Abstract
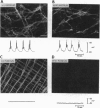
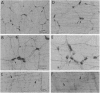
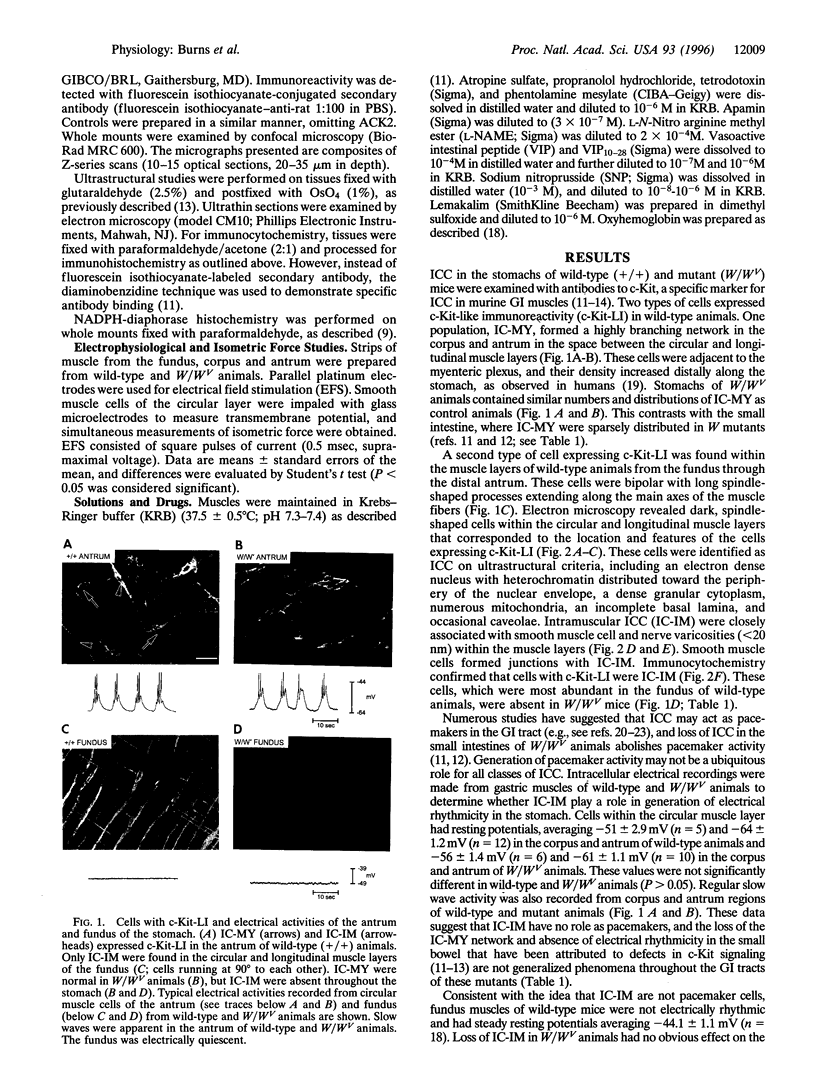
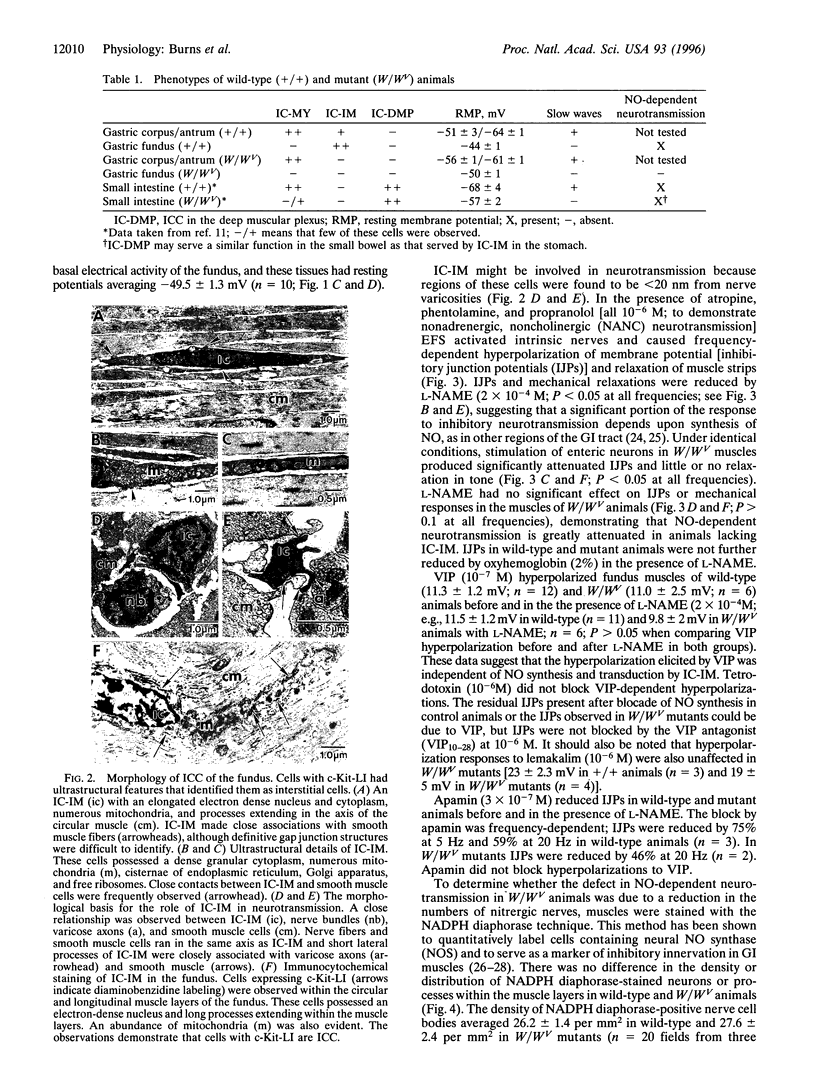
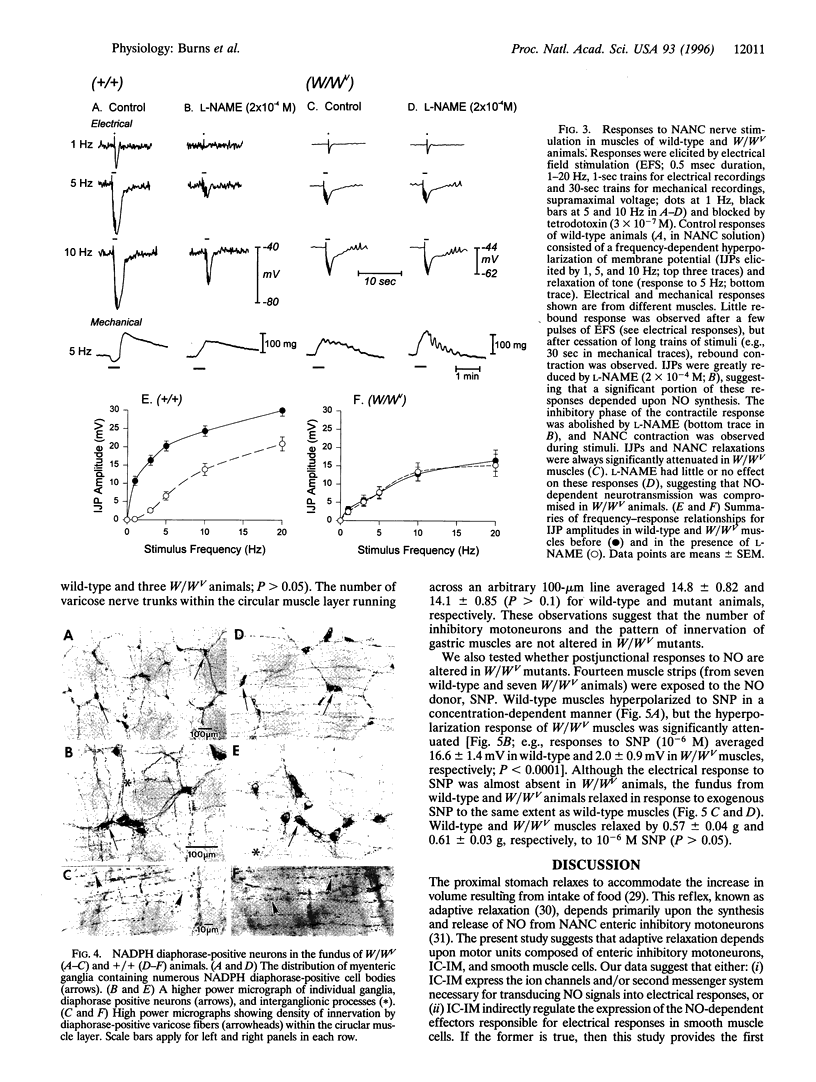
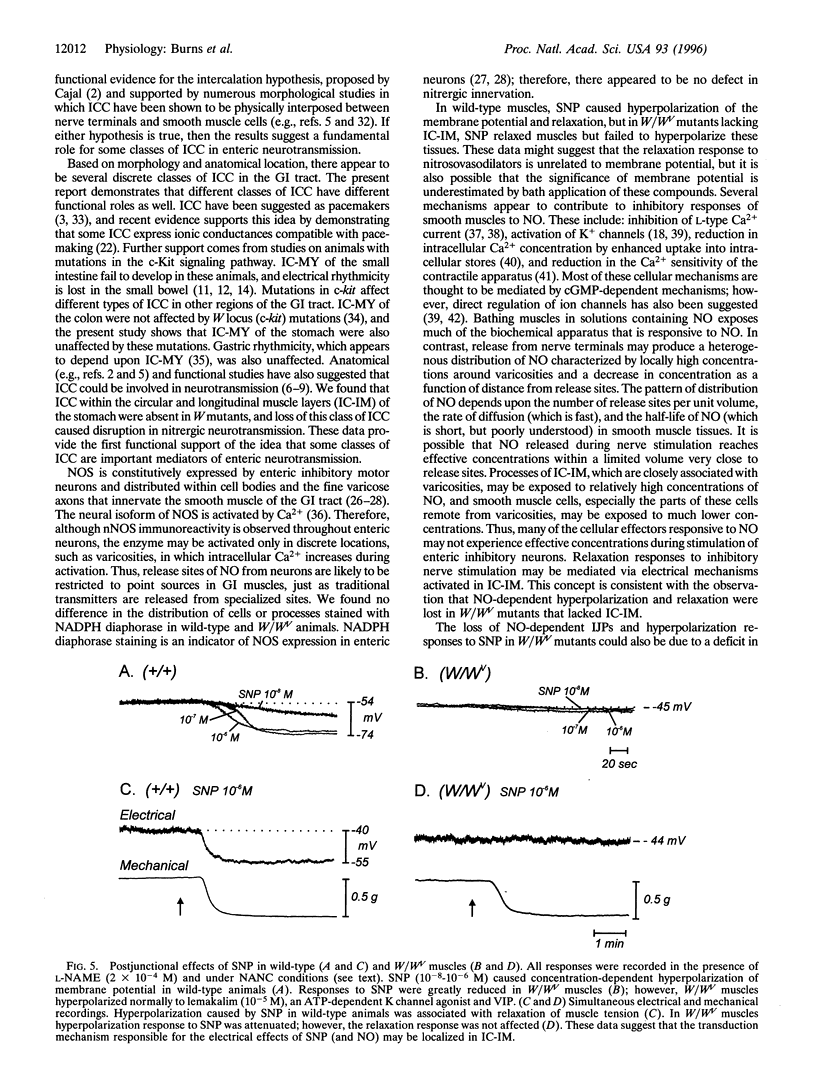
The structural relationships between interstitial cells of Cajal (ICC), varicose nerve fibers, and smooth muscle cells in the gastrointestinal tract have led to the suggestion that ICC may be involved in or mediate enteric neurotransmission. We characterized the distribution of ICC in the murine stomach and found two distinct classes on the basis of morphology and immunoreactivity to antibodies against c-Kit receptors. ICC with multiple processes formed a network in the myenteric plexus region from corpus to pylorus. Spindle-shaped ICC were found within the circular and longitudinal muscle layers (IC-IM) throughout the stomach. The density of these cells was greatest in the proximal stomach. IC-IM ran along nerve fibers and were closely associated with nerve terminals and adjacent smooth muscle cells. IC-IM failed to develop in mice with mutations in c-kit. Therefore, we used W/W(V) mutants to test whether IC-IM mediate neural inputs in muscles of the gastric fundus. The distribution of inhibitory nerves in the stomachs of c-kit mutants was normal, but NO-dependent inhibitory neuro-regulation was greatly reduced. Smooth muscle tissues of W/W(V) mutants relaxed in response to exogenous sodium nitroprusside, but the membrane potential effects of sodium nitroprusside were attenuated. These