Abstract
Telomere maintenance by the conventional DNA replication machinery and telomerase is assisted by specialized DNA helicases, nucleases and telomere binding proteins. Here, we identify the THO components at telomeres and define critical roles of this complex in telomere stability. Deletion of the THO-subunit THP2 leads to telomere shortening. We discover that telomeres contain RNA:DNA hybrid structures or R-loops which involve the long-noncoding RNA TERRA and which accumulate in thp2-Δ cells. Telomere length is not restored by R-loop removal upon RNase H overexpression, but by deletion of Exonuclease 1 (Exo1). Replication stress further enhances the short telomere phenotype of THP2 mutants. Similar events occur upon induced transcription of TERRA and genetic analysis links Thp2 to TERRA function. Altogether, our data indicate that THO, through the interplay with TERRA, regulates chromosome end processing activities and prevents interference with semiconservative DNA replication of telomeric DNA.
Keywords: DNA replication stress, R-loops, telomere instability, TERRA, THO
Introduction
Stable maintenance of telomeric DNA is critical for cell survival as telomeres protect chromosome ends from DNA end joining, homologous recombination, DNA end degradation and checkpoint signalling (Sfeir and De Lange, 2012). Telomere replication involves the conventional DNA replication machinery, which replicates the bulk of the telomeric DNA and telomerase (Teixeira et al, 2004). Telomerase counteracts telomere shortening that occurs at the distal ends of telomeres due to the end replication problem and nucleolytic trimming of chromosome ends. Semiconservative DNA replication of telomeric DNA is challenging and replication forks frequently stall when encountering telomeric DNA (Ivessa et al, 2002; Makovets et al, 2004; Azvolinsky et al, 2006). Efficient telomere replication requires the double-strand telomere binding protein Taz1 in fission yeast (Miller et al, 2006) and the orthologous TRF1 in mammals (Sfeir et al, 2009). The telomeric CST complex also promotes telomere replication (Qi and Zakian, 2000; Grossi et al, 2004; Casteel et al, 2009; Gu et al, 2012; Huang et al, 2012; Stewart et al, 2012; Wu et al, 2012). In addition, several helicases are critical for telomere replication. This includes Rrm3 and Pif1 in Saccharomyces cerevisiae and Werner, RTEL1 and BLM in human cells (Ivessa et al, 2002; Crabbe et al, 2004; Sfeir et al, 2009; Paeschke et al, 2011; Vannier et al, 2012). These helicases may facilitate replication by removing complex DNA structures such as G-quadruplexes or T-loops in telomeric DNA and they might dissolve tightly associated proteins or telomeric RNA from telomeres for replication.
Telomeric repeat containing RNA (TERRA) has the potential to perturb telomere maintenance. Its increase at chromosome ends in RNA surveillance mutants correlated with frequent telomere loss events (Azzalin et al, 2007), which preferentially occurred at telomeres replicated by leading-strand synthesis (Chawla et al, 2011). Furthermore, TERRA is a potent inhibitor of human telomerase in vitro (Schoeftner and Blasco, 2008; Redon et al, 2010) whereas in vivo TERRA transcription in S. cerevisiae induces telomere shortening through stimulation of telomere resection by Exonuclease 1 (Exo1) (Pfeiffer and Lingner, 2012).
The THO complex is evolutionary conserved. In the nucleus, it promotes efficient packaging of nascent messenger RNAs into ribonucleoprotein complexes. In S. cerevisiae, the minimal THO complex consists of Tho2, Hpr1, Mft1 and Thp2. THO enhances normal pre-mRNA processing and mRNA export (Rondon et al, 2010). Transcription of the PHO5 gene with the strong GAL1 promoter in hpr1-Δ cells promoted R-loop formation and hyper-recombination (Huertas and Aguilera, 2003). R-loops are structures where an RNA strand is base paired with the template DNA strand of a DNA duplex, leaving the displaced non-template DNA strand single-stranded. Deletion of other THO components also showed hyper-recombination that was linked to defects in R-loop removal. THO is not essential for growth in yeast though HPR1- or THO2-deletion mutants grow slowly. Cells defective in two THO components (hpr1-Δ mft1-Δ cells) show a synthetic growth defect in the absence of a proper S-phase checkpoint (Gomez-Gonzalez et al, 2009). THO mutants are also slightly sensitive to drugs that produce replicative stress, such as hydroxyurea (HU). These data indicated that R-loops mediate replication stress (Gomez-Gonzalez et al, 2009). Roles of THO for non-coding RNAs have not been described yet.
Here, we discover THO as an important and novel component of telomeres. In the absence of the THO-subunit Thp2, telomeres suffer from telomere shortening. We also demonstrate that telomeric R-loops accumulate in thp2-Δ cells but R-loops are not responsible for telomere shortening. Instead, THP2 loss leads to increased telomere resection by Exo1, an effect that is also induced upon induction of TERRA transcription. DNA replication stress enhances THP2 deletion and TERRA-mediated telomere shortening, indicating that TERRA can interfere with semiconservative DNA replication. Our data demonstrate that THO promotes telomere maintenance through the control of TERRA biogenesis and Exo1-mediated resection.
