Abstract
MicroRNAs (miRNAs) have recently emerged as key regulators of metabolism. However, their potential role in the central regulation of whole-body energy homeostasis is still unknown. In this study we show that the expression of Dicer, an essential endoribonuclease for miRNA maturation, is modulated by nutrient availability and excess in the hypothalamus. Conditional deletion of Dicer in POMC-expressing cells resulted in obesity, characterized by hyperphagia, increased adiposity, hyperleptinemia, defective glucose metabolism and alterations in the pituitary–adrenal axis. The development of the obese phenotype was paralleled by a POMC neuron degenerative process that started around 3 weeks of age. Hypothalamic transcriptomic analysis in presymptomatic POMCDicerKO mice revealed the downregulation of genes implicated in biological pathways associated with classical neurodegenerative disorders, such as MAPK signaling, ubiquitin–proteosome system, autophagy and ribosome biosynthesis. Collectively, our results highlight a key role for miRNAs in POMC neuron survival and the consequent development of neurodegenerative obesity.
Abbreviations: 3V, third ventricle; Acp2, acid phosphatase 2, lysosomal; ACTH, adrenocorticotropic hormone; Ago2, Argonaute 2; AgRP, agouti-related protein; AP, adenopituitary; ARC, arcuate nucleus of the hypothalamus; AUC, area under the curve; CART, cocaine and amphetamine-related transcript; CNS, central nervous system; CRH, corticotropin releasing hormone; Crhr1, corticotrophin releasing hormone receptor 1; Cx, Cortex; DIO, diet-induced obesity; Fa, Fascicular zone; Gapdh, Glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; Gh, growth hormone; Gl, Glomerular zone; Hprt, Hypoxanthine guanine phosphoribosyl transferase; IL, intermediate lobe; IP, intraperitoneal; LH, lateral hypothalamus; MC3R, melanocortin receptor 3; MC4R, melanocortin receptor 4; miRISC, miRNA-induced silencing complexes; miRNA, microRNA; Me, Medula; MZ, Marginal Zone; Myc, myelocytomatosis oncogene; Naglu, alpha-N-acetylglucosaminidase; Nhlrc1, NHL repeat containing 1; NP, neurohypophysis; NPY, neuropeptide Y; NS, not significant; Ntrk2, Neurotrophic tyrosine kinase, receptor, type 2; Park2, Parkin; Pit1, pituitary-specific transcription factor 1; POMC, pro-opiomelanocortin; POMCDicerKO, mice lacking Dicer in POMC-expressing cells; PVN, paraventricular nucleus; qPCR, quantitative real-time PCR; Re, Reticular zone; Rps9, ribosomal protein S9; Rps24, ribosomal protein S24; Tpit, T box transcription factor; Tshβ, thyroid-stimulating hormone β chain; UD, undetectable; UPS, ubiquitin proteosome system; UTR, untranslated region; VMH, ventromedial hypothalamus; YFP, yellow fluorescent protein.
Keywords: microRNA, Dicer, Hypothalamus, POMC, Obesity, Neurodegeneration
1. Introduction
MicroRNAs (miRNAs) are short, non-coding RNAs that have recently emerged as key posttranscriptional regulators. The biogenesis of the majority of miRNAs is achieved through a canonical process by which long primary transcripts undergo nuclear processing by the enzymes Drosha and DGCR8 to produce stem-loop precursors (pre-miRNAs) [1]. These pre-miRNAs are exported to the cytoplasm and further processed by a complex containing Dicer. The activity of Dicer is essential for producing the mature form of miRNAs that eventually enter the miRNA-induced silencing complex (miRISC) [1]. In general lines, miRNAs interact with the 3′ untranslated region (UTR) of specific target mRNAs in a sequence-specific manner causing repression of protein translation and/or degradation of mRNA [2].
Functional studies are beginning to reveal relevant roles for miRNAs in a number of biological processes and their involvement in pathological conditions such as heart diseases, cancer, metabolic conditions and neurological disorders [3,4]. In the central nervous system (CNS), the study of cell and regional-specific deletions of Dicer using the cre/lox system resulted in a wide range of anatomical and behavioral phenotypes, but overall revealed a fundamental role for miRNAs in neural cell proliferation, differentiation and survival [5–9]. Furthermore, different timings of Dicer deletion indicated the activation of time-dependent functions of miRNAs [10,11]. Collectively, these reports highlight the importance of miRNAs in neuronal development and the activation of different programs during embryonic or post-natal life.
The hypothalamus is a key CNS area implicated in the regulation of whole-body energy homeostasis [12]. The regulation of this biological process is achieved through a complex sensing of hormones and nutrient-related signals, followed by its integration and coordination of precise neurochemical and neurophysiological responses. These effector mechanisms are critically mediated by specific populations of neurons of the arcuate nucleus of the hypothalamus (ARC). In particular, neurons co-expressing orexigenic neuropeptides agouti-related protein (AgRP) and neuropeptide Y (NPY), along with neurons co-expressing anorexigenic pro-opiomelanocortin (POMC) precursor and cocaine and amphetamine-related transcript (CART), have been extensively implicated in the regulation of appetite, body weight, glucose and lipid metabolism [12–14]. Interestingly, recent studies suggest important functions for miRNAs upon metabolic regulation in peripheral tissues such as liver [15], adipose tissue [16] and pancreas [17], but have not investigated their role in hypothalamic neurons involved in energy homeostasis control.
Here we demonstrate that Dicer is expressed in relevant ARC neurons implicated in energy balance and that its expression is regulated by nutrient availability. Furthermore, our data also shows that ablation of Dicer-dependent miRNAs in POMC-expressing cells leads to pituitary dysplasia, adrenal hypofunction and neurodegenerative obesity.
2. Materials and methods
2.1. Animals and diets
C57Bl/6 and ob/ob mice were purchased from Harlan Europe. Indicator mice were generated by crossing POMC-cre mice [18,19] with Z/EG mice [20] and AgRP-cre mice [18,19] with ROSA26-YFP mice [21]. To generate mice lacking Dicer specifically in POMC neurons, POMC-cre mice were mated with mice carrying a loxP-flanked Dicer1 allele (Jackson Laboratory, Bar Harbor, USA). Mouse colonies were maintained by breeding male POMC+/− DicerloxP/loxP with female POMC−/− DicerloxP/loxP. Mice were maintained on a 12 h-light/12 h-dark cycle with free access to water and standard chow (2014; Teklad). Diet-induced obesity (DIO) was achieved by the administration of high-fat diet (D12451; 45% Kcal derived from fat, Research Diets, New Brunswick, USA) to male C57Bl/6 at 6 weeks of age for 12 consecutive weeks. All animal procedures were approved by the Animal Experimentation Ethics Committee of the University of Barcelona and performed according to European Community and local guidelines.
