polyunsaturated fatty acids (PUFAs),7, 8, 9 particularly long-chain Ω-3 fatty acids (FAs). These Ω-3 FAs have protective benefits against cardiovascular disease, obesity and diabetes10, 11 which continue to be much more prevalent in aboriginal populations.1, 12, 13 The environment around remote communities of northwestern Ontario provides access to a wide range of fish, mammals, birds and wild berries that can be consumed throughout the year.14 However, these wild foods are far from being accessible in sufficient amount to all community members. Ascertaining the extent to which these foods are consumed is challenging owing to their seasonal availability, environmental factors and life circumstances that impact access to land-based foods. This unique context makes accurate and objective assessments of overall food consumption difficult, which is exacerbated by language/cultural barriers and reporting bias.
Methods to assess dietary intake using isotopic and lipid markers may assist in mitigating challenges with communication and reporting. It is well established that isotopic enrichment and FA profiles of consumed foods are reflected in the organism and can be quantified in blood, expired gases and tissue biopsies.15, 16, 17, 18, 19 For example, some studies have used stable isotope ratios of carbon (13C/12C) and nitrogen (15N/14N) to estimate the relative consumption of sugars and sweeteners made from corn and sugar cane20 and the consumption of specific types of proteins between groups of individuals.15, 17, 18, 20, 21 Similarly, intervention studies in humans and in other animals showed that dietary FAs are reflected in the FA profile of membrane phospholipids (PLs).22, 23, 24, 25 Traditionally, the hunter/gatherer diet north of the 49th parallel was rich in proteins (∼35% of total energy intake (E)), rich in lipids (∼43% E), and low in carbohydrates (CHO; ∼22% E).26 The CHO consumed were mainly from C3 photosynthesis plants low in carbon-13 as sugars from corn and sugar cane were not available.26, 27, 28 Proteins and lipids were obtained from hunting and fishing and constituted at least 70% of their subsistence.26 Proteins from wild meats, especially fish, are naturally enriched in 15N (∼14 δ15N‰) compared with market meats (∼4 δ15N‰).21 Moreover, high wild meat consumption provides a greater diversity of FA intake.9 For example, PUFA intake of hunter–gatherer societies was estimated at 27 g·day−1 with 42% of linoleic acid, 41% of alpha-linolenic acid, 11% of 20–22C Ω-6 FAs and 6% of 20–22C Ω-3 FAs for a Ω-3 PUFA balance of 47%.29 Whether the combined measurements of isotopic enrichment (13C and 15N) and FA profiles would allow researchers to discriminate between dietary practices in First Nations communities of Northwestern Ontario remains to be determined.
The purpose of this study was to quantify differences in the quality of CHO, lipid and protein consumed in two Oji-Cree communities of northwestern Ontario. More specifically, 13C enrichment (in expired CO2, plasma and hair), 15N enrichment (in hair) and FA profiles in plasma PLs were quantified in men and in women consuming various amounts of wild food. Based on the combined results obtained by a number of researchers,15, 17, 18, 20, 21, 22, 23, 24, 25, 30 we anticipated that wild food consumers would show lower 13C enrichment in all compartments measured, higher 15N enrichment and higher PUFA levels in plasma PL.
Methods
Participants
This study was conducted according to the guidelines in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Ottawa Research Ethics Board and Health Canada Ethics Board. Written informed consent was obtained from all volunteers. The study was conducted in Wapekeka (Angling Lake) and Kasabonika Lake First Nations in northwestern Ontario, Canada. Inclusion criteria required that individuals be Aboriginal, over 18 years, non-pregnant and free of type 1 diabetes. The number of individuals participating in each community (39 in Wapekeka and 32 in Kasabonika) represents ∼9% of the eligible population in Kasabonika and 24% of the eligible population in Wapekeka at the time of the study. In order to potentiate differences in dietary behaviour between individuals, data collection was performed over a period of 2 months when wild food consumption was known to be high in these communities (late September to early November 2007). Participants were recruited using a mixed-method ethnological approach combining semi-structured interviews, food frequency questionnaires, 3-day dietary records and 24-h dietary recalls. As presented in Table 1, the 71 participants who participated in this study were separated into three groups according to the estimated frequency of wild food consumption: once a week or more (WF3), between once a week and once a month (WF2) and once a month or less (WF1).
Table 1. Characterization of participants for wild food categories WF1 (⩽ once a month), WF2 (once a week>WF2>once a month) and WF3 (⩾ once a week) dietary categories.
| WF1 n=28 (13♂,15♀) | WF2 n=22 (6♂,16♀) | WF3 n=21 (12♂,9♀) | |
|---|---|---|---|
| Age (y) | 41±2 | 41±2 | 51±4 ya,b |
| Weight (kg) | 89±3 | 85±3 | 95±3 |
| BMI (kg m−2) | 32±1 | 31±1 | 34±1 |
Abbreviation: BMI, body mass index.
Significantly different from WF1.
Significantly different from WF2.