Results
Identification of the THO complex at telomeres
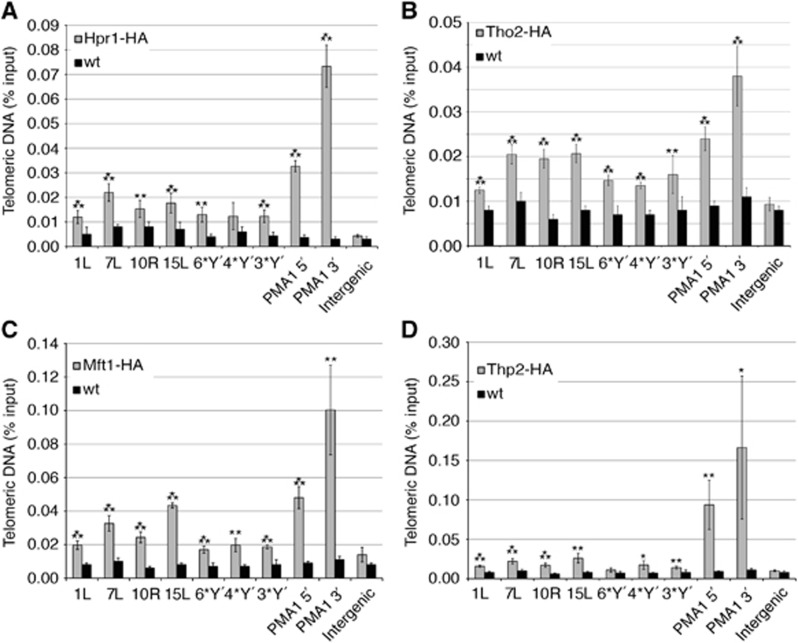
In a paper submitted elsewhere, we identified all THO subunits as components of human telomeres (Grolimund et al, submitted). This prompted us to investigate whether telomere association of THO is conserved in S. cerevisiae. Therefore, we tagged the four THO genes HPR1, THO2, MFT1 and THP2 at their 3′ termini in the endogeneous loci with three HA tags. Chromatin immunoprecipitation (ChIP) was carried out using anti-HA antibodies (Figure 1). The presence of telomeric DNA in the immunoprecipitates was tested by quantitative (q) PCR using primers that specifically amplified subtelomeric sequences present at different telomeres. Untagged strains served as a negative control. With all tagged strains, telomeric DNA was immunoprecipitated and the signals for most chromosome ends were significantly above the untagged controls (Figure 1). All four THO components were also detected at the PMA1 locus as expected, with increased presence towards the 3′ end of the gene (Zenklusen et al, 2002). On the other hand, no interaction was detected at an intergenic locus that is not transcribed. Therefore, all four THO subunits associate with telomeres.
Figure 1.
The THO complex is at telomeres. Hpr1-HA, Tho2-HA, Mft1-HA and Thp2-HA strains (in A, B, C and D, respectively) were grown to exponential phase and HA-associated chromatin was immunoprecipitated. A wild-type (wt) strain served as a negative control. The immunoprecipitated telomeres were amplified by qPCR with primer pairs specific for X-only telomeres 1L, 7L, 10R, 15L and for three (3*Y′), four (4*Y′) or six (6*Y′) different Y′ telomeres and expressed as a percentage of the input. Amplification of the 5′-end (PMA1 5′) and the 3′-end (PMA1 3′) of PMA1 served as positive controls for THO association (Zenklusen et al, 2002). An untranscribed intergenic sequence (intergenic) on chromosome 4R (nucleotides 1 516 119 to 1 516 229) served as a negative control. Values of three independent biological replicates with standard deviation are shown. Statistical analyses were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01).
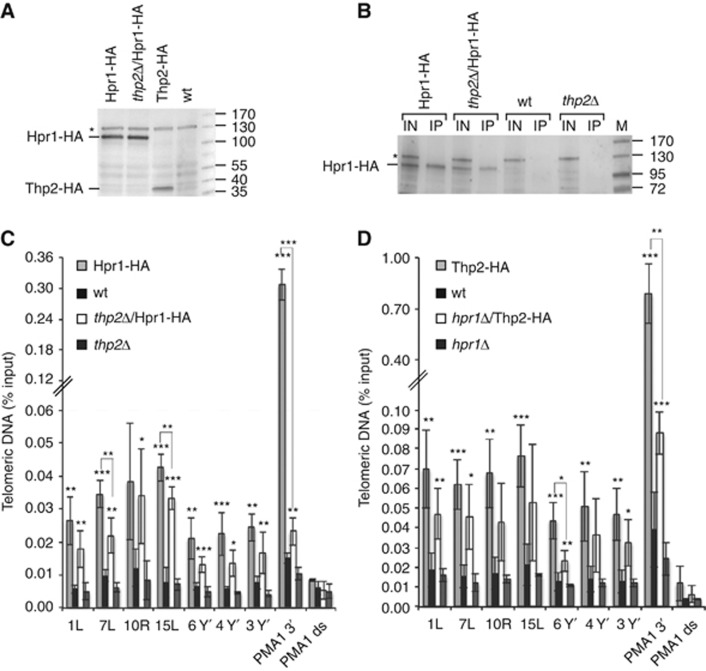
We also tested whether the associations of Hpr1 and Thp2 with telomeres are interdependent (Figure 2). While overall expression levels of Hpr1-HA are not influenced by the absence of THP2 (Figure 2A), chromatin enriched Hpr1 was slightly reduced (Figure 2B). The telomeric ChIP signal obtained with Hpr1-HA in a thp2-Δ strain was reduced at most telomeres but not abolished (Figure 2C). Similarly, deletion of HPR1 reduced but did not abolish the telomeric ChIP signal obtained with Thp2-HA (Figure 2D). We conclude that Thp2 and Hpr1 reinforce their presence at telomeres but telomere association can occur independently. Similar results were obtained for 3′ end of the PMA1 gene but the interdependence of Thp2 and Hpr1 for binding this region of the genome appeared to be more pronounced as signal reduction was more substantial upon deletion of either of the two partners of the THO complex.