2.2. In vivo physiological studies
Body weights were measured weekly. Food intake was measured in singly-housed mice for 5 consecutive days. For fast-refeeding studies, mice were fasted for 16 h and refed with a pre-weight amount of chow. Food intake was measured at the indicated time points. Adiposity was measured as epididymal fat-pad weight in relation to total body weight and expressed as percentage. Blood trygliceride concentration was assessed using the Accutrend Plus GCT system (Roche Diagnostics, Spain). Blood glucose levels were determined using a glucometer (Arkray, Shiga, Japan). Glucose-tolerance tests were conducted in 16 h-fasted mice by intraperitoneal (IP) administration of glucose (2 g/Kg). Area under the curve was calculated above baseline glucose. Insulin sensitivity tests were performed in 4 h-food deprived mice by IP administration of insulin (0.375 UI/Kg). ELISA kits were used to determine plasma insulin and leptin (Crystalchem Inc., Downers Grove, USA), corticosterone (Immuno Diagnostic System, Germany) and catecholamines (Labor Diagnostika Nord, Germany).
2.3. Hypothalamic immunohistochemistry
Mice were transcardially perfused with 4% paraformaldehyde, over-night fixed, cryoprotected in 30% sucrose and frozen. Brains were cut into 25 μm-thick slices using a sliding microtome and immunohistochemistry performed as previously described [22,23]. Antibodies used were: anti-Dicer (1:100, Santa Cruz Biotechnology, Santa Cruz, USA), anti-POMC (1:1000, Phoenix Pharmaceuticals, Burlingame, USA) and anti-rabbit Alexa Fluor 488 or 595 (1:400, Molecular Probes, Grand Island, USA). Imaging was performed using a Leica DMI6000B microscope.
2.4. Neuron counts
Sections throughout the ARC from control and POMCDicerKO mice were collected in 3 series as described [22]. The distribution and number of POMC neurons were counted in one series in a blinded fashion. This result was multiplied by 3 to account for the 3 series.
2.5. Pituitary and adrenal gland immunohistochemistry
Pituitaries and adrenals from 4% paraformaldehyde-perfused mice were fixed overnight and maintained in 70% ethanol until paraffin inclusion. To prevent staining variations from slide to slide, 3 μm sections of 3 controls and 3 POMCDicerKO mice were mounted in parallel in the same slide and stained with Haematoxylin–Eosin [24]. For immunohistochemistry, antigens were recovered with microwave treatment (5 pulses×5 min/each) in TE buffer and Envision kit (DAKO, Glostrup, Denmark) for blocking. Primary antibodies were incubated for 1 h at 37 °C (anti-ACTH) or overnight at 4 °C (all other antibodies). Antibodies used were: monoclonal anti-ACTH (1:3000, DAKO) and rabbit (National Institute of Diabetes and Digestive and Kidney diseases) anti-GH (1:3000), anti-PRL (1:1500), anti-TSH (1:1000), anti-LH (1:10,000) and anti-FSH (1:4000).
2.6. RNA purification and gene expression analysis by quantitative real-time PCR (qPCR).
Hypothalami and pituitaries were harvested and immediately frozen in liquid nitrogen. mRNA was isolated using Trizol (Invitrogen, Carlsbad, USA). Retrotranscription was performed in 1 μg of RNA using the Taqman Retrotranscription kit (Applied Biosystems, Foster City, USA). QPCR was carried out using proprietary Taqman gene expression assays (Applied Biosystems) for Dicer (Mm00521715_m1), Drosha (Mm01310009_m1), Dgcr8 (Mm01146851_m1), Ago2 (Mm00838341_m1), Agrp (Mm00475829_g1), Cart (Mm00489086_m1), Pomc (Mm00435874_m1), Npy (Mm00445771_m1), Crh (Mm01293920_s1), Mc3r (Mm00434876_s1), Mc4r (Mm00457483_s1), Tpit (Mm00453377_m1), Pit1 (Mm00476852_m1), Crhr1 (Mm00432670_m1), Gh (Mm01258409_g1), Tshβ (Mm00437190_m1), Ntrk2 (Mm00435422_m1), Myc (Mm00487804_m1), Naglu (Mm00479175_m1), Acp2 (Mm00651503_m1), Nhlrc1 (Mm00614667_s1), Park2 (Mm00450186_m1), Rps24 (Mm01623058_s1) and Rps9 (Mm00850060_s1). Probes for Hprt (Mm00446968_m1) or Gapdh (Mm99999915_g1) were used to adjust for total RNA content. mRNA levels were measured using the ABI Prism 7900 HT system (Applied Biosystems).
2.7. Global transcriptomic analysis
Total RNA was extracted from hypothalamic microdissections of 2-week old control and POMCDicerKO mice using the miRNAeasy mini kit (Qiagen, Venlo, The Netherlands) and RNeasy micro spin columns (Qiagen). RNA was hybridized to mouse 430 2.0 genechips (Affymetrix, Santa Clara, USA). Background adjustment, normalization and data summarization were performed by evaluation of .cell files by Robust Multi-array Analysis (RMA) [25] using the Affy package [26] from bioconductor [27] on R language. Raw and processed data passed successfully several quality controls as described previously [28]. In order to increase the sensitivity of the analysis and reduce background noise, those genes that were called Absent (calculated with the MAS5 algorithm from the simpleAffy package [29] in at least two microarrays in both experimental groups) were removed. Differentially expressed genes were considered when presented a t-student P-value≤0.05. Those genes were represented in a heatmap using dChip software [30] and KEGG pathway analysis was performed with DAVID Bioinformatic tool [31,32], which provides typical gene-term analysis to identify relevant biological pathways associated with a particular gene list. Raw and processed data has been deposited in GEO database with the accession number GSE38672.
2.8. Statistics
Data are expressed as mean±SEM. P values were calculated using non-parametric Mann–Whitney test. P≤0.05 was considered significant.