Sample collection
Volunteers were asked to report at the nursing station (Kasabonika) and clinic (Wapekeka) located in each community at ∼0900 following 24 h without strenuous excercise and a 12 h fast. First, height and weight were measured and used to calculate body mass index (BMI) by dividing body weight (in kilograms, kg) by square height (in metres, m). For the collection of expired CO2, participants exhaled in a 10 ml vacutainer tube containing no additives. Tubes were sealed with a rubberized cap and secured in place with paraffin tape for transport to the University of Ottawa (Ottawa, Ontario, Canada). Blood samples were taken from the antecubital vein (left arm) in 4 ml vacutainers containing an anticoagulant (ethylenediaminetetraacetic acid). Upon collection, blood samples were immediately placed on ice and centrifuged at 3500 g. Plasma and red blood cells were separated and frozen at −20 °C. At the end of the study, samples were transported frozen to a −80 °C freezer at University of Ottawa (Ottawa, Ontario) until analysis. Hair samples were collected from the nape of the neck as near as possible to the scalp using stainless steel scissors, and were sealed separately in labelled bags for safe transportation to the University of Ottawa. Hair was then cleaned by soaking in a 2:1 chloroform:methanol solution to remove any residues and then rinsed several times with distilled water. Samples were thoroughly dried before performing any analysis.
Sample analysis
13C/12C ratio in expired CO2. 13C enrichment in expired CO2 was measured at the GEOTOP UQAM/McGill Stable Isotope Laboratory (Université du Québec à Montréal, Montréal, Canada) in a Prism mass spectrometer (VG, Manchester, UK). In plasma glucose. 1 ml of plasma was deproteinized, and the glucose was then separated by double-bed ion exchange chromatography by running the supernatants through superimposed columns (resins: AG 50 W-X8 H+, 200–400 mesh and AG 1-X8 chloride, 200–400 mesh), as previously described by Peronnet et al.31 Following evaporation, glucose was combusted (60 min at 400 °C) in the presence of copper oxide. CO2 was recovered and measured at the GEOTOP UQAM/McGill Stable Isotope Laboratory (Université du Québec à Montréal, Montréal, Canada) in a Prism mass spectrometer (VG, Manchester, UK). Isotope ratios in hair. 13C and 15N enrichment in hair was measured at the G.G. Hatch Stable Isotope Laboratory, University of Ottawa. Cleaned hair samples (∼0.6 milligram) were cut, weighed and placed into tin capsules (8 × 5 millimetres (mm), Isomass Scientific (Calgary, AB, Canada). Isotopic enrichment was determined by the analysis of CO2 and N2 produced by flash combustion at 1800 °C on a CE 1110 Elemental Analyzer followed by gas chromatographic separation and on-line analysis by continuous-flow with a DeltaPlus Advantage isotope ratio mass spectrometer coupled with a ConFlo interface. Data were normalized using internal standards previously calibrated with International standards IAEA-CH-6, IAEA-NBS22, IAEA N1, IAEA-N2, USGS-40 and USGS-41.
Isotopic composition in hair samples, in expired CO2 and in CO2 obtained from plasma glucose was expressed as δ values (‰) compared with the V-PDB standard for 13C and atmospheric N2 for 14N, respectively:32, 33
where X is 13C and 15N and R is the corresponding ratio of 13C/12C and 15N/14N in the sample and standard, respectively.
FA profiles of plasma PL
Circulating lipids were extracted twice in chloroform–methanol (2:1 v/v) from 200 μl of plasma collected in the fasting state according to the Folch method.34 Extracted lipids were then suspended in chloroform in which PLs were separated by filtration on Superclean solid-phase extraction tubes (1 ml LC-NH2; Sigma-Aldrich, St Louis, MO, USA) as previously described35 and then eluted with methanol. Two hundred micro liters of margaric acid (17:0; 30 μg per 100 μl hexane) was added as an internal standard and PLs were then trans-esterified with acetyl chloride in methanol for 2 h at 90 °C. After evaporation, the newly formed FAs methyl ester were dissolved in 60 μl isooctane and 2 μl were injected in a Hewlett-Packard gas chromatograph (HP 9890 with HP 7683B autosampler) equipped with a flame-ionization detector and a 60 m fused silica column (DB-23; J&W Scientific, Folsom, CA, USA). The carrier gas was nitrogen and detector gases were hydrogen and air. Injection port temperature was set at 220 °C and the detector at 240 °C. The temperature was 185 °C for 35 min after injection, raised to 210 °C at a rate of 5 °C min−1, and kept at 210 °C for an additional 10 min. Exact retention times of each FA were determined with pure standards (Sigma-Aldrich). Plasma PL concentrations were measured by colorimetry using the PL-C microtiter procedures kit (Wako Diagnostics, Chemicals, Richmond, VA, USA). Because samples were not store in transported in N2 filled vacutainers, it is possible that some hydrolysis of PL occurred resulting in a release of FAs. These FAs would not have been included in the PL fraction following the filtration through the solid-phase extraction tubes (described above). However, it was assumed that the hydrolysed PL would release FAs in the same proportion as what remained in the non-hydrolysed PL fraction. FA profiles were presented as per cent contribution of individual FA to total plasma PL-FA. Only FAs accounting for >5% of total PL-FA were presented in this analysis. Degree of unsaturation of PL-FA was calculated using the following formula:36
Statistical analysis
Data were expressed as mean+s.e. A one-way ANOVA with between-subjects design was used to analyse differences in 13C/12C ratios, 15N/14N ratios and relative contributions of individual FAs to total PL-FA. Bonferonni's multiple comparisons post hoc test was used, where applicable. A two-tailed P-value of <0.05 was considered significant. All analyses were performed using SPSS for Windows (version 16.0; SPSS Inc., Chicago, IL, USA) or GraphPad Prism version 5.00 for Windows (GraphPad, San Diego, CA, USA). All parameters were normally distributed (according to the Shapiro–Wilk test) and the homogeneity of variance between our groups was equal, based on the Levene's test. Despite limited sample size, the effect size for the differences observed between our groups was large (that is, d>0.8). Because 25 of the 71 participants suffered from type 2 diabetes mellitus, we conducted a chi-square test and Pearson correlations between HOMA-IR to determine whether this chronic disease could influence the results of this study. We found that proportion of type 2 diabetic individuals did not differ between food groups (chi-square=2.72, P=0.26). In addition, isotopic and FA markers did not correlate with HOMA-IR scores (P=0.47–0.98). Based on these two results, we confirm that our findings remain the same regardless of whether type 2 diabetic individuals are included in the analyses.