Figure 2.
Hpr1 is present at telomeres in thp2-Δ cells and Thp2 is present at telomeres in hpr1-Δ cells. (A) Deletion of THP2 does not influence the amount of Hpr1-HA in whole-cell protein extracts. Whole-cell protein extracts were prepared from strains grown to exponential phase in YPD at 30°C. Western blot analysis determined the amount of Hpr1-HA and Thp2-HA protein. An unspecific band is marked by an asterisk and was used as a loading control. Marker is given in kDa. (B) Deletion of THP2 does influence the specific pull-down efficiency of Hpr1-HA in ChIP experiments as determined by western blotting. After crosslink reversal, input (IN) and immunoprecipitates (IP) were analysed by PAGE. The Hpr1-HA protein band is highlighted by a line; an unspecific band is marked by an asterisk. Marker is given in kDa. (C) Hpr1-HA is associated with telomeres in thp2-Δ cells. Hpr1-HA and thp2-Δ/Hpr1-HA cells were grown to exponential phase, crosslinked and HA-associated chromatin was immunoprecipitated. The untagged wild-type (wt) and thp2-Δ strains served as negative controls. The immunoprecipitated telomeres 1L, 7L, 10R, 15L, 3*Y′, 4*Y′ and 6*Y′ were amplified by qPCR and are expressed as a percentage of the input. Amplification of the 3′end of PMA1 served as a positive control for Hpr1 association as in Figure 1. Hpr1 is not enriched at an untranscribed locus downstream of PMA1 (PMA1 ds, see Supplementary Figure S5B). Values of three independent biological replicates with standard deviation are shown. Statistical analyses are as in Figure 1. (D) Thp2-HA is associated with telomeres in hpr1-Δ cells. Thp2-HA and hpr1-Δ/Thp2-HA strains were grown and the HA-associated chromatin was detected as in (C). The wt and hpr1-Δ strains served as negative controls. Statistical analyses were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01).
Telomeres contain R-loops that are repressed by THO
The THO complex is involved in RNA maturation and R-loop removal (see Introduction). In order to test for roles of THO in R-loop removal at telomeres, we generated strains in which either HPR1 or THP2 was deleted. Nucleic acids were isolated and DNA immunoprecipitation (DIP) was carried out using a monoclonal antibody (S9.6), which specifically recognizes DNA/RNA hybrids (Boguslawski et al, 1986; Hu et al, 2006). The S9.6 antibody has successfully been used in a large variety of studies to detect R-loops in several species at different genomic loci (Szekvolgyi et al, 2007; El Hage et al, 2010; Mischo et al, 2011; Skourti-Stathaki et al, 2011; Wahba et al, 2011; Ginno et al, 2012; Stirling et al, 2012). We determined the presence of telomeric R-loops in the immunoprecipitates by qPCR using different subtelomeric primers as above. As a control, we treated isolated nucleic acids with RNase H in vitro prior to immunoprecipitation, which destroys the RNA moiety in DNA/RNA hybrids. The qPCR analysis (Figure 3A and B) demonstrated specific enrichment of telomeric nucleic acids in the fractions obtained with the S9.6 monoclonal antibody over mouse IgG control. Furthermore, the signals were significantly reduced in the RNase H-treated samples, indicating that we detected R-loops with this technique and that R-loops are present at telomeres in wild-type cells (Figure 3A and B). The signals for telomeric R-loops roughly doubled in hpr1-Δ and thp2-Δ strains, indicating that THO suppresses R-loops at chromosome ends.
Figure 3.
Deletion of HPR1 or THP2 increases DNA:RNA hybrid (R-loops) formation at telomeres. (A) Deletion of HPR1 increases R-loop formation at telomeres. Three independent colonies of wt and hpr1Δ mutants were used to immunoprecipitate R-loops using the S9.6 antibody. Treating one half of each sample with RNase H and using an unspecific IgG antibody served as controls for the specificity of the antibody. The immunoprecipitated telomeres 1L, 7L, 10R, 15L, 3*Y′, 4*Y′ and 6*Y′ were amplified by qPCR and expressed as a percentage of input. Amplification of PMA1 3′ served as a positive control for R-loop formation as in Figure 1. Values of three independent biological replicates with standard deviation are shown. PMA1 controls and statistical analyses are as in Figure 1. (B) Deletion of THP2 increases R-loop formation at telomeres. Three independent colonies of a wt strain and of a thp2-Δ mutant were analysed by DIP as described in (A). Statistical analyses were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01).