3. Results
3.1. Dicer is expressed in hypothalamic POMC and AgRP neurons
To initially investigate a potential role for Dicer in the central regulation of energy balance, we assessed its anatomical distribution in the hypothalamus of control C57Bl/6 mice by immunohistochemistry. Dicer protein was broadly expressed in medial hypothalamic areas implicated in energy homeostasis control, including the paraventricular, lateral and ventromedial hypothalamus (Figure 1A–C). We next asked whether Dicer was expressed in POMC and AgRP neurons. To address this question, we generated mice expressing green fluorescent protein (GFP) or yellow fluorescent protein (YFP) specifically in POMC or AgRP neurons and performed Dicer colocalization studies. The specificity of Dicer antibody was previously reported [33] and omission of primary antibody in our experimental settings produced no signal (data not shown). Consistent with a ubiquitous expression of Dicer, we found colocalization in most POMC (93.6±0.2%, Figure 1D–F) and AgRP neurons (93.8±1.5%, Figure 1G–I). These results indicate that Dicer is expressed in key hypothalamic nuclei and neuron populations implicated in energy homeostasis control.
Figure 1.
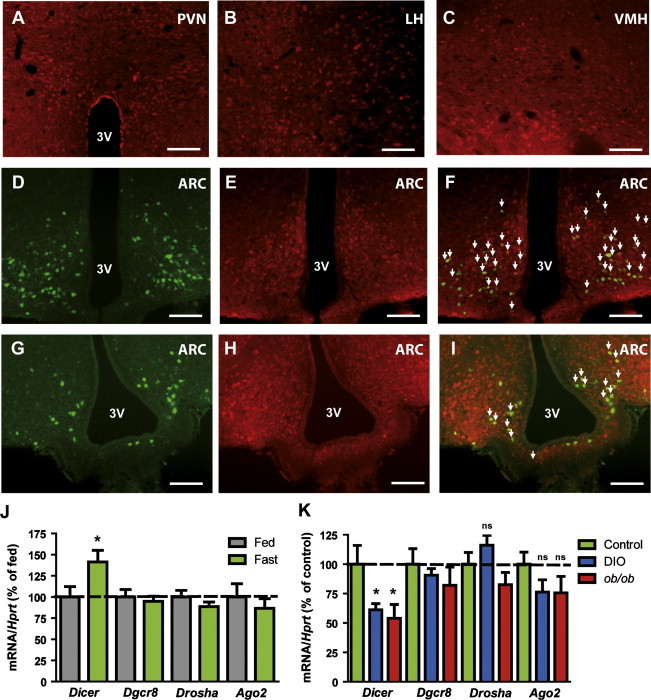
Dicer is expressed in the mediobasal hypothalamus and is modulated by nutrient availability. Representative immunofluorescence images of Dicer staining (red) in the paraventricular (A), lateral (B) and ventromedial (C) hypothalamus of male C57Bl/6 control mice. (D–F) Representative immunofluorescence images of the ARC from indicator POMC-Z/EG mice, showing POMC neurons (D, green), Dicer staining (E, red) and merged images (F). (G–I) Representative immunofluorescence images of the ARC from indicator AgRP-YFP mice, showing AgRP neurons (G, green), Dicer staining (H, red) and merged images (I). Arrows show colocalization. PVN: paraventricular nucleus. LH: lateral hypothalamus. VMH: ventromedial hypothalamus. ARC: arcuate nucleus of the hypothalamus. Scale bars: 100 μm. 3V: third ventricle. (J) Expression analysis of miRNA processing enzymes in the hypothalamus of 12-week old male C57Bl/6 control mice under fed and fasting (24h) conditions (n=11–12 mice/group) assessed by qPCR. (K) Expression analysis of miRNA processing enzymes in the hypothalamus of 18-week old male DIO C57Bl/6 control mice and ob/ob mice (n=5–7 mice/group) under fasting (24 h) conditions assessed by qPCR. Probe for Hprt was used to adjust for total RNA content. Data are expressed as mean±SEM. *P<0.05; ns: not significant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Dicer expression in the hypothalamus is regulated by nutrient availability
Next, we assessed whether hypothalamic Dicer expression was modulated by acute changes in nutrient availability in 12-week old male control C57Bl/6 mice. Fasting caused specific upregulation of Dicer mRNA in the hypothalamus, as expression of other miRNA biogenesis genes (Dgcr8, Drosha and Argonaute 2 (Ago2, a core component of the miRISC complex)) was unaltered (Figure 1J) and Dicer transcript was unchanged in other brain areas such as the hindbrain (Fed: 100±6%; Fasted: 112±14%; n=5–6 mice/group, NS).
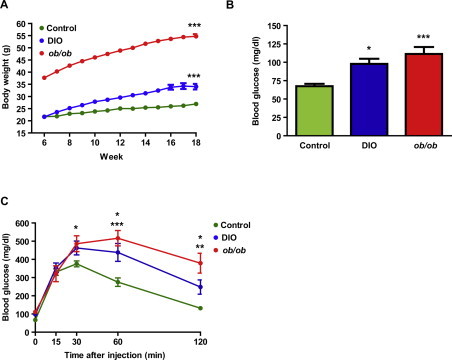
We also examined the effect of nutrient excess upon the hypothalamic expression of the same genes in diet-induced (DIO) and genetic (ob/ob) models of obesity. At the time of the study (18 weeks of age), both experimental models exhibited obesity, fasting hyperglycemia and glucose intolerance (Supplementary Figure 1). Dicer expression was reduced in hypothalami from both fasted DIO and ob/ob mice when compared to control mice under the same nutritional status (Figure 1K). No significant changes were seen in the expression of Dgcr8, Drosha and Ago2 (Figure 1K). Together, these results suggest that Dicer expression is modulated by nutrient availability and that might be a physiologically relevant enzyme implicated in the hypothalamic control of energy balance.
3.3. Dicer deficiency in POMC-expressing cells leads to energy homeostasis alterations
To further understand the role of hypothalamic miRNAs upon whole-body energy balance control, we crossed POMC-cre mice [18,19] with floxed Dicer mice [34] to generate animals lacking Dicer in POMC neurons (hereafter referred as POMCDicerKO). Dicer mRNA expression was significantly decreased in the hypothalamus from 12-week old (control: 100±6%; POMCDicerKO: 77±5%; n=6 mice/genotype, P<0.01 mutant mice, confirming appropriate tissue-specific reduction).
Postnatal POMCDicerKO mice appeared to be normal, showing equivalent body weights as compared with control littermates (Figure 2A). However, from 6 weeks of age onwards, mutant mice exhibited increased body weight. By 12 weeks of age, POMCDicerKO mice were ∼13% heavier than control counterparts (Figure 2A) and showed increased epididymal fat weight (Figure 2B) suggesting higher adiposity. Consistent with this phenotype, plasma leptin (Figure 2C) and blood triglycerides concentration (Figure 2D) were significantly increased in mutant mice. POMCDicerKO mice displayed hyperphagia (Figure 2E) and increased rebound food intake after a fast-refeeding paradigm (Figure 2F). Twelve-week old mutant mice also displayed hyperglycemia under fed and fasting conditions (Figure 3A), hyperinsulinemia (Figure 3B), glucose intolerance (Figure 3C; Area under the curve (AUC) control: 23,995±652 mg/dl/120 min; AUC POMCDicerKO: 28,352±725 mg/dl/120 min; n=6–8 mice/genotype, P<0.001) and insulin resistance (Figure 3D). Defective glucose homeostasis in POMCDicerKO mice was secondary to obesity development, since mutant mice at 6 weeks of age did not exhibit altered glucose metabolism parameters (Figure 3E–H). Female POMCDicerKO mice showed essentially the same phenotype as males, in terms of both energy balance and glucose metabolism (data not shown).