Results
Participants
Differences in wild food categories, age, sex ratios, weight and body mass index between individuals and within each food behaviour group are presented in Table 1. Even though no difference in body weight (P=0.13) and body mass index (P=0.10) were found between groups, WF1 were 1.8 years and 12.1 years younger than WF2 (P=0.02) and WF3 (P=0.02), respectively.
Isotopic composition
Differences in 13C/12C ratios for expired CO2, plasma glucose and hair for WF1, WF2 and WF3 are presented in Figure 1a. No differences in the 13C/12C ratio was found in expired CO2 with δ13C values averaging −23.4±0.2‰ for WF1, −23.3±0.2‰ for WF2 and −23.9±0.2‰ for WF3 (P=0.16). In contrast, the average plasma glucose 13C/12C ratio was lower in WF1 (δ13C of −24.1±0.5‰) than in WF3 (δ13C of −26.3±0.8‰ P=0.05). Values in WF2 for this compartment (δ13C of −24.3±0.8‰) were not different from WF1 (P=0.16) and WF3 (P=1.00). Similar results were found for 13C/12C ratios in hair where WF1 displayed a lower average (δ13C of −19.2±0.1‰) than in WF3 (δ13C of −19.5±0.1‰ P=0.019). No differences were observed between WF2 (δ13C of −19.2±0.1‰) and WF1 (P=0.13) and between WF2 and WF3 (P=1.00).
Figure 1.
(a) δ13C±s.e. (‰, hair, expired CO2 and plasma) and (b) hair δ15N±WFSE (‰) for WF1, WF2 and WF3 food consumption groups. Values are means±s.e. a, significantly different from WF1.
Differences in 15N/14N ratios found in hair in WF1, WF2 and WF3 are presented in Figure 1b. As for 13C/12C ratios in plasma glucose and hair, a significant difference was observed for the 15N/14N ratio in WF1 (δ15N of 9.6±0.1‰) compared with WF3 (δ15N of 10.1±0.1‰) (P=0.006). Values in WF2 (δ15N of 9.9±0.1‰) were not different from WF1 (P=0.90) or WF3 (P=0.116).
FA profiles of plasma PLs
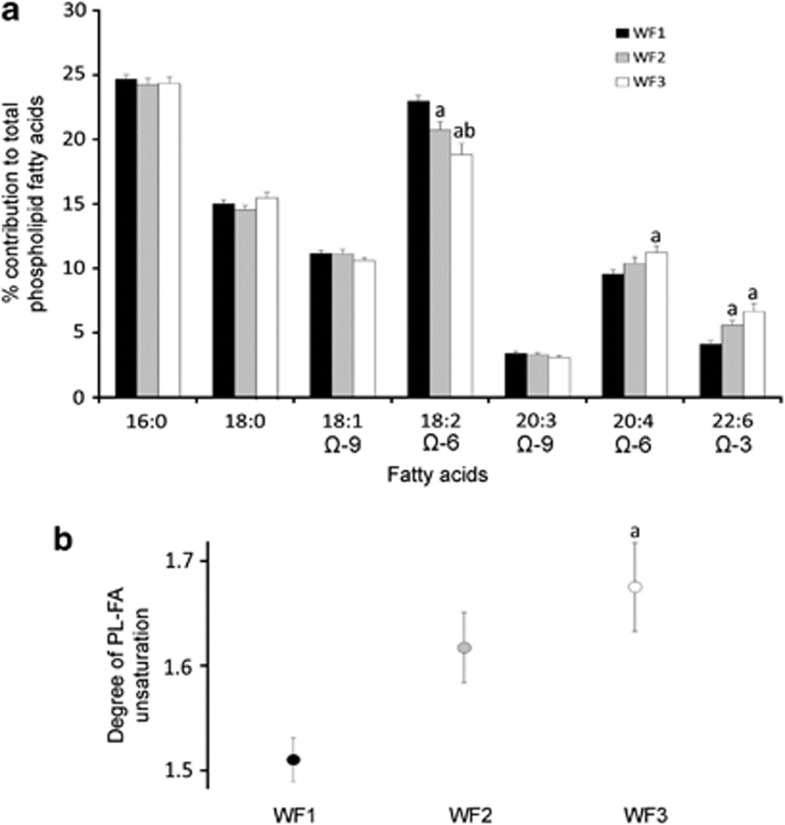
Differences in the FAs composition and degree of PL-FA unsaturation for WF1, WF2 and WF3 are shown in Figure 2. There were no differences in the per cent contribution of 16:0 (P=0.76), 18:0 (P=0.18), 18:1 Ω-9 (P=0.22) and 20:3 Ω-9 (P=0.34) to total PL-FA between WF1 (24.7±0.3%, 15.0±0.3%, 11.2±0.2% and 3.4±0.2%, respectively), WF2 (24.2±0.5%, 14.5±0.3%, 11.1±0.3% and 3.3±0.2%, respectively) and