In order to confirm these data and to manipulate R-loop formation in vivo, we ectopically overexpressed Rnh1, one of the major yeast RNase H enzymes from a Gal promoter (Crouch et al, 2001). DIP analysis demonstrated that Rnh1 overexpression efficiently reduced R-loops in vivo in wild-type cells as well as in the thp2-Δ strain (Figure 4A). On the other hand, when deleting the RNase H enzyme genes rnh1 and rnh201 in yeast, the DIP signal for telomeres increased (Supplementary Figure S1). Altogether, these results demonstrate that R-loops exist at telomeres in yeast and that R-loop abundance is suppressed by the Tho components Hpr1 and Thp2, as well as by RNase H enzymes.
Figure 4.
Overexpression of RNase H1 in vivo reduces telomeric R-loops and TERRA. (A) Overexpressed RNase H1 reduces R-loops at telomeres in wt and thp2-Δ strains. Cells were grown in YPD or in YPGal at 25°C to either repress or induce RNase H1. R-loops were immunoprecipitated and analysed as in Figure 3. Values of three independent biological replicates with standard deviation are shown. Statistical analyses are as in Figure 1. (B) TERRA levels are reduced in thp2-Δ cells upon RNase H1 overexpression. Strains were grown as above and TERRA transcribed from telomeres 1L, 7L, 10R, 13R, 15L, 3*Y′, 4*Y′, 6*Y′ and RNA derived from PMA1 3′ were quantified by qRT-PCR. RNA levels relative to wt of three independent biological replicates normalized against actin with standard deviations are shown. Statistical analyses were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01).
Measurement of TERRA levels in wild-type and thp2-Δ cells further demonstrated that RNase H1 overexpression reduces the amount of TERRA in thp2-Δ cells ∼1.5–3-fold depending on telomere identity (Figure 4B). This therefore indicates that a substantial fraction of TERRA molecules is present in an R-loop configuration in thp2-Δ cells. In wild-type cells, on the other hand, RNase H1 overexpression reduced TERRA only at some telomeres indicating that most TERRA molecules are not part of R-loops in wild-type cells.
Telomere shortening in thp2-Δcells revealed by single telomere sequence analysis
We used single telomere sequence analysis in THO deletion strains in order to test its roles in telomere maintenance (Forstemann et al, 2000; Teixeira et al, 2004). Individual cells were clonally expanded for ∼40 generations, telomere 1L was amplified by telomere PCR and individual telomere sequences were aligned. Sequence comparison of sister telomeres allows distinction of regions that are identical due to faithful replication by semiconservative DNA replication. Sequence divergence at telomere ends on the other hand indicates action of telomerase, which adds in yeast irregular GT1–3 repeats or DNA recombination events (Forstemann et al, 2000; Teixeira et al, 2004). For each experimental condition, we analysed at least 30 sequences from three expanded clones and calculated the average telomere lengths as well as the percentage of the undiverged (red) and diverged (blue) portion of the sequences (Figure 5). As expected, wild-type telomeres showed after clonal expansion a telomere proximal region that was identical between all sisters and diverged distal ends at which telomerase had acted (Figure 5A). Telomeres at the left arm of chromosome 1 in hpr1-Δ cells were ∼15 bp longer, had a slightly enlarged diverged zone but otherwise resembled wild-type telomeres (Supplementary Figure S2A). The telomere length analysis of bulk telomeres by Southern blotting confirmed slight telomere lengthening (Supplementary Figure S2B).
Figure 5.
Exo1 mediates telomere shortening in thp2-Δ cells independently of R-loop formation. (A) Telomere 1L is shorter in thp2-Δ, which does not depend on R-loops. To overexpress RNase H1, three independent colonies of a wt+pYGW-RNH1 strain and of a thp2-Δ+pYGW-RNH1 strain were grown in YPD (−RNase H1 OE) or YPGal (+RNase H1 OE) at 25°C. Genomic DNA was extracted and analysed by telomere PCR for telomere 1L. The PCR products were TOPO cloned and sequenced. Ten sequences per clone were analysed. Given are the sequence reads for all three clones per condition. Each bar represents the amplified undiverged (red) and diverged (blue) portion of the telomeric tract of telomere 1L. The average percentage per telomere of undiverged (red number in brackets) and diverged (blue number in brackets) telomeric tract is given for each strain. Statistical analyses for differences in telomere length were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01). (B) Deletion of EXO1 compensates the short telomere phenotype of thp2-Δ. Three independent clones of the exo1-Δ and thp2-Δ exo1-Δ strains were grown at 25°C to OD600 0.4. The cultures were then treated with 100 mM hydroxyurea (HU) for 2 h. Genomic DNA was extracted and analysed as above. (C) Replicative stress induced by HU treatment leads to further telomere shortening in thp2-Δ cells. wt and thp2-Δ cells were grown and analysed as described above. (D) RNase H1 overexpression does not rescue the short telomeres of thp2-Δ cells that are treated with HU. The analysis was performed as in (A) with strains overexpressing RNase H1. Statistical analyses in (C) and (D) were done as in (A).
