Abstract
Despite glycemic control, evidence suggests that mortality and morbidity remain high in diabetes. Regulatory agencies deem, therefore, additional safety trials necessary for the approval of new antidiabetic drugs. Nevertheless, markers of cardiovascular risk, which can be used as response predictors, are not available. In contrast with current efforts on further understanding of glucose–insulin homeostasis, a model-based approach is required to assess the correlation between hyperglycemia and cardiometabolic phenotypes, enabling prediction of the underlying cardiovascular risk.
The Chicken and The Egg
Type 2 diabetes is characterized by the resistance of the body tissues to utilize insulin and promote glucose disposal [S1,S2]. There has been a long-standing assumption that normoglycemia is a condition sine qua non to stabilize disease progression and prevent or reduce morbidity [S3], which has led to the development of pharmacological interventions, either as single agents or combination therapy, which are aimed primarily at maintaining normoglycemia. Recently, new treatments have started to target incretins, which enhance insulin secretion [S4], but such a strategy also relies on the same assumptions regarding the role of glucose homeostasis [S5]. In contrast with the traditional views about the disease progression, it appears that diabetes may not be the cause but rather a consequence of an underlying cardiometabolic dysfunction. Oncoming evidence suggests that metabolic syndrome1 is a cluster of abnormalities that, if present concomitantly, increases the risk of developing both diabetes and subsequent cardiovascular complications. Throughout the course of the disease, the vast majority of subjects develop symptoms of diabetes, including hyperglycemia, elevated triglycerides, low levels of high-density lipoprotein, and increase in blood pressure and waist circumference (Figure 1). Others with different metabolic phenotypes will present abnormalities in lipids or blood pressure in the absence of diabetes,2 which suggests that diabetes may be just one component of the progression of the metabolic syndrome. In fact, to understand the association between cardiovascular risk and diabetes, one needs to establish a logical relationship between cause and effect, namely, whether the onset of diabetes should be considered the cause or the consequence of a wider underlying dysfunction (see glossary and definitions in Supplementary Boxes 1 and 2 online).
Figure 1.
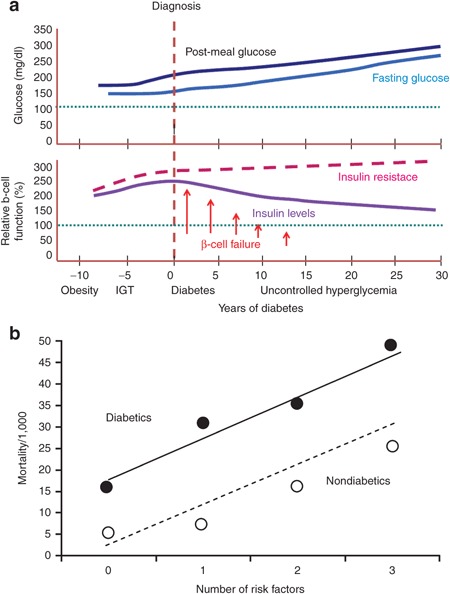
Disease progression and mortality risk in type 2 diabetes. (a) Time course of glucose and insulin under different states in type 2 diabetes. Insulin resistance is apparent well before the onset of type 2 diabetes and is characterized by a poor glycemic control. The increase of the β-cell function acts as a compensatory effect to stabilize glucose levels in the blood. However, as one progresses toward the development of type 2 diabetes, the persistent insulin resistance causes the exhaustion of the pancreas and prevents secretion of more new insulin. (b) Synergic effect of diabetes mellitus associated with other cardiovascular factors. Cardiovascular risk is significantly increased in type 2 diabetics, and hence dealing with the factors contributing to the risk may have a great impact on the progression of the disease in this population. A more prompt and aggressive management of these factors is often required in diabetic patients. It has been estimated that for each level of abnormality of each risk factor, type 2 diabetics present a risk of coronary artery disease that is two to four times greater. IGT, impaired glucose tolerance.
Reprinted with permission from refs. [S6,S19].