Figure 2.
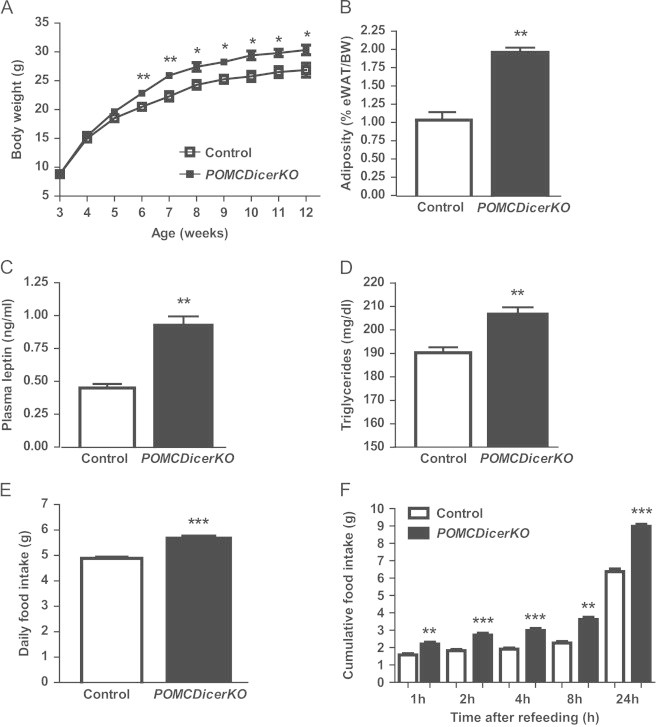
POMCDicerKO mice exhibit increased body weight.(A) Body weight profiles of male control and POMCDicerKO mice.(B) Epididymal fat weight relative to body weight, (C) plasma leptin, (D) blood triglycerides, (E) daily food intake and (F) fast-refeeding test in 12–14 week old male control and POMCDicerKO mice. eWAT: epididymal white adipose tissue. BW: body weight. Data are expressed as mean±SEM. N=6–8 mice/genotype. *P<0.05; **P<0.01; ***P<0.001.
Figure 3.
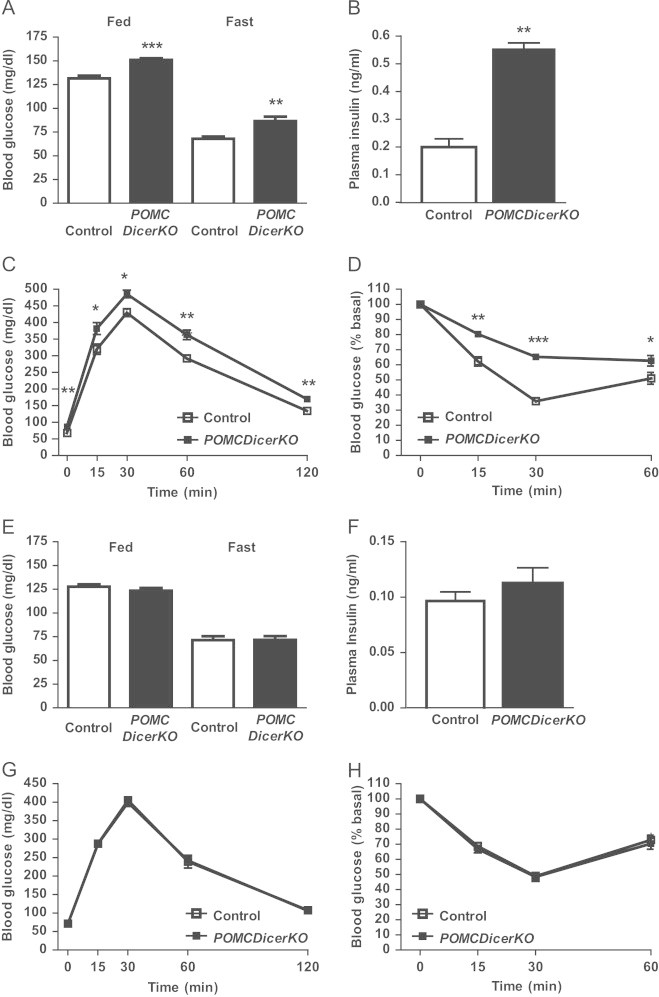
POMCDicerKO mice exhibit glucose metabolism alterations. (A) Blood glucose levels under fed and fasting conditions and (B) fasting plasma insulin in 12–14 week-old male control and POMCDicerKO mice. Glucose metabolism was assessed by (C) glucose tolerance and (D) insulin sensitivity tests in 12–14 week-old male control and POMCDicerKO mice. (E) Blood glucose levels under fed and fasting conditions and (F) fasting plasma insulin in 6 week-old male control and POMCDicerKO mice. Glucose metabolism was assessed by (G) glucose tolerance and (H) insulin sensitivity tests in 6 week-old male control and POMCDicerKO mice. Data are expressed as mean±SEM. N=6–8 mice/genotype. *P<0.05; **P<0.01; ***P<0.001.