In thp2-Δ cells, however, telomeres were ∼60 nucleotides shorter than in wild-type cells at telomere 1L with similar proportions of diverged and non-diverged zones (Figure 5A). Similar telomere shortening was seen by Southern blot analysis (Supplementary Figure S2B) at other chromosome ends consistent with published results (Askree et al, 2004). Telomere shortening in thp2-Δ cells was independent of growth temperature despite the fact that telomeres in wild-type cells shorten slightly when cells are grown at higher temperatures (Supplementary Figure S2C and D; Paschini et al, 2012). Furthermore, the telomere length effects were recapitulated by single telomere sequence analysis at telomere 6R (Supplementary Figure S3A). Since R-loops accumulate at telomeres in thp2-Δ cells we tested whether R-loop reduction by Rnh1 overexpression would rescue the short telomere phenotype. Rnh1 overexpression in wild-type and thp2-Δ cells caused a slight increase in repeat divergence, but it did not markedly influence telomere length (Figure 5A). This indicates that R-loops have no major impact on telomere length. We also tested for changes of TERRA levels from different chromosome ends in the different mutant situations by quantitative real-time PCR (qRT-PCR) but observed no major effects (Supplementary Figure S4). However, the short telomere phenotype of thp2-Δ cells was fully suppressed by deletion of Exonuclease 1 (EXO1) as telomere length in exo1-Δ cells and thp2-Δ exo1-Δ cells was identical (Figure 5B). This indicates that Thp2 protects chromosome ends from Exo1. This phenotype is reminiscent of TERRA overexpressing cells, which also suffer from telomere shortening due to the partial loss of protection from Exo1 (Pfeiffer and Lingner, 2012).
Since THO is required to prevent DNA replication stress (Gomez-Gonzalez et al, 2009) we tested how treatment with low doses of HU, which leads to slowed/stalled replication fork events but did not impede proliferation (Supplementary Figure S3B), would affect telomere maintenance. Addition of 100 mM HU for 2 h to the medium prior to telomere length analysis did not markedly affect telomere maintenance in wild-type cells (Figure 5C). However, HU treatment of thp2-Δ cells for 2 h led to a considerable telomere shortening by ∼40 bp at telomere 1L and 6R (Figure 5C; Supplementary Figure S3A). This fast shortening that occurred in approximately one cell cycle cannot be explained by telomerase inhibition. Instead, it indicates that Thp2 is required for efficient and complete semiconservative replication of telomeric DNA in the occurrence of replication stress. We tested whether R-loop reduction by Rnh1 overexpression would rescue the telomere shortening phenotype in thp2-Δ cells treated with HU (Figure 5D). Rnh1 overexpression caused a slight increase in repeat divergence as in the absence of HU, but telomere shortening was not rescued. Deletion of EXO1 led to a considerable telomere length increase (Figure 5B), but it also did not fully rescue the telomere shortening that can be attributed to HU treatment. These data indicate that Thp2 promotes telomere maintenance by at least two separate pathways. It protects telomeres from Exo1 and it protects telomeres from additional shortening that occurs upon DNA replication stress.
Telomere instability in thp2-Δcells is recapitulated by TERRA induction
THO and thus Thp2 play critical roles in transcription and maturation of many RNAs. The association of the complex with telomeres suggested direct roles of THO and in particular Thp2 for telomere stability possibly by impacting on TERRA maturation. This notion was supported by our observation that TERRA levels are reduced upon overexpression of RNase H1 in thp2-Δ cells (Figure 4B). We therefore tested whether induction of TERRA transcription at chromosome 1L could recapitulate the telomere phenotypes of thp2-Δ cells. For this, we used a doxycycline regulatable promoter that was inserted upstream of the natural TERRA transcription start site at telomere 1L. This telomere is referred to as TetO7-1L (Figure 6A top; Pfeiffer and Lingner, 2012). Overexpression of TERRA upon removal of doxycycline for 30 generations (Supplementary Figures S5A and S6) lead to telomere shortening in cis at telomere 1L as described previously (Figure 6A; Pfeiffer and Lingner, 2012). The 2-h HU treatment further shortened telomere TetO7-1L under TERRA-induced but not TERRA-repressed conditions (Figure 6A). Therefore, replication stress-induced telomere shortening is TERRA mediated. Furthermore, we observed several complete telomere-loss events in which the entire TG tract was lost. However, since they also occurred at TetO7-1L upon HU treatment under TERRA-repressed conditions their significance is not clear. The analysis of telomeres in exo1-Δ cells confirmed that TERRA induction leads to Exo1-dependent telomere shortening as described previously (Pfeiffer and Lingner, 2012; Figure 6B). HU treatment-induced shortening was partially but not fully rescued by EXO1 deletion when comparing TERRA-1L-expressing to TERRA-1L-repressed cells (Figure 6B). Curiously, we observed that HU treatment of exo1-Δ cells caused slight telomere lengthening by unknown mechanisms (Figures 5B and 6B), which however was not seen in EXO1 wild-type cells. As for thp2-Δ cells, R-loops do not appear to play a role in TERRA-mediated telomere shortening as their abundance did not increase upon TERRA overexpression (Supplementary Figure S5B). Altogether, TERRA induction resembled THP2 deletion in these experiments. When we induced TERRA in thp2-Δ cells, the telomere shortening effects were additive (Figure 6C). Therefore, loss of telomere protection from Exo1 in thp2-Δ cells becomes exacerbated upon TERRA induction.
Figure 6.