From a drug development perspective, evidence indicates that glucose homeostasis, and in particular hemoglobin glycation, has limited prognostic value for the prediction of cardiovascular morbidity and mortality [S6],3 which is associated with different disease phenotypes, including cardiac/vascular hypertrophy, congestive heart failure, stroke, and cardiovascular death [S7,S8]. As a consequence, regulatory agencies have introduced additional prerequisites for the evaluation of the safety of new antidiabetic agents. These requirements impose the need for large clinical trials and systematic assessment of cardiovascular risk in addition to the individual metabolic profile.
Clearly, biomarkers of pharmacology and prognostic markers of long-term response are required to prevent lengthy trials and exposure of patients to potentially ineffective therapies. A promising method to identify such markers is the parameterization of the pathophysiological processes underlying the observed cardiometabolic phenotypes, which can be defined according to the degree of impairment in glycemic control and/or hyperinsulinemia, excess visceral fat, dyslipidemia, and obesity.4
In this article, we propose the use of model-based approaches as a tool to predict the cardiovascular risk through the characterization of the underlying cardiometabolic phenotypes. The availability of such models can provide the basis for evidence synthesis, i.e., integrating new and historical data during the development of new molecules. Moreover, it offers the possibility to explore the prognostic value of markers of cardiovascular disease among the patient population.
Cardiovascular Risk
Clinical data suggest that it is the underlying metabolic syndrome and not diabetes per se that triggers an increased risk of cardiovascular disease [S9,S10], and studies indicate that lowering of glucose levels may have no or an adverse impact on cardiovascular events [S11–S14]. It is striking that the cardiovascular risk in diabetic patients is similar to that in nondiabetic subjects who suffer from a myocardial infarction.5 Epidemiological data also show that aggressive glycemic control does not decrease the cardiovascular risk, but can be even harmful in the presence of cardiovascular disease [S15].
Recently, a debate has arisen about the potential role of the pharmacological interventions, rather than the disease itself, as the primary trigger for cardiovascular events. Various studies have explored the correlation between a strict glycemic lowering or fasting glucose control and their implications in overall cardiovascular risk [S12,S16,S31]. The results suggest that a neutral or considerably delayed protective effect of treatment exists on micro- and macrovascular structures. However, other studies have failed to establish a direct link between glycemic control and increased cardiovascular risk [S13,S14] (Figure 2).
Figure 2.
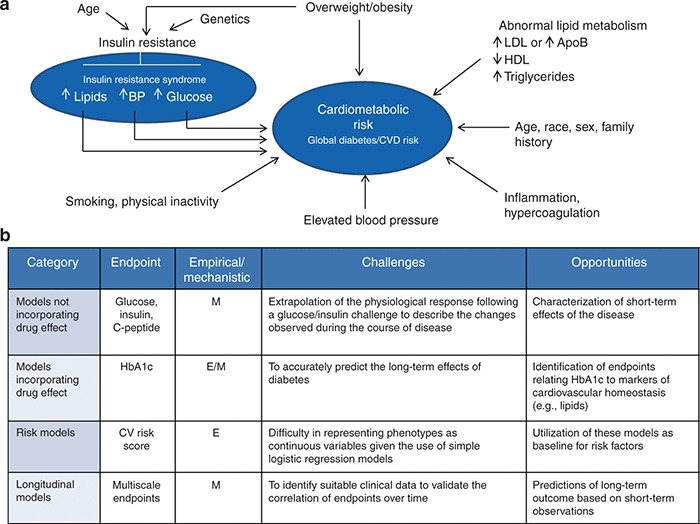
Cardiometabolic phenotypes and integration of cardiometabolic risk into PKPD, risk and longitudinal (disease) models. (a) factors that predict global diabetes mellitus, cardiovascular disease risk, and other manifestations of metabolic syndrome (insulin resistance syndrome). (b) a model-based approach enables integration of the relevant factors associated with the glucose–insulin homeostasis dysfunction and risk factors related to different cardiometabolic phenotypes. Multiscale models represent today the state of the art in establishing correlations between short- and long-term disease outcomes. ApoB, apolipoprotein B; BP, blood pressure; E, empirical; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, mechanistic. Reprinted with permission from refs. [S9,S10].