data suggest that IC-IM play a critical serial role in NO-dependent neurotransmission: the cellular mechanism(s) responsible for transducing NO into electrical responses may be expressed in IC-IM. Loss of these cells causes loss of electrical responsiveness and greatly reduces responses to nitrergic nerve stimulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belai A., Schmidt H. H., Hoyle C. H., Hassall C. J., Saffrey M. J., Moss J., Förstermann U., Murad F., Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett. 1992 Aug 31;143(1-2):60–64. doi: 10.1016/0304-3940(92)90233-w. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984 Mar;246(3 Pt 1):G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Faussone Pellegrini M. S., Cortesini C., Romagnoli P. Sull'ultrastruttura della tunica muscolare della porzione cardiale dell'esofago e dello stomaco umano con particolare riferimento alle cosiddette cellule interstiziali di Cajal. Arch Ital Anat Embriol. 1977;82(2):157–177. [PubMed] [Google Scholar]
- Faussone-Pellegrini M. S., Pantalone D., Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989 Jul;21(3):439–460. [PubMed] [Google Scholar]
- Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995 Mar;24(3):159–187. doi: 10.1007/BF01181533. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kubota M., Szurszewski J. H. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986 Mar;372:501–520. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Thuneberg L., Klüppel M., Malysz J., Mikkelsen H. B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995 Jan 26;373(6512):347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Imaizumi M., Hama K. An electron microscopic study on the interstitial cells of the gizzard in the love-bird (Uroloncha domestica). Z Zellforsch Mikrosk Anat. 1969;97(3):351–357. doi: 10.1007/BF00968841. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S. D., Campbell J. D., Carl A., Sanders K. M. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol. 1995 Dec 15;489(Pt 3):735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P., Ward S. M., Carl A., Norell M. A., Sanders K. M. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K., Sanders K. M. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993 Jan;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- PATON W. D., VANE J. R. Analysis of he responses of the isolated stomach to electrical stimulation and to drugs. J Physiol. 1963 Jan;165:10–46. doi: 10.1113/jphysiol.1963.sp007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser C. L. Rhythmic electrical and mechanical activity in stomach of toad and frog. Am J Physiol. 1995 Sep;269(3 Pt 1):G386–G395. doi: 10.1152/ajpgi.1995.269.3.G386. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover N. G., Horowitz N. N., Sanders K. M. Calcium oscillations in freshly dispersed and cultured interstitial cells from canine colon. Am J Physiol. 1992 Mar;262(3 Pt 1):C589–C597. doi: 10.1152/ajpcell.1992.262.3.C589. [DOI] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Sanders K. M. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol. 1992;54:439–453. doi: 10.1146/annurev.ph.54.030192.002255. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. W., Xue C., Ward S. M., de Vente J., Sanders K. M. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993 Sep;56(2):513–522. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Prosser C. L., Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986 Mar;250(3 Pt 1):G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Torihashi S., Ward S. M., Nishikawa S., Nishi K., Kobayashi S., Sanders K. M. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995 Apr;280(1):97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burns A. J., Torihashi S., Harney S. C., Sanders K. M. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995 Dec;269(6 Pt 1):C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burns A. J., Torihashi S., Sanders K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994 Oct 1;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Xue C., Shuttleworth C. W., Bredt D. S., Snyder S. H., Sanders K. M. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992 Aug;263(2 Pt 1):G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- Young H. M., Furness J. B., Shuttleworth C. W., Bredt D. S., Snyder S. H. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry. 1992 May;97(4):375–378. doi: 10.1007/BF00270041. [DOI] [PubMed] [Google Scholar]
- Young H. M., McConalogue K., Furness J. B., De Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993 Jul;55(2):583–596. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]