WF3 (24.3±0.5%, 15.5±0.4%, 10.6±0.2% and 3.0±0.2%, respectively) (Figure 2a). The relative contribution of 18:2 Ω-6 to total PL-FA decreased progressively with increasing level of wild food consumption from 23.0±0.5% in WF1 to 20.7±0.7% in WF2 to 18.8±0.9% in WF3 (P<0.001). Conversely, per cent contribution of 20:4 Ω-6 to total PL-FA increased overall from WF1 (9.6±0.3%) to WF3 (11.2±0.5%) (P=0.03). No difference in this FA was found between WF2 (10.4±0.5%) and WF1 (P=0.60) or WF3 (P=0.57). Finally, per cent contribution of 22:6 Ω-3 to total PL-FA was lower in WF1 (4.1±0.2%) when compared with WF3 (6.6±0.6%) (P<0.0001). However, no difference for 22:6 Ω-3 was observed between WF2 (5.6±0.4%) and WF1 (P=0.27) although this FA was lower in WF2 than in WF3 (P=0.04). As shown in Figure 2b, degree of unsaturation of PL-FA increased from WF1 (1.51±0.02%) to WF3 (1.68±0.04%) (P=0.001). No difference in degree of unsaturation was found between WF2 (1.62±0.03%) and WF1 (P=0.38) or WF3 (P=0.12).
Figure 2.
(a) Per cent contribution of individual fatty acids to total phospholipids (%PL-FA) and (b) degree of unsaturation (DU) of PL-FA measured in WF1, WF2 and WF3 food consumption groups. Only fatty acids accounting for >2% of total PL-FA are presented. Values are means±s.e. a, significantly different from WF1; and b, significantly different from WF2.
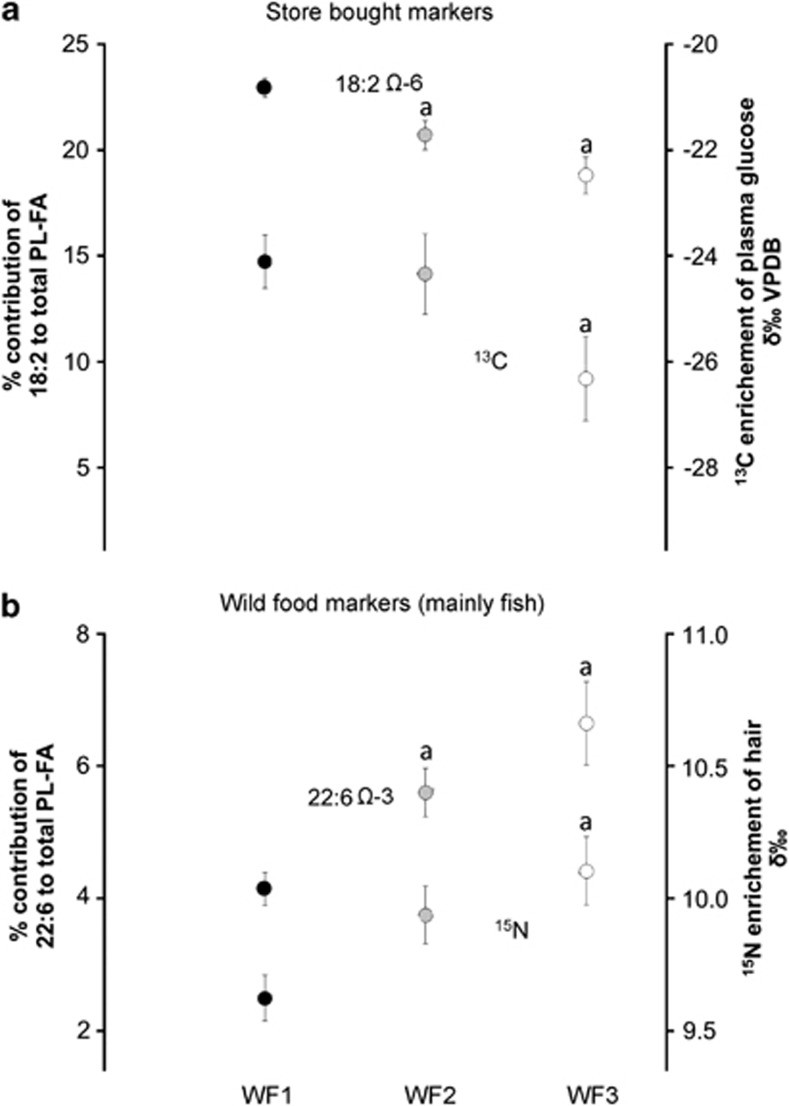
Figure 3 summarizes and illustrates findings for isotopes ratios and FA profiles of plasma PLs as they relate to store-bought markers (13C/12C and 18:2 Ω-6) and fish consumption markers (15N/14N and 22:6 Ω-3). For store-bought food markers, 13C/12C ratios and the relative contribution of 18:2 Ω-6 to total PL-FA decreased overall from WF1 to WF3 (P=0.05 and P<0.0001, respectively). For fish consumption markers, 15N/14N ratios and the relative contribution of 22:6 Ω-3 to total PL-FA increased overall from WF1 to WF3 (P=0.006 and P<0.0001, respectively). In addition, 13C/12C ratios and the relative contribution of 18:2 Ω-6 to total PL-FA as well as 15N/14N ratios and the relative contribution of 22:6 Ω-3 to total PL-FA were significantly correlated (r=0.51, P<0.0001 and r=0.58, P<0.05, respectively).