A shift in the paradigm, currently applied to the development of novel antidiabetic agents, is required to take into account the overall metabolic phenotype and cardiovascular risk.
An approach similar to existing efforts in the treatment of neuroendocrine diseases [S17] can be envisaged in which the metabolic syndrome underlying the symptomatology of diabetes is only one of the various components of a multidimensional disorder [S18].
Identifying Prognostic Markers of Treatment Outcome
Type 2 diabetes accelerates the process of vascular vulnerability and disturbed endothelial function through different mechanisms [S19], including (i) anomalies in lipoprotein concentration and composition; (ii) its association with hypertension, insulin resistance, and hyperinsulinemia; (iii) protein glycosylation in plasma and arterial wall; and (iv) lipid oxidation and proinflammatory state. On the other hand, differently from most endocrine diseases, the clinical evolution of symptoms is considerably slow rendering the evaluation of long-term treatment outcome rather challenging. Such a slow progression imposes the identification of markers with both predictive and prognostic value. Conceptually, this implies a detailed understanding of the processes that occur at different time scales during the course of disease. In this context, a meaningful start would involve exploring the relationship between diabetes, lipids, obesity, and atherosclerosis, which seem to be part of a single continuum in the pathophysiology of the disease at histological and functional levels.
Recent findings on statins have led to the hypothesis that cardioprotection may depend on the timely management of disrupted lipid metabolism.6 It is clear that the majority of obese diabetic patients who show a lipid dysfunction will be exposed to a higher risk for cardiovascular diseases [S20]. This hypothesis is further corroborated by the fact that dyslipidemia is often accompanied by obesity, which in turn leads to atherosclerosis and consequently to a potentially higher cardiovascular risk than that in normal weight individuals. More recent data reveal that the metabolically healthy but obese phenotype is a benign condition, with a better prognosis for mortality and morbidity than metabolically abnormal obese individuals [S21]. Given that most interventions are focused on glycemic control, it remains unclear whether progressive secondary cardiometabolic changes (e.g., lipids) are associated with an increased cardiovascular risk.
Ultimately, the development of new biomarkers would involve identifying high-risk individuals who may benefit from a reduction in modifiable risk factors, including treatments aimed at changes in life style and pharmacological interventions. Nevertheless, accurate evaluation of the benefit–risk balance of treatments aimed at prophylaxis and/or modification of disease progression will remain a burden by using empirical protocols, even when longitudinal follow-up is well designed and outcomes strictly defined.7 The spectrum of factors preceding the onset and progression of the metabolic syndrome is too wide to stratify in traditional clinical and epidemiological trials [S22,S23], leading to associations without the required evidence for causation [S24,S25].8
From Experiments to Models in Diabetes
The use of systems biology and systems pharmacology in conjunction with control theory and statistical methods has allowed the development of modeling approaches to better understand the biological processes in healthy and disease conditions [S26,S27]. In contrast with traditional meta-analysis, this has offered the opportunity to describe processes in a mechanistic or semi-mechanistic manner (see Supplementary Data online). In diabetes, the parameterization of insulin–glucose homeostasis has provided evidence for the progressive nature of the disease and highlighted the potential role of other organs (other than liver and pancreas) to fully characterize the underlying pathophysiology (see Supplementary Figure S1 online).9
Despite these advancements, prediction of disease progression may not be possible unless diagnostic procedures are complemented by prognostic measures. Current models assume diabetes as the central component of a progressive metabolic dysfunction, whereas in reality, it may be the consequence of a primary disorder.
