Manipulating energy expenditure is one frequently employed strategy to combat obesity, but fundamental components of the cross-talk between key organs burning calories and the central nervous system (CNS) control of systemic energy metabolism are still missing.
Adipose tissue secretes leptin in proportion to the amount of body fat, potentially as a lipostatic signal, to regulate food intake and body weight via action in the central nervous system (CNS), thereby maintaining energy homeostasis. Leptin function is predominantly mediated by leptin receptor (LepR) expressing cells in the brain. In contrast to well-studied leptin sensing signaling pathways in discrete brain regions, the mechanistic underpinnings of how efferent signals from the CNS exactly mediate leptin action to regulate peripheral metabolism is less clear.
Although ablation of the leptin or leptin receptor (LepR) gene has profound phenotypic effects, specific deletion of LepR signaling in AgRP or POMC neurons (which are the primary leptin target neurons in the hypothalamus) does not mimic the obese phenotype(s) to the same extent [1,2], demonstrating the complexity of the system. Therefore, continued dissection of the complex communication and circuit wiring of hypothalamic leptin target neurons appears to be necessary to establish translationally meaningful understanding of the neuronal network that mediates leptin's pleiotropic actions on metabolic homeostasis. Vong et al. [3] previously showed that LepR neurons can be broadly distinguished in glutamatergic and GABAergic, and that a large effect of leptin action may be mediated via GABA. In this issue of Molecular Metabolism, Xu et al. [4] now expand our knowledge on key aspects of leptin downstream in the CNS by showing that a specific subset of metabolic in vivo responses to leptin are mediated by glutamatergic signaling from LepR expressing neurons.
Xu and colleagues generated and studied mice with a specific deletion of Vglut-2, the crucial transporter for glutamate, in LepR positive neurons (CKO mice). These CKO mice turned out to be moderately obese, which appeared to result from a reduction in energy expenditure (EE). Reduced capacity for non-shivering thermogenesis was found in brown adipose tissue of these mice, possibly caused by low sympathetic tone. Defective control of thermogenesis in CKO mice was most pronounced in the dark phase and in response to food shortage.
The authors discuss that the recurrent and episodic dips in body temperature, metabolic rate, and activity observed in CKO mice resemble a “torpor-like” state. Torpor, found among most mammalian groups including primates, represents an extreme form of controlled metabolic depression to survive harsh environmental conditions. Dissecting the neuronal and humoral factors involved in mammalian torpor is an ongoing challenge, and leptin is thought to play a key role in this process. Specifically, low leptin levels seem to be permissive for torpor entry. In mice, full expression of torpor involves a controlled reduction of metabolic rate by 30–50% (relative to basal metabolic rate) over a period of 3–10 h. As a consequence, body temperature drops well below 30 °C. Although these phenotypic characteristics are not met by the CKO mice the results by Xu and colleagues raise the exciting perspective that glutamatergic output from LepR neurons is part of a neuronal circuit that may ultimately shed light on the path that leads to fasting induced torpor.
Interestingly, the study of Xu et al. also showed that the CKO mice, in comparison to their wild-type controls, displayed altered metabolic patterns during the dark period, expressed as fluctuating amplitude of body temperature toward the end of the light- and throughout the entire dark period. Periodic fluctuations in body temperature and energy expenditure usually follow a day–night rhythm, which is generated by a so called “master clock”, which is believed to reside in the suprachiasmatic nuclei (SCN) of the hypothalamus [5]. The exact CNS pathway by which the SCN co-modulates energy expenditure is still unclear. While GABAergic neurons are abundant in the SCN, significantly fewer glutamatergic neurons are present. Thus, in the light of the biological clock controlling sympathetic and parasympathetic pre-autonomic neuronal activity in the paraventricular nuclei (PVN), GABAergic transmission represents a major part of the mechanism which gates the timing information from SCN to the PVN. Glutamatergic signaling appears to play a comparably minor role in this process [6]. However, it remains a possibility that a combined GABAergic–glutamatergic regulatory circuit also plays a significant role in fine-tuning the mediation of leptin signals to control EE. Among these circuits, the “minor” glutamatergic outputs would play an essential role in maintaining a biorhythmic pattern of EE. One also has to consider that circulating leptin displays a day-night rhythm with a peak around the beginning of the dark period, as shown in nocturnal rodents [7]. In that regard, the current study breaks new ground by exploring whether rhythmic leptin inputs into specific subsets of lepR cells adjust glutamate release to specifically control EE during resting and active period shifts, integrating responses to food deprivation and other physiological challenge (Fig. 1).