Figure 3.
Isotopic and fatty acid markers of (a) store-bought and (b) wild food consumption (focus on fish). Values are means±s.e. a, significantly different from WF1.
Discussion
The purpose of this study was to determine whether analysis of isotopic and FA profiles could provide more comprehensive information to discriminate between various levels of wild food consumption in isolated First Nations communities of northwestern Ontario. As anticipated, isotopic results showed that 13C/12C ratios were lower and 15N/14N higher in participants consuming wild food at least once a week (Figure 1). In addition, FA results indicated that the relative contribution of 20:4 Ω-6 and 22:6 Ω-3 to total PL-FA was higher and 18:2 Ω-6 lower in wild food consumers (Figure 2). Together, these findings confirm that isotopic and lipid markers discriminate between the different wild food groupings in these two First Nations communities (Figures 1, 2, 3). Such an assessment is important to determine the relationship between dietary practices and the development/treatment of chronic diseases.
Isotopic dietary markers
The traditional First Nations diet continues to be undermined by the access to low quality market foods in northern Canadian First Nations communities.37 The calorie dense, low quality market foods that are presently available in the two First Nations communities depicted in this study are rich in CHO derived from corn that contains a high natural abundance of 13C (δ13C of −11‰).20, 38 In contrast, traditional hunter–gatherer diet consists of plants relying on C3-photosynthesis with a lower natural abundance of 13C (δ13C of −25‰). Based on these important differences in 13C enrichment, many have advocated the value of carbon isotope ratios (13C/12C) as a means to differentiate dietary preferences/behaviours.15, 17, 18, 20 In our study, results indicated that differences in the quality of CHO consumed between participants were sufficient to obtain significant differences in average 13C/12C ratios in blood or hair between low- (WF1; less than once a month) and high-wild food consumers (WF3; more than once a week). However, 13C enrichment was not sensitive enough to discriminate between the quality of CHO consumed in WF1 and WF3 from the intermediate wild food consumers (WF2; less than once a week but more than once a month). In addition, contrary to what was anticipated, breath CO2 did not allow us to differentiate the three levels of wild food consumption. This might not be that surprising as 13CO2 abundance in breath CO2 is not only influenced by diet but also by the 13C abundance of the substrate being oxidized.38, 39 As fuel selection was not quantified in this study, it is difficult to speculate on whether the lack of differences in 13C/12C between groups is due to differences in substrate utilization.39 Nevertheless, findings from this study show that 13C/12C ratios in blood glucose and in hair are the most reflective of dietary practices in these communities.
In addition to 13C/12C ratios, nitrogen isotope ratios (15N/14N) have proven to be a powerful dietary marker for wild food and fish consumption in this study. This outcome corroborates several other studies that have shown hair δ15N to track animal protein consumption among vegans, vegetarians and omnivores.15, 17, 18 Compared with vegans and vegetarians, omnivores are feeding at the highest trophic level and thus have a greater enrichment of δ15N in their hair due to higher animal protein intake. As expected, in our study, individuals consuming greater amounts of wild fish and hunted meats display higher hair δ15N values. More specifically, community members that consumed wild meat more than once a week (WF3) had higher δ15N values than participants who consumed wild meats less than once a month (WF1). However, as for 13C/12C ratios in blood and hair, variations in diet were not sufficient to find differences between WF2 and the two other groups. It is important to note that higher δ15N values observed in the WF3 are likely attributed to the high consumption of wild fish. In these two communities, wild fish is a staple food available in large amounts most of the year.