Telomerase counteracts TERRA-induced telomere shortening by Exo1 and replication stress increases TERRA-mediated telomere shortening. (A) TERRA transcription induces telomere shortening and divergence of telomere sequences. Replication stress enforces TERRA-mediated telomere shortening. DNA was extracted from four independent clones of the TetO7-1L strain (see scheme at the top of the figure) grown at 30°C on YPD plates with (−TERRA) or without (+TERRA) Dox for 25 generations. After an additional growth in liquid culture to an OD600 of 0.5, 100 mM of hydroxyurea (HU) or H2O was added for 2 h at 25°C and DNA was analysed by telomere PCR for telomere 1L as described in Figure 5. Each bar represents the amplified subtelomeric sequence (−156 nts, green), and the undiverged (red) and diverged (blue) portion of the telomeric tract of telomere 1L under the given conditions. Statistical analyses are as in Figure 5. (B) TERRA-mediated increase in telomere sequence divergence depends on Exo1. Replication stress and TERRA transcription in the absence of EXO1 lead to telomere shortening in cis. Given are the sequence reads for telomere 1L of three independent clones of exo1-Δ/TetO7-1L grown and analysed as in (A). (C) TERRA expression in the absence of THP2 leads to telomere shortening of telomere 1L and telomere sequence divergence. The sequence reads of telomere 1L from three independent clones of thp2-Δ/TetO7-1L grown and analysed as in (A) are shown. (D) Sequence divergence upon TERRA transcription is reduced but not abolished in the absence of RAD52. Given are the sequences of telomere 1L of three independent clones of rad52-Δ/TetO7-1l grown and analysed as in (A). (E) Telomerase is needed to counteract TERRA transcription-mediated telomere shortening. The sequences of telomere 1L of three independent clones of est1-Δ/TetO7-1L grown and analysed as in (A) are shown. Statistical analyses were calculated using the Student’s t-test (*P<0.05, **P<0.03 and ***P<0.01).
The single telomere sequence analysis also revealed increased length of repeat divergence upon TERRA induction in wild-type cells (Figure 6A). The increased repeat divergence was reduced but not abolished upon deletion of RAD52, which is required for homologous recombination (Figure 6D). Therefore, we also tested whether TERRA affects the occurrence of telomere repeat divergence in est1-Δ cells that lack telomerase activity (Figure 6E). Sequence alignment of individual chromosome ends after clonal expansion for 25 generation showed a slightly increased frequency of telomeres with diverged chromosome end sequences upon TERRA induction from 30% in TERRA-repressed cells (17 of 56) to 43% in TERRA-induced cells (26 of 61). However, this trend to an increased divergence is not statistically significant and may not fully reflect the true increase in homologous recombination, as recombination between accurately aligned sister telomeres is not expected to introduce sequence divergence. Overall, our data suggest that telomere shortening upon TERRA transcription is mostly counteracted by telomerase, but that the enhancement of recombination at distal telomere ends may also contribute to telomere maintenance.
Discussion
In this paper, we uncover crucial functions of the THO complex and in particular the Thp2 component for telomere maintenance and normal telomere structure. First, we show that R-loops exist at yeast telomeres and that in the absence of the THO components Thp2 or Hpr1, telomeric R-loops accumulate. R-loops are known to promote genome instability as they are recombinogenic and they can block incoming DNA replication forks (Gomez-Gonzalez et al, 2011; Wahba et al, 2011; Bermejo et al, 2012; Stirling et al, 2012). However, the single telomere sequence analysis did not reveal increased telomere breakage and healing events in thp2-Δ cells, which should have been detected by increased fractions of telomeres with large diverged telomeric tracts. Thus, it seems that telomeres are more tolerant to R-loops than other regions of the genome.
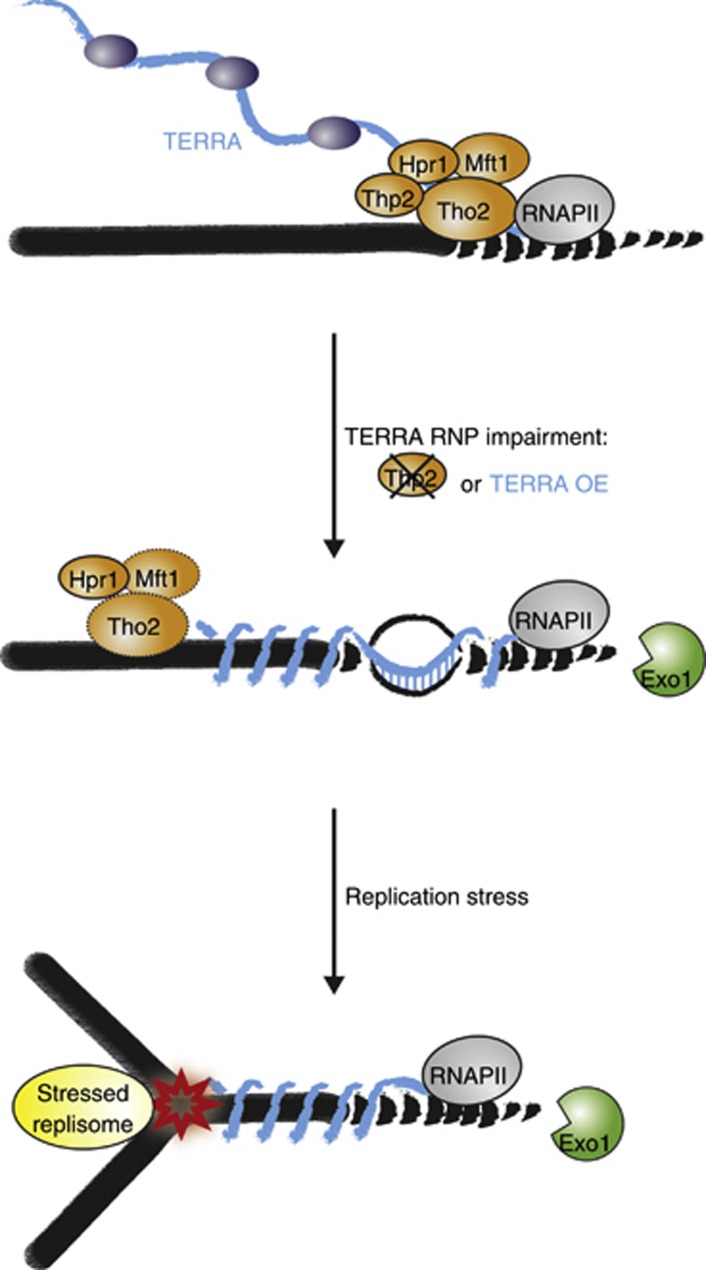