3.4. POMCDicerKO mice exhibit alterations in pituitary-adrenal axis function
POMC promoter drives cre recombinase expression in the pituitary and the hypothalamus [18], and therefore we undertook studies to assess these tissues. Pituitary Dicer mRNA was reduced by ∼40% in 12-week old male mutant mice (control: 100±5%; POMCDicerKO: 59±5%; n=5 mice/genotype, P<0.001). Immunohistochemical studies revealed lack of pituitary intermediate lobe (Figure 4A) and a dramatic reduction of adrenocorticotropic hormone (ACTH) positive cells in the adenopituitary of POMCDicerKO mice (control: 1078±74 cells; POMCDicerKO: 46±7 cells; n=3 mice/genotype, P<0.01 and Figure 4B). Interestingly, mutant mice showed small clusters of ACTH-positive cells in the marginal zone (Figure 4B, insets), suggesting de novo regeneration from progenitor cells [35]. Staining for growth hormone (GH), thyroid-stimulating hormone (TSH), prolactin (PRL), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) was unaltered (Figure 4C–G). Consistently, expression of melanotroph and corticotroph specific genes such as Pomc, T box transcription factor (Tpit ) and corticotrophin releasing hormone receptor 1 (Crhr1) were significantly downregulated (Figure 4H), while essential genes for the generation of other pituitary lineages such as pituitary-specific transcription factor 1 (Pit-1), growth hormone (Gh) and thyroid-stimulating hormone beta chain (Tshβ) were unchanged in POMCDicerKO mice (Figure 4H). Adrenal gland morphology was also abnormal in POMCDicerKO mice, exhibiting reduced size and cortex hypoplasia affecting the fascicular and reticular zones (Figure 5A and B). Basal plasma corticosterone levels were reduced by ∼80% and the response to stress was blunted (Figure 5C). However, adrenal medullas were normal with strong positivity for tyrosine hydroxylase (data not shown). Plasma epinephrine was slightly reduced while norepinephrine and dopamine unaltered (Figure 5D). Collectively, these results indicate that deletion of Dicer in pituitary POMC-expressing cells lead to dysfunction of the pituitary-adrenal axis and secondary hypoadrenalism.
Figure 4.
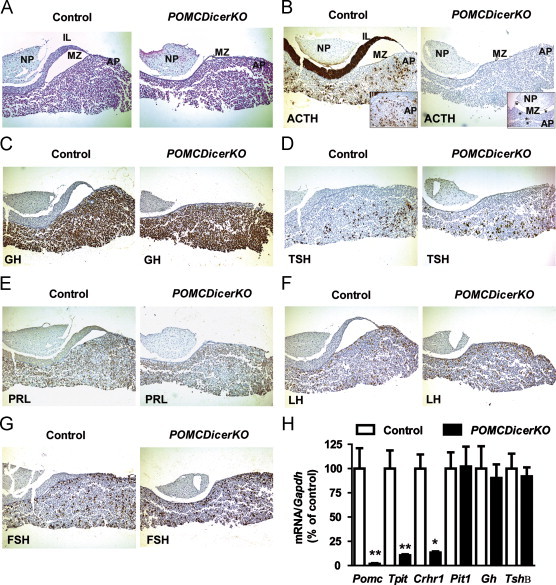
POMCDicerKO mice exhibit pituitary dysplasia. (A) General pituitary morphology assessed by Haematoxylin-Eosin staining. Hormone-specific pituitary immunohistochemistry was performed for (B) ACTH, (C) GH, (D) TSH (E), PRL (F) LH and (G) FSH. Representative images from 12-week old male control and POMCDicerKO mice are shown (n=3 mice/genotype). IL: intermediate lobe. NP: neurohypophysis. MZ: Marginal Zone. AP: adenopituitary. All images were taken at 60x except inserts at 120x. (H) Pituitary gene expression profile in 12-week old male control and POMCDicerKO mice (n=5 mice/genotype) assessed by qPCR. Probe for Gapdh was used to adjust for total RNA content. Data are expressed as mean±SEM. *P<0.05; **P<0.01.
Figure 5.
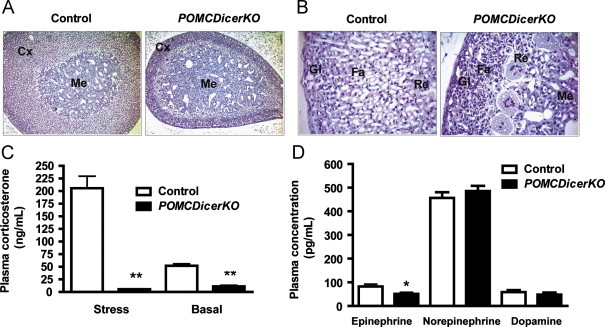
POMCDicerKO mice exhibit adrenal hypofunction. (A, B) Adrenal gland Haematoxylin–Eosin staining. Representative images from 12-week old male control and POMCDicerKO mice are shown (n=3 mice/genotype). Me: Medula. Cx: Cortex. Gl: Glomerular zone. Fa: Fascicular zone. Re: Reticular zone. (C) Plasma corticosterone, (D) epinephrine, norepinephine and dopamine in 12-week old male control and POMCDicerKO mice. Data are expressed as mean±SEM. N=6–7 mice/genotype. *P<0.05; **P<0.01.
3.5. POMCDicerKO mice exhibit POMC neuron degeneration
To further investigate the mechanisms underlying the obese phenotype, we next analyzed the expression of neuropeptides and relevant melanocortin pathway genes in the hypothalamus of 12-week old male control and POMCDicerKO mice. Pomc and Cart mRNA levels were undetectable (Figure 6A), while AgRP and Npy transcripts were reduced in mutant mice (Figure 6A). The expression levels of corticotropin-releasing hormone (Crh), melanocortin receptor 3 (Mc3r) and 4 (Mc4r) were also reduced (Figure 6B).
Figure 6.
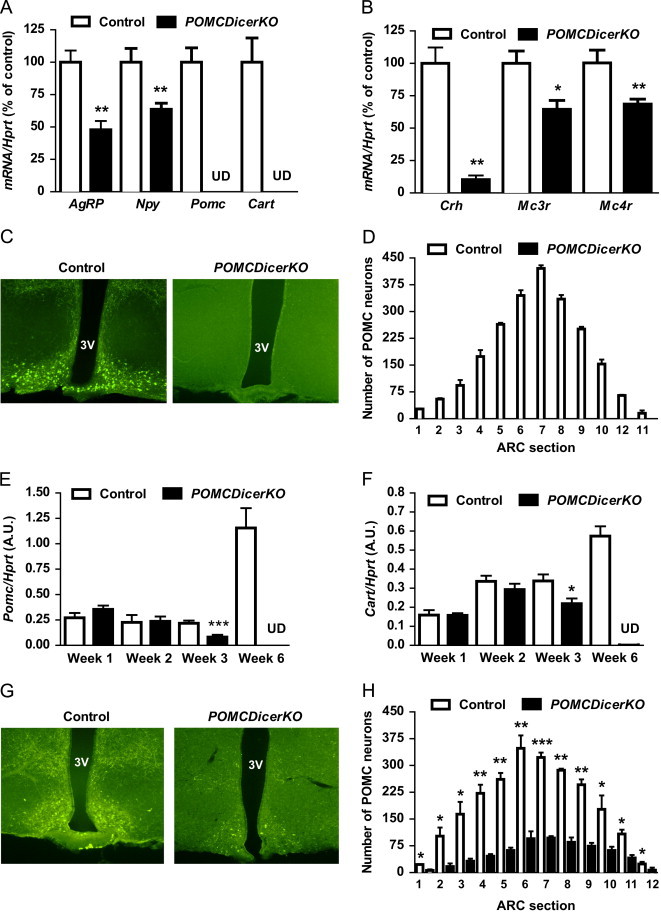
Dicer deletion in POMC neurons leads to neurodegeneration. (A) Hypothalamic neuropeptides and (B) melanocortin pathway genes in 12-week old male control and POMCDicerKO mice assessed by qPCR (n=6–8 mice/genotype). (C) POMC immunofluorescence and (D) neuron counts throughout the ARC of 12-week old male control and POMCDicerKO mice (n=3 mice/genotype). (E) Temporal pattern of Pomc and (F) Cart mRNA expression in the hypothalamus from control and POMCDicerKO mice during post-natal and early adulthood (n=5–9 mice/genotype). (G) POMC immunofluorescence and (H) neuron counts throughout the ARC of 3-week old male control and POMCDicerKO mice (n=3 mice/genotype). 3V: third ventricle. UD: undetectable. Mean±SEM. *P<0.05; **P<0.01; ***P<0.001.