FA dietary markers
In addition to higher levels of 15N isotope, wild foods are particularly rich in 20–22C Ω-3 FAs.9, 21, 40, 41 Previous work has shown that PL-FA were highly reflective of FA intake for 3–6 weeks prior to the blood draw.42, 43 This is mainly due to the fact that proportion of PUFA in plasma PL is tightly regulated and approximate 40% of total FA.44 Here, results showed that 22:6 Ω-3 (6.6±0.6% PL-FA) and 20:4 Ω-6 (11.2±0.5% PL-FA) were, respectively, 1.6-times and 1.2-times higher, whereas 18:2 Ω-6 was 1.2-times lower (18.8±0.9% PL-FA) in WF3 when compared with WF1 (4.1±0.2, 9.6±0.3, 23.0±0.5% PL-FA, respectively). In the intermediate WF2, measurements of PL-FA were sensitive enough to discriminate the relative contribution of 18:2 Ω-6 from that of the two extreme groups. For 22:6 Ω-3, values for WF2 were different from those found for WF3 (Figure 2a). In addition, essential FA and degree of unsaturation were, respectively, 1.2- and 1.1-times higher between WF3 (23.4±1.1% PL-FA and 1.68±0.04) and WF1 (18.9±0.6% PL-FA and 1.51±0.02). Together, these results indicate that measurements of PL-FA composition reflect the dietary practices of community members, they also suggest strongly the provenance of foods consumed (that is, wild food or store-bought).
Wild food vs store-bought
When compared with westernized foods obtained in stores and restaurants, adherence to land based foods is often recognized to provide several health benefits for indigenous populations.1, 2, 45, 46, 47, 48 Figure 3 summarizes key results from the presented study as they relate markers of store-bought vs wild food consumption. Community store-bought food is highly processed and is particularly rich in linoleic acid (18:2 Ω-6) derived from soybean oil and rich in refined sugar derived from corn and sugar cane containing high level of 13C label.20, 21, 49 In contrast, wild foods have higher 20–22C Ω-3 FAs, much lower linoleic acid and lower α-linolenic acid29 and contain virtually no refined sugars (δ13C∼−25‰). In addition, proteins from wild meats, especially fish, are naturally enriched in 15N (δ15N ∼14‰) compared with market meats (δ15N ∼4‰).21 Although historical diet was entirely land based, most individuals living in remote First Nations communities today have to rely on store-bought foods to sustain their dietary needs and complement wild food consumption. Interestingly, the parallel increase in 22:6 Ω-3 and δ15N of values strongly suggest that fish is a key source of meat for wild food consumers in these communities; a finding confirmed by the information obtained during the interviews. Clearly, our study showed that isotopes and FA measurements can be used to establish significant differences in the relative contribution of store-bought vs wild food consumption in First Nations communities. However, it seems that this is only possible when extremes in wild food consumption are considered; that is, wild food more than once a week or wild food less than once a month. Results show that only 22:6 Ω-3 and δ13C can be differentiated between intermediate WF2 and high wild food consumers in WF3. This finding exposes the importance of carefully recruiting participants in research attempting to quantify the potentially beneficial effects of wild food consumption on rates of chronic diseases. Typically, studies interested in estimating dietary pattern within a population would use epidemiological methodologies involving excessively large cohorts (ex: >10 000 samples) and food frequency questionnaires.50, 51 However, this approach is difficult in remote, isolated First Nations communities where available cohorts are small and where it is difficult to assess precisely the dependence on locally harvested/hunted foods.46 In this context, many researchers interested in assessing dietary intake rely on self-reporting by participants which has obvious limitations. Dietary assessment tools such as individual interviews, food frequency questionnaires and dietary recalls are essential to understanding dietary behaviours; however, they can be problematic owing to, among other things, under-reporting,30 individual privacy, bias, cost, and time required of both participants and researchers.21, 52, 53, 54, 55 Although dietary marker-based methods provide less qualitative information, they are relatively free of any bias56 and can help to validate dietary surveys by identifying inconsistencies between tissue concentrations and dietary intake.
Conclusion
Stable isotopes and FA profiles as dietary markers have been used widely in both human and animal dietary studies, however none have successfully combined these methods to assess well-defined dietary behaviours in northern Canadian First Nations communities. With large differences in dietary behaviours observed within First Nations communities, analysis of stable isotopes and FA profiles of PLs can provide quantitative information about an individual's diet and can contribute greatly to Aboriginal health and nutrition studies by providing a ‘non-invasive, simple, yet powerful tool for monitoring dietary pattern'.21 The use of these methods can be directly applied to assessing dietary behaviours within and between First Nations communities. Knowing the close relationship between dietary intake and the development/prevention of obesity and obesity-related diseases, it is critical to accurately measure the composition of diet for individuals in their specific environments. This information will be key for the development of region specific chronic disease prevention strategies.
Acknowledgments
The National First Nations Environmental Contaminant Program (NFNECP), the Natural Sciences and Engineering Research Council (NSERC) provided financial support for this study. We are grateful to Margaret Kenequanash and the Shibogama First Nations Council. Many thanks to the participants, band officers and nurses from Kasabonika and Wapekeka First Nations who contributed greatly to this project. We also thank Bernard Pinet for measuring and analysing FA profiles and his contribution in writing this manuscript and Dr Jean-Michel Weber for his advice regarding lipid analysis. TS conducted the field study, sample preparation, stable isotope and mercury analysis, data analysis and led the writing; FH, JMB, MAR and PI proposed and designed the study, and contributed to the writing; and, SP and EMK participated in the field study and contributed to the writing. In memory of Dr Georges Zwingelstein who has shared generously and unconditionally with FH his knowledge (passion) of lipid biochemistry and science.