Second, we show that a significant telomere shortening occurs in thp2-Δ cells, which was not reversed upon R-loop reduction by RNase H overexpression. We show that the telomere shortening was due to the increased accessibility of chromosome ends to Exo1 (Figure 5). The same Exo1-mediated telomere shortening was obtained upon induced expression of TERRA. Since THO is involved in RNA maturation it seems likely that THO dysfunction perturbs TERRA maturation, which in turn leads to chromosome end resection by Exo1 (Pfeiffer and Lingner, 2012). We have not detected dramatic changes in TERRA levels in thp2-Δ cells nor was TERRA length affected when analysed on northern blots (data not shown). However, we find that TERRA levels are significantly reduced in thp2-Δ cells that overexpressed RNase H1 whereas TERRA levels were only slightly reduced in wild-type cells that overexpressed this enzyme. As RNase H destroys RNAs that are present as R-loops this indicates that TERRA is present in the telomeric R-loops that accumulate in thp2-Δ cells. Therefore, in the absence of Thp2, TERRA is not properly assembled into native ribonucleoprotein particles (Rougemaille et al, 2008). Unassembled, nascent TERRA may be impaired in localization and thus remain entangled around the DNA template and interfere with formation of telomeric chromatin and fully capped chromosome ends (Figure 7).
Figure 7.
Model for THO function at telomeres. The THO complex localizes to telomeres and assists TERRA packaging into RNPs. In the absence of Thp2, telomeric R-loops accumulate and unassembled, nascent TERRA that may remain entangled around the DNA template accumulates. Failure of TERRA maturation or unscheduled TERRA expression interferes with chromosome end cap formation and protection from the telomere end-trimming enzyme Exo1, which induced telomere shortening. Upon replication stress, perturbed replication forks may collide with TERRA-entangled DNA. Enhanced telomere shortening of TERRA-entangled telomeres upon fork stalling is partially but not solely mediated by Exo1. The additional shortening may be due to enhanced telomere erosion or incomplete DNA replication of chromosome ends. R-loops are not responsible for Exo1- and replication stress-induced telomere shortening.
Third, we uncovered that unscheduled TERRA transcription or its defective maturation in thp2-Δ cells interferes with stalled DNA replication forks at telomeres. Strikingly, a short 2-h HU treatment was sufficient to induce a significant telomere shortening from distal ends in thp2-Δ cells or TERRA-overexpressing cells. This shortening was not due to increased occurrence of R-loops. However, as the telomere shortening was partially rescued in exo1-Δ cells, the shortening was partially but probably not solely mediated by Exo1. We therefore propose that misregulation of TERRA RNP formation and possibly entanglement of telomeric DNA by nascent, unassembled TERRA might interfere with the stability of stalled forks (Figure 7) or replication fork restart though we cannot exclude other scenarios. Consistent with our findings are also the previous observations of Aguilera and colleagues that THO mutants rely on an intact S-phase checkpoint (Gomez-Gonzalez et al, 2009). Since telomeres are difficult to replicate and replication forks often stall, the THO complex may be particularly important for their replication. Notably, however, the telomeric phenotypes of thp2-Δ and hpr1-Δ cells are strikingly different. While both mutants accumulate telomeric R-loops only thp2-Δ cells suffer from short telomeres. Furthermore, Thp2 is present at telomeres in hpr1-Δ cells and Hpr1 still associates with telomeres in thp2-Δ cells. This therefore suggests that in thp2-Δ and hpr1-Δ cells, THO subcomplexes or subunits can function on their own at chromosome ends and contribute to telomere integrity. To conclude, our data uncover that tight regulation of TERRA transcription and RNA maturation by the THO complex are crucial determinants of telomere stability. It will be fascinating to dissect the roles of THO at mammalian telomeres that may pose particular challenges for DNA replication, as TERRA and their telomeric tracts are 10–100 × longer than in budding yeast.