Further development of model-based approaches faces a conceptual challenge in that it requires changes in the design of protocols and data collection to ensure causal inferences about the relationship between hyperglycemia and cardiometabolic phenotypes. Once such data become available, we anticipate that multiscale models will enable integration of the various dimensions of disease, from the mechanism of action and the underlying pathophysiological processes all the way to the overall outcome of interest (see Supplementary Figure S2 online).10 A first step should be the inclusion of lipids as primary clinical endpoints, which together may provide the basis for parameterizations that capture second- and higher-order processes, describing the impact of interacting factors (e.g., obesity) in a continuous manner.
Conclusion and Perspectives
The association of antidiabetic agents with an increased risk of cardiovascular diseases [S28] has become critical for the development of new molecules in the treatment of diabetes. As a result, there is an emerging need to characterize the disease beyond the time scales used in conventional protocols. The use of empirical protocols to assess cardiovascular risk in patients clearly entails large complexity and adds long safety trials that may last for several years, without necessarily providing further insight into the processes that determine disease progression.
Undoubtedly, parameterization of the traits determining cardiometabolic phenotypes will be essential to ensure a mechanistic description of the underlying biological processes (see Supplementary Figures S3 and S4 online). Lessons can be learned from other therapeutic areas [S29,S30] where pharmacometrics and systems pharmacology are commonly used to describe disease progression and discriminate drug from system-specific effects. Concepts integrating the time course of disease with drug action could be extended to diabetes to unravel the link between the timing of an intervention and its impact on the disease's progression [S30]. Such models may also facilitate the characterization of drug combinations, providing a stronger rationale for dose selection for different phenotypes or subgroups in the population. Given the well-known relationship between lipids and cardiovascular risk, it is appealing to consider the interplay between lipids and hemoglobin glycation as the starting point for further modeling efforts. The adoption of lipids as biomarkers may also explain why certain long-term studies have failed to demonstrate increases in cardiovascular risk.
Conflict of Interest
Both authors are employed by GlaxoSmithKline, Uxbridge, Middlesex, UK. The views and opinions presented in this manuscript do not reflect the company position. The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the contribution of Malcolm Young, Jeff Wald, and Derek Nunez for their valuable insight into the issues discussed in this manuscript.
Supplementary Material
References
- Taylor J.Y., Kraja A.T., de las Fuentes L., Stanfill A.G., Clark A., Cashion A. An overview of the genomics of metabolic syndrome. J. Nurs. Scholarsh. 2013;45:52–59. doi: 10.1111/j.1547-5069.2012.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Engelsen C., Gorter K.J., Salome P.L., Rutten G.E. Development of metabolic syndrome components in adults with a healthy obese phenotype. A three year follow-up. Obesity (Silver Spring) 2013;21:1025–1030. doi: 10.1002/oby.20049. [DOI] [PubMed] [Google Scholar]
- Pradhan A.D., Rifai N., Buring J.E., Ridker P.M. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am. J. Med. 2007;120:720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de las Fuentes L., de Simone G., Arnett D.K., Dávila-Román V.G. Molecular determinants of the cardiometabolic phenotype. Endocr. Metab. Immune Disord. Drug Targets. 2010;10:109–123. doi: 10.2174/187153010791213119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm L.H., et al. LIFE Study Group Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- Booth G.L., Kapral M.K., Fung K., Tu J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- Tseng C.H. Pioglitazone and bladder cancer in human studies: is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities. J. Formos. Med. Assoc. 2012;111:123–131. doi: 10.1016/j.jfma.2011.10.003. [DOI] [PubMed] [Google Scholar]
- King G., Imai K., Stuart E.A. Misunderstandings between experimentalists and observationalists about causal inference. J. R. Statist. Soc. A. 2008;171:481–502. [Google Scholar]
- Ajmera I., Swat M., Laibe C., Le Novère N., Chelliah V. The impact of mathematical modelling on the understanding of diabetes and related complications. CPT Pharmacometrics Syst. Pharmacol. 2013;2:e54. doi: 10.1038/psp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H.P., Hill R.G.The nature of disease and the purpose of therapy. Drug Discovery and Development: Technology in Transition p. 25eds. Hill R.G.Elsevier Health Sciences, London; 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