Given that miRNAs are implicated in neuronal development, survival and function we hypothesized that undetectable Pomc and Cart expression in the hypothalamus from POMCDicerKO mice (Figure 6A) was the consequence of POMC neuron degeneration (both neuropeptides colocalize in ARC POMC neurons). To test this hypothesis, we performed a systematic immunohistochemical analysis throughout the ARC in 12-week old POMCDicerKO mice and failed to detect POMC neurons or POMC-positive fibers (Figure 6C and D). No gross neuroanatomical abnormalities were observed. To determine the temporal pattern of POMC neuron disappearance, we conducted gene expression studies during post-natal development and early adulthood. As described [36], hypothalamic Pomc mRNA in control mice was stable during the lactation period and increased between weaning and early adulthood (Figure 6E). In POMCDicerKO mice, Pomc expression was unaltered during the first two weeks of life but was significantly reduced at 3 weeks of age and undetectable at 6 weeks of age (Figure 6E). A similar pattern of expression was observed for Cart (Figure 6F). Consistent with this data, immunohistochemistry studies showed a significant ∼70% reduction in POMC neuron number in the ARC from 3-week old POMCDicerKO mice (Figure 6G and H). The density of POMC-positive fibers was also dramatically reduced (Figure 6G). Collectively, our results show evidence of altered POMC neuron function and features, associated with the development of a pathological condition suggestive of selective neurodegeneration.
In an attempt to reveal the molecular alterations leading to POMC neurodegeneration, we performed a global transcriptomic analysis in hypothalamic microdissections from presymptomatic 2-week old control and POMCDicerKO mice. We found 246 downregulated and 157 upregulated genes in hypothalami from POMCDicerKO mice when compared to controls (Figure 7A and Supplemental Tables 1 and 2). To establish potential dysregulated pathways, we conducted gene ontology and KEGG pathway analyses. Downregulated categories included MAPK signaling, lysosome, ubiquitin-mediated proteolysis and ribosome (Figure 7B). Representative genes for each biological pathway were validated by qPCR (Figure 7C). Expression of transcriptional markers for astrocytes (Gfap, Aldh1L1) and microglia (Cd34, Itgam, Aif, Emr1) were unaltered (data not shown) suggesting that, at least at this stage, gliosis or microglial infiltration were not associated with POMC neuron degeneration.
Figure 7.
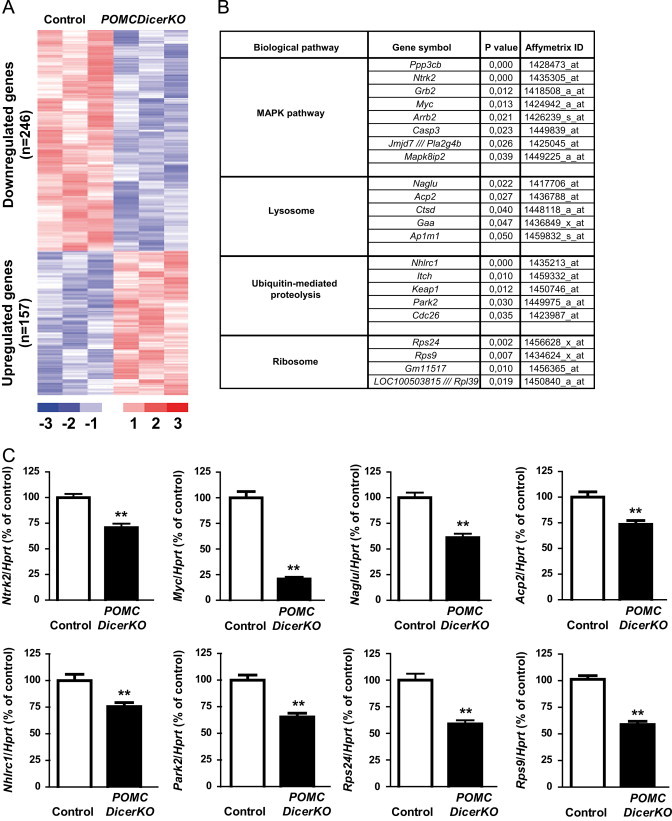
Hypothalamic global transcriptomic studies in POMCDicerKO mice. (A) Heatmap representation of relative gene expression changes in hypothalamic microdissections from 2-week old male control and POMCDicerKO mice (n=3 mice/genotype). (B) Representative downregulated genes by KEGG pathway analysis. (C) qPCR validation of representative genes found to be downregulated by microarray analysis. Data are expressed as mean±SEM. **P<0.01.
4. Discussion
A large body of evidence indicates that miRNAs play a major role in proliferation, differentiation and survival of a wide range of neuronal and non-neuronal cell-types of the CNS [5–9]. Consistent with these observations, deletion of Dicer in hypothalamic POMC neurons leads to neurodegeneration, a term which refers to loss of both neuronal function and structure. This process starts between 2 and 3 weeks of age and is completed around 6 weeks of age. This data indicate that Dicer-derived miRNAs are essential for survival and maintenance of POMC neurons during post-natal and early adulthood, but are not necessary to establish the POMC neuron lineage. This notion is in line with other conditional Dicer knock-out models that also suggest the absence of any relevant function for Dicer/miRNAs in early cell-fate decisions. However, it cannot be ruled out a potential role for Dicer-independent miRNAs in the establishment of the POMC neuron lineage.