The authors declare no conflict of interest.
References
- Young TK, Reading J, Elias B, O'Neil JD. Type 2 diabetes mellitus in Canada's first nations: status of an epidemic in progress. CMAJ. 2000;163:561–566. [PMC free article] [PubMed] [Google Scholar]
- Gittelsohn J, Wolever TM, Harris SB, Harris-Giraldo R, Hanley AJ, Zinman B. Specific patterns of food consumption and preparation are associated with diabetes and obesity in a Native Canadian community. J Nutr. 1998;128:541–547. doi: 10.1093/jn/128.3.541. [DOI] [PubMed] [Google Scholar]
- Harris SB, Gittelsohn J, Hanley A, Barnie A, Wolever TM, Gao J, et al. The prevalence of NIDDM and associated risk factors in native Canadians. Diabetes Care. 1997;20:185–187. doi: 10.2337/diacare.20.2.185. [DOI] [PubMed] [Google Scholar]
- Garriguet D. Obesity and the eating habits of the Aboriginal population. Health Rep. 2008;19:21–35. [PubMed] [Google Scholar]
- Imbeault P, Haman F, Blais JM, Pal S, Seabert T, Krummel EM, et al. Obesity and type 2 diabetes prevalence in adults from two remote first nations communities in northwestern ontario, Canada. J Obes. 2011;2011:267509. doi: 10.1155/2011/267509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Haman F, Robidoux MA. The costs of local food procurement in two northern indigenous communities in Canada. Food Foodways. 2013;21:132–152. [Google Scholar]
- Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how. Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr. 2002;76:85–92. doi: 10.1093/ajcn/76.1.85. [DOI] [PubMed] [Google Scholar]
- Kuhnlein HV, Chan HM, Leggee D, Barthet V. Macronutrient, mineral and fatty acid composition of Canadian Arctic traditional food. J Food Compos Anal. 2002;15:545–566. [Google Scholar]
- Kuhnlein HV, Receveur O, Soueida R, Egeland GM. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J Nutr. 2004;134:1447–1453. doi: 10.1093/jn/134.6.1447. [DOI] [PubMed] [Google Scholar]
- Seo T, Blaner WS, Deckelbaum RJ. Omega-3 fatty acids: molecular approaches to optimal biological outcomes. Curr Opin Lipidol. 2005;16:11–18. doi: 10.1097/00041433-200502000-00004. [DOI] [PubMed] [Google Scholar]
- Ayach BB, Korda H. Type 2 diabetes epidemic in First Nations people of Canada. Ethn Dis. 2010;20:300–303. [PubMed] [Google Scholar]
- Tjepkema M, Wilkins R, Goedhuis N, Pennock J. Cardiovascular disease mortality among First Nations people in Canada, 1991-2001. Chronic Dis Inj Can. 2012;32:200–207. [PubMed] [Google Scholar]
- Robidoux MA, Imbeault P, Blais JM, Pal S, Seabert T, Krümmel E, et al. Traditional foodways in two contemporary Northern First Nations communities. Can J Native Stud. 2012;32 [Google Scholar]
- Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135:1515–1520. doi: 10.1093/jn/135.6.1515. [DOI] [PubMed] [Google Scholar]
- Bol R, Pflieger C. Stable isotope (13C, 15N and 34S) analysis of the hair of modern humans and their domestic animals. RCM. 2002;16:2195–2200. doi: 10.1002/rcm.706. [DOI] [PubMed] [Google Scholar]
- O'Connell TC, Hedges RE. Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol. 1999;108:409–425. doi: 10.1002/(SICI)1096-8644(199904)108:4<409::AID-AJPA3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Petzke KJ, Boeing H, Metges CC. Choice of dietary protein of vegetarians and omnivores is reflected in their hair protein 13C and 15N abundance. RCM. 2005;19:1392–1400. doi: 10.1002/rcm.1925. [DOI] [PubMed] [Google Scholar]
- Nardoto GB, Silva S, Kendall C, Ehleringer JR, Chesson LA, Ferraz ES, et al. Geographical patterns of human diet derived from stable-isotope analysis of fingernails. Am J Phys Anthropol. 2006;131:137–146. doi: 10.1002/ajpa.20409. [DOI] [PubMed] [Google Scholar]
- Jahren AH, Saudek C, Yeung EH, Kao WH, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–1384. doi: 10.1093/ajcn/84.6.1380. [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Yai Y, O'Brien DM. Age-related variation in red blood cell stable isotope ratios (delta13C and delta15N) from two Yupik villages in southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66:31–41. doi: 10.3402/ijch.v66i1.18222. [DOI] [PubMed] [Google Scholar]
- Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia. 2001;44:312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- Abbott SK, Else PL, Atkins TA, Hulbert AJ. Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim Biophys Acta. 2012;1818:1309–1317. doi: 10.1016/j.bbamem.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Nagahuedi S, Popesku JT, Trudeau VL, Weber JM. Mimicking the natural doping of migrant sandpipers in sedentary quails: effects of dietary n-3 fatty acids on muscle membranes and PPAR expression. J Exp Biol. 2009;212 (Pt 8:1106–1114. doi: 10.1242/jeb.027888. [DOI] [PubMed] [Google Scholar]
- Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- Kuhnlein HV, Receveur O, Chan HM. Traditional food systems research with Canadian indigenous peoples. Int J Circumpolar Health. 2001;60:112–122. [PubMed] [Google Scholar]
- Eaton SB, Eaton SB, 3rd, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev Nutr Diet. 1998;83:12–23. doi: 10.1159/000059672. [DOI] [PubMed] [Google Scholar]
- Vessby B. Dietary fat and insulin action in humans. Br J Nutr. 2000;83 (Suppl 1:S91–S96. doi: 10.1017/s000711450000101x. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Rheaume N, Lavoie C, Hillaire-Marcel C, Massicotte D. Oral [13C]glucose oxidation during prolonged exercise after high- and low-carbohydrate diets. J Appl Physiol. 1998;85:723–730. doi: 10.1152/jappl.1998.85.2.723. [DOI] [PubMed] [Google Scholar]
- Craig H. The geochemistry of stable isotopes. Geochim Cosmochim Acta. 1953;3:53–92. [Google Scholar]
- Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Ann Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Vaillancourt E, Weber JM. Lipid mobilization of long-distance migrant birds in vivo: the high lipolytic rate of ruff sandpipers is not stimulated during shivering. J Exp Biol. 2007;210 (Pt 7:1161–1169. doi: 10.1242/jeb.003012. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Batal M, Gray-Donald K, Kuhnlein HV, Receveur O. Estimation of traditional food intake in indigenous communities in Denendeh and the Yukon. Int J Circumpolar Health. 2005;64:46–54. doi: 10.3402/ijch.v64i1.17953. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Klein PD, Watkins JB, Heim T, MacLean WC., Jr. 13C abundances of nutrients and the effect of variations in 13C isotopic abundances of test meals formulated for 13CO2 breath tests. Am J Clin Nutr. 1980;33:2375–2385. doi: 10.1093/ajcn/33.11.2375. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Brown C, Nakamura K, Nakagawa A, Mazzeo RS, Brooks GA, et al. Influence of metabolic fuel on the 13C/12C ratio of breath CO2. Biomed Mass Spectrom. 1984;11:557–561. doi: 10.1002/bms.1200111103. [DOI] [PubMed] [Google Scholar]
- Appavoo D, Kubow S, Kuhnlein HV. Lipid composition of indigenous foods eaten by the Sahtù (Hareskin) Dene-Metis of the Northwest Territories. J Food Compos Anal. 1991;4:107–119. [Google Scholar]
- van der Merwe NJ, Williamson RF, Pfeiffer S, Thomas SC. Allegretto KO. The Moatfield ossuary: isotopic dietary analysis of an Iroquoian community, using dental tissue. J Anthropol Archaeol. 2003;22:245–261. [Google Scholar]
- Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of Fatty acids and insulin action. Ann N Y Acad Sci. 2002;967:183–195. doi: 10.1111/j.1749-6632.2002.tb04275.x. [DOI] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- O'Dea K, Spargo RM. Metabolic adaptation to a low carbohydrate-high protein ('traditional') diet in Australian Aborigines. Diabetologia. 1982;23:494–498. doi: 10.1007/BF00254297. [DOI] [PubMed] [Google Scholar]
- Kuhnlein HV, Receveur O. Dietary change and traditional food systems of indigenous peoples. Ann Rev Nutr. 1996;16:417–442. doi: 10.1146/annurev.nu.16.070196.002221. [DOI] [PubMed] [Google Scholar]
- Haman F, Fontaine-Bisson B, Batal M, Imbeault P, Blais JM, Robidoux MA. Obesity and type 2 diabetes in Northern Canada's remote First Nations communities: the dietary dilemma. Int J Obes (Lond) 2010;34 (Suppl 2:S24–S31. doi: 10.1038/ijo.2010.236. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta med Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, McCullough ML. Dietary pattern analysis for the evaluation of dietary guidelines. Asia Pacific J Clin Nutr. 2008;17 (Suppl 1:75–78. [PubMed] [Google Scholar]
- Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- Becker W, Welten D. Under-reporting in dietary surveys–implications for development of food-based dietary guidelines. Public Health Nutr. 2001;4:683–687. doi: 10.1079/phn2001154. [DOI] [PubMed] [Google Scholar]
- Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, et al. Structure of dietary measurement error: results of the OPEN biomarker study Am J Epidemiol 200315814–21.discussion 22-6. [DOI] [PubMed] [Google Scholar]
- Shai I, Rosner BA, Shahar DR, Vardi H, Azrad AB, Kanfi A, et al. Dietary evaluation and attenuation of relative risk: multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall questionnaires: the DEARR study. J Nutr. 2005;135:573–579. doi: 10.1093/jn/135.3.573. [DOI] [PubMed] [Google Scholar]
- Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- Willett W. Dietary fat and breast cancer. J Natl Cancer Inst. 1991;83:1035–1036. doi: 10.1093/jnci/83.14.1035. [DOI] [PubMed] [Google Scholar]