Figure 1.
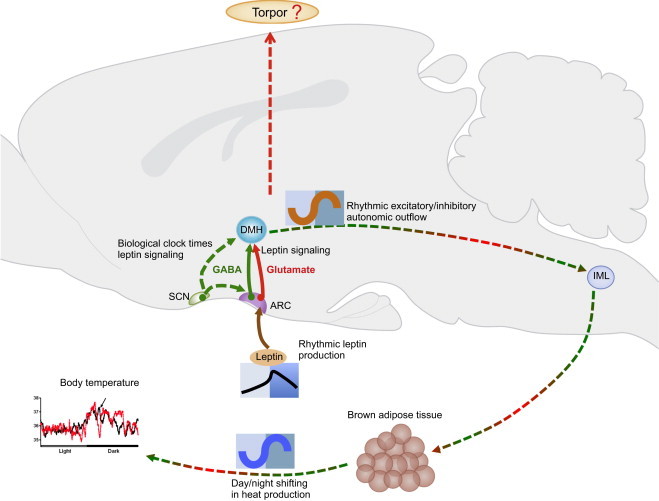
A schematic of the physiological and behavioral responses mediated via inhibitory or excitatory output from leptin receptor expressing cells in the central nervous system. While GABA released from leptin receptor expressing cells in the hypothalamus governs leptin's stimulatory effect on thermogenesis, glutamatergic signalling may tune the GABA signal, thus generating a rhythmic fluctuation in heat production. Such glutamatergic tuning could be directly regulated by rhythmic leptin feedback from the circulation, or indirectly timed by the master clock located in the suprachiasmatic nuclei via its connection to the arcuate nuclei or dorsomedial nuclei in the hypothalamus. Modulation of glutamatergic output from leptin receptor expressing cells may also be involved in gating entry into fasting-induced torpor, the most extreme form of transient metabolic suppression in mammals.
Based on their findings, the authors suggest that leptin regulation of EE by glutamatergic leptin receptor expressing neurons may act on downstream circuits controlled by the SCN, for example the dorsomedial hypothalamic nuclei (DMH). This is supported by previous studies revealing the sympathetic outflow from DMH to control brown adipose tissue thermogenesis [8,9]. The next missing link to address is how such signaling affects EE and thermogenesis and whether these signals are integrated in a specific circadian paradigm. One hypothesis worth testing would be that the DMH synchronizes timing information from the SCN and in response to leptin signals in order to accomplish appropriate regulation of thermogenesis. The studies by Xu et al. provide pioneering observations substantiating the link between leptin and control of energy metabolism and further uncover the complexities of the CNS neurotransmission involved.
Conflict of interest
None declared.
Footnotes
This Commentary refers to “Glutamate release mediates leptin action on energy expenditure, by Yuanzhong Xu et al.”, http://dx.doi.org/10.1016/j.molmet.2013.01.004.
References
- 1.Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.van deWall E., Leshan R., Xu A.W., Balthasar N., Coppari R., Liu S.M. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vong L., Ye C., Yang Z., Choi B., Chua S., Jr., Lowell B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Kim ER, Zhao R, Myers MG, Muenzberg-Gruening H, Tong Q.Glutamate release mediates leptin action on energy expenditure. Mol Metab, in this issue. [DOI] [PMC free article] [PubMed]
- 5.Moore R.Y., Eichler V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 6.Kalsbeek A., Foppen E., Schalij I., Van Heijningen C., van der Vliet J., Fliers E. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One. 2008;3:e3194. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalsbeek A., Fliers E., Romijn J.A., La Fleur S.E., Wortel J., Bakker O. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 8.Enriori P.J., Sinnayah P., Simonds S.E., Garcia Rudaz C., Cowley M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Kerman I.A., Laque A., Nguyen P., Faouzi M., Louis G.W. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