POMC neuron loss is paralleled by a ∼40–50% reduction in Npy and AgRP transcript expression. This observation could be the consequence of altered NPY/AgRP neurocircuitry, but preliminary data suggest that the distribution of AgRP-positive fibers throughout the ARC is unaltered in POMCDicerKO mice (M.S. and M.C., unpublished results). This data suggest that decreased expression of these orexigenic neuropeptides may reflect a compensatory mechanism to counteract the orexigenic tone induced by POMC neuron loss, rather than alterations in the establishment of the AgRP neuronal network.
Our global transcriptomic analysis showed that the altered early molecular neurodegenerative events found in POMCDicerKO mice were linked to defective MAPK signaling, lysosome, ubiquitin proteosome system (UPS) and ribosome function. Interestingly, these pathways have been associated with classical neurodegenerative disorders. For example, UPS and autophagy dysfunction have been widely implicated in the accumulation of damaged organelles and protein aggregates in Parkinson's and Alzheimer diseases [37,38]. Parkin expression, a key component of the E3 ubiquitin ligase complex and the most common genetic alteration in autosomal recessive Parkinson's disease [39,40], was reduced in POMCDicerKO mice. Furthermore, recent evidences indicate that ribosome biogenesis and delivery to specific areas is essential for neuronal growth, maintenance and synaptic plasticity and, therefore, failure of ribosome production may underlie neurodegeneration. In fact, nucleolus dysfunction has been reported in a number of neurodegenerative disorders [41]. The role of the ERK/MAPK pathway in neurons is more controversial, but commonly associated with neuronal survival, proliferation, differentiation, synaptic plasticity [42] as well as neuroprotective processes [43,44]. Collectively, these results suggest that the POMC degeneration observed in POMCDicerKO mice shares striking similarities with common neurodegenerative disorders.
Several studies have recently linked hypothalamic neurodegeneration with obesity [45,46], and it has been recently reported that high-fat diet administration leads to reduced POMC neuron number in the ARC [47,48]. Furthermore, either acute [49] or progressive [19] POMC neuron loss results in body weight gain. Consistent with these observations, POMC neurodegeneration in POMCDicerKO mice resulted in increased orexigenic tone and obesity, characterized by hyperphagia, increased adiposity and secondary alterations in glucose metabolism. Although the magnitude and onset of the phenotypes exhibited by POMC ablated mice are different, acute POMC ablation, either in the adult [49] or during development (present manuscript), lead to a similar phenotype suggesting lack of neuronal plasticity and developmental compensations [50].
In relation to pituitary function, our gene expression analysis showed reduced expression of specific melanotroph and corticotroph genes (Pomc, Tpit and Crhr1) and lack of ACTH staining in POMCDicerKO mice. These results indicate survival defects of pituitary melanotrophs and corticotrophs, which is consistent with the lack of the intermedial lobe and the deletion of Dicer specifically in these cell types. The abnormal functionality of the pituitary–adrenal axis was further confirmed by secondary alterations in adrenal gland morphology and reduced plasma concentrations of corticosterone and epinephrine in POMCDicerKO mice. Most forms of human and rodent obesity are associated with high glucocorticoid levels, while corticosterone deficiency induces weight loss and hypophagia [51]. Therefore, it is very unlikely that hypocortisolism contributes to the obese phenotype observed in mice lacking Dicer in POMC-expressing cells. In fact, restoration of circulating corticosterone in POMC knock-out [52] or POMC-neuron ablated mice [19] leads to a more pronounced hyperphagia and obese phenotype. Although the defects in the neuroendocrine axis are likely to be a direct consequence of Dicer deletion in the pituitary, we cannot exclude that part of these alterations are due to hypothalamic defects since Crh expression, a neuropeptide implicated in the pituitary synthesis of ACTH, was decreased in POMCDicerKO mice.
Recent reports have demonstrated that miRNAs are critical regulators of glucose and lipid metabolism and therefore have been suggested to play a role in the development of metabolic conditions such as obesity, diabetes and metabolic syndrome [4]. Our results show that Dicer is expressed in critical hypothalamic areas and neurons implicated in energy balance control, and that its expression in the hypothalamus is modulated by nutrient availability, pathophysiological conditions of nutrient excess and genetic obesity. Interestingly, Dicer gene expression has been also reported to be reduced in liver and muscle biopsies from insulin-resistant obese patients [53]. Thus, our results suggest a potential role for Dicer in the hypothalamic regulation of energy homeostasis in adult mice. Unfortunately, due to early POMC neuron degeneration, this hypothesis could not be addressed using our POMCDicerKO model and should be formally investigated using alternative approaches such as viral-mediated knock-down strategies or inducible cre/lox system.
In conclusion, this study demonstrates that deletion of Dicer in POMC neurons leads to neurodegeneration and early onset of obesity and associated metabolic alterations. These data highlight the importance of miRNAs in the survival of hypothalamic POMC neurons and their involvement in the maintenance of key neuronal circuits implicated in energy balance regulation during development. POMCDicerKO mice could represent a useful model for the study of early-onset neurodegenerative obesity.
Conflict of interest
None declared.
Acknowledgments
We are grateful to Dr Gregory S Barsh (Standford University) for providing Pomc-cre and Agrp-cre mouse lines and Dr Franck Costantini (Columbia University) for providing YFP indicator mice. The authors also thank Mercè Martín for assistance with the confocal microscope, Ana Senra for assistance with immunohistochemistry and members from the Diabetes and Obesity Laboratory for helpful discussions and advice. This work has been supported by Grants from the Spanish Ministry of Economy and Competitiveness PI10/01074 (MC) and BFU2010-16652 (CVA) and Xunta de Galicia 09CSA011208PR. Marc Schneeberger is a recipient of an undergraduate grant from the University of Barcelona. Marc Claret is a recipient of a Miguel Servet contract (CP09/00233) from the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness. Montserrat García-Lavandeira is a recipient of an Isabel Barreto grant (Xunta de Galicia). These grants are co-financed by the European Regional Development Fund “A way to build Europe”. This work was carried out in part at the Esther Koplowitz Centre, Barcelona.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at 10.1016/j.molmet.2012.10.001.
Appendix A. Supporting information
Figure S1.
References
- 1.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews Genetics. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 2.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annual Review of Biochemistry. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Non-coding RNAs in human disease. Nature Reviews Genetics. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 4.Rottiers V., Naar A.M. MicroRNAs in metabolism and metabolic disorders. Nature Reviews Molecular Cell Biology. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J., Inoue K., Ishii J., Vanti W.B., Voronov S.V., Murchison E. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer A., O'Carroll D., Tan C.L., Hillman D., Sugimori M., Llinas R. Cerebellar neurodegeneration in the absence of microRNAs. Journal of Experimental Medicine. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin D., Shin J.Y., McManus M.T., Ptacek L.J., Fu Y.H. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Annals of Neurology. 2009;66:843–857. doi: 10.1002/ana.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao J., Wu H., Lin Q., Wei W., Lu X.H., Cantle J.P. Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration. Journal of Neuroscience. 2011;31:8306–8319. doi: 10.1523/JNEUROSCI.0567-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis T.H., Cuellar T.L., Koch S.M., Barker A.J., Harfe B.D., McManus M.T. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. Journal of Neuroscience. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Bian S., Hong J., Kawase-Koga Y., Zhu E., Zheng Y. Timing specific requirement of microRNA function is essential for embryonic and postnatal hippocampal development. PloS One. 2011;6:e26000. doi: 10.1371/journal.pone.0026000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konopka W., Kiryk A., Novak M., Herwerth M., Parkitna J.R., Wawrzyniak M. microRNA loss enhances learning and memory in mice. Journal of Neuroscience. 2010;30:14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 13.Sisley S., Sandoval D. Hypothalamic control of energy and glucose metabolism. Reviews in Endocrine & Metabolic Disorders. 2011;12:219–233. doi: 10.1007/s11154-011-9189-x. [DOI] [PubMed] [Google Scholar]
- 14.Dieguez C., Vazquez M.J., Romero A., Lopez M., Nogueiras R. Hypothalamic control of lipid metabolism: focus on leptin, ghrelin and melanocortins. Neuroendocrinology. 2011;94:1–11. doi: 10.1159/000328122. [DOI] [PubMed] [Google Scholar]
- 15.Moore K.J., Rayner K.J., Suarez Y., Fernandez-Hernando C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annual Review of Nutrition. 2011;31:49–63. doi: 10.1146/annurev-nutr-081810-160756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor R.A., Choi M.S. microRNAs in the regulation of adipogenesis and obesity. Current Molecular Medicine. 2011;11:304–316. doi: 10.2174/156652411795677990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Valverde S.L., Taft R.J., Mattick J.S. MicroRNAs in beta-cell biology, insulin resistance, diabetes and its complications. Diabetes. 2011;60:1825–1831. doi: 10.2337/db11-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu A.W., Kaelin C.B., Takeda K., Akira S., Schwartz M.W., Barsh G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. Journal of Clinical Investigation. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu A.W., Kaelin C.B., Morton G.J., Ogimoto K., Stanhope K., Graham J. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biology. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak A., Guo C., Yang W., Nagy A., Lobe C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 21.Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claret M., Smith M.A., Batterham R.L., Selman C., Choudhury A.I., Fryer L.G. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. Journal of Clinical Investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claret M., Smith M.A., Knauf C., Al-Qassab H., Woods A., Heslegrave A. Deletion of Lkb1 in pro-opiomelanocortin neurons impairs peripheral glucose homeostasis in mice. Diabetes. 2011;60:735–745. doi: 10.2337/db10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canibano C., Rodriguez N.L., Saez C., Tovar S., Garcia-Lavandeira M., Borrello M.G. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO Journal. 2007;26:2015–2028. doi: 10.1038/sj.emboj.7601636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Gautier L., Cope L., Bolstad B.M., Irizarry R.A. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 27.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altirriba J., Barbera A., Del Zotto H., Nadal B., Piquer S., Sanchez-Pla A. Molecular mechanisms of tungstate-induced pancreatic plasticity: a transcriptomics approach. BMC Genomics. 2009;10:406. doi: 10.1186/1471-2164-10-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson C.L., Miller C.J. Simpleaffy: a BioConductor package for affymetrix quality control and data analysis. Bioinformatics. 2005;21:3683–3685. doi: 10.1093/bioinformatics/bti605. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Wong W.H. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proceedings of the National Academy of Sciences of U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 32.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynn F.C., Skewes-Cox P., Kosaka Y., McManus M.T., Harfe B.D., German M.S. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes. 2007;56:2938–2945. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- 34.Harfe B.D., McManus M.T., Mansfield J.H., Hornstein E., Tabin C.J. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proceedings of the National Academy of Sciences of the U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Lavandeira M., Quereda V., Flores I., Saez C., Diaz-Rodriguez E., Japon M.A. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PloS One. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remmers F., Delemarre-van de Waal H.A. Developmental programming of energy balance and its hypothalamic regulation. Endocrine Reviews. 2011;32:272–311. doi: 10.1210/er.2009-0028. [DOI] [PubMed] [Google Scholar]
- 37.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W. Regulation of mammalian autophagy in physiology and pathophysiology. Physiological Reviews. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 38.Marino G., Madeo F., Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Current Opinion in Cell Biology. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 40.Lucking C.B., Durr A., Bonifati V., Vaughan J., De Michele G., Gasser T. Association between early-onset Parkinson's disease and mutations in the parkin gene. New England Journal of Medicine. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 41.Hetman M., Pietrzak M. Emerging roles of the neuronal nucleolus. Trends in Neurosciences. 2012;35:305–314. doi: 10.1016/j.tins.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweatt J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Current Opinion in Neurobiology. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Hetman M., Kanning K., Cavanaugh J.E., Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Journal of Biological Chemistry. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 44.Maher P., Dargusch R., Bodai L., Gerard P.E., Purcell J.M., Marsh J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Human Molecular Genetics. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu K.Y., Garza J.C., Lu X.Y., Barsh G.S., Kopito R.R. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proceedings of the National Academy of Sciences of U S A. 2008;105:4016–4021. doi: 10.1073/pnas.0800096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Susaki E., Kaneko-Oshikawa C., Miyata K., Tabata M., Yamada T., Oike Y. Increased E4 activity in mice leads to ubiquitin-containing aggregates and degeneration of hypothalamic neurons resulting in obesity. Journal of Biological Chemistry. 2010;285:15538–15547. doi: 10.1074/jbc.M110.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moraes J.C., Coope A., Morari J., Cintra D.E., Roman E.A., Pauli J.R. High-fat diet induces apoptosis of hypothalamic neurons. PloS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 50.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 51.la Fleur S.E. The effects of glucocorticoids on feeding behavior in rats. Physiological Behavior. 2006;89:110–114. doi: 10.1016/j.physbeh.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 52.Smart J.L., Tolle V., Low M.J. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. Journal of Clinical Investigation. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pihlajamaki J., Lerin C., Itkonen P., Boes T., Floss T., Schroeder J. Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell Metabolism. 2011;14:208–218. doi: 10.1016/j.cmet.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