Materials and methods
Yeast strains, growth conditions and plasmids
Yeast strains were derived from BY4741 and are listed in Supplementary Table S1. Strains were cultured in YPD at 25 or 30°C on a shaker (220 r.p.m.) unless stated otherwise. Growth in YPD medium or on YPD plates supplemented with 10 μg/ml Dox is referred to as +Dox. Induction of TERRA from telomere 1L of the inducible (TetO7-1L) strain was performed by streaking single colonies for 25 generations on −Dox YPD plates before additional culture to OD600 of 0.6–0.8 (RNA) or overnight (ON) (DNA) (Pfeiffer and Lingner, 2012). For the overexpression of RNase H1, cells were grown ON at 25°C in YPGal (2% galactose and 1% raffinose; promoter induced) or YPD (promoter repressed) until they reached OD600 0.8. For HU treatment, single colonies were grown ON at 25°C, diluted into two separate vials and grown again to an OD600 of 0.5 at 25°C before HU (+HU; final concentration 100 mM) or H2O (–HU) were added and growth was continued for 2 h. Lithium acetate transformation of yeast strains was performed as described (Ito et al, 1983). Oligonucleotides used in this study are listed in Supplementary Table S2. Plasmids used in this study are listed in Supplementary Table S3.
The EST1 ORF was replaced with a HISMX6 cassette in LVPS-31 using a PCR fragment obtained with oKF147/oKF148 DNA primers and pFA6a-HISMX6 as a template (Longtine et al, 1998). The RAD52 ORF was deleted as above using oJC221/oJC222 DNA primers, resulting in LVPS-184/185. The THP2 ORF was deleted in LVPS-31 as described above using oJC94/oJC95 primers and JCY248 template, resulting in LVPS-164. The THP2 ORF was deleted in BY4741 using oJC138/oJC139 primers, resulting in JCY248. The EXO1 ORF was replaced by a KANMX6 cassette in BY4741, obtained with o1365/o1366 primers and pFA6a-KANMX6 as a template (Longtine et al, 1998), resulting in JCY254. Deletion of the THP2 ORF in BY4741 with a KANMX6 cassette using oJC138/oJC139 primers resulted in JCY173. The THP2 ORF was deleted in yNI590 as described above using oJC138/oJC139 primers, resulting in JCY104. Mating and sporulation of JCY248 and JCY254 generated JCY252. The HPR1 ORF was replaced in BY4741 with a KANMX6 cassette using oJC132/oJC133 primers and pFA6a-KANMX6 as a template, resulting in JCY108. The HA-tagged HPR1, THO2, MFT1 and THO2 gene constructs express C-terminally tagged proteins and were obtained using pFA6a-3HA-HISMX6 as a template and oJC111/oJC112, oJC118/oJC119, oJC120/oJC121 or oJC122/oJC123 as primers, resulting in JCY81, JCY106, JCY109 and JCY111. The THP2 ORF was deleted in JCY81 with a KANMX6 cassette using oJC138/oJC139 primers and pFA6a-KANMX6 as a template, resulting in JCY137. Mating and sporulation of JCY111 and JCY171 resulted in JCY243. Transformation of BY4741 and JCY173 with plasmid pYGW-RNH1 (gift from Robert J Crouch) resulted in JCY237 and JCY241. JCY305 was obtained by replacing the KANMX6 cassette of rnh1::KANMX6 in strain YBL582 with a NAT cassette using plasmid p4339. The RNH201 ORF was replaced in JCY305 with a KANMX6 cassette using oJC234/oJC235 as primers and YBL435 as a template, resulting in LVPS-199/200/201.
Chromatin immunoprecipitation
ChIP was performed as described (Luke et al, 2008; Pfeiffer and Lingner, 2012). In brief, strains were grown in 200 ml rich medium at 30°C to an OD600 of 0.8–0.9. Cells were crosslinked with 1.2% formaldehyde for 25 min (Hpr1-HA, Tho2-HA, Mft1-HA, Thp2-HA ChIP) at 25°C, quenched, washed and lysed. Soluble crosslinked chromatin was obtained upon centrifugation (15 900 g, 10 min, 4°C) and resuspended in 2 ml FA lysis buffer containing 0.26% SDS and sonicated for 15 min (Hpr1-HA, Tho2-HA, Mft1-HA, Thp2-HA ChIP) (30 s ON, 60 s OFF; high; Diagenode Bioruptor). The cell debris was removed by centrifugation at 15 900 g for 15 min at 4°C and the chromatin extract was again sonicated for 15 min (30 s ON, 60 s OFF; high; Diagenode Bioruptor), yielding DNA fragment sizes of 150–500 bp. For immunoprecipitation of Hpr1-HA, Tho2-HA, Mft1-HA and Thp2-HA, the chromatin extracts (1 mg protein/ml) were incubated with 6 μl α-HA 16B12 mouse antibody (Covance) in the presence of 25 μl (bed volume) Protein A Sepharose (GE Healthcare) ON at 4°C on a rotating wheel. A mock IP lacking the antibody or an untagged strain served as controls. In all, 50 μl of the chromatin extract was taken as an input sample. Immunoprecipitates were washed, eluted and the crosslink was reversed as described (Luke et al, 2008). The crosslink of the input samples was reversed simultaneously and the immunoprecipitated DNA was quantified by real-time PCR as described (Pfeiffer and Lingner, 2012). Primer concentrations, sequences and amplified products are listed in Supplementary Table S2. In all, 10 μl of the input and 20 μl of the immunoprecipitates were used for western blot analysis after reversal of the crosslink.
Western blot analysis
Western blotting was performed as described (Pfeiffer and Lingner, 2012). In brief, culture samples of 1.5 OD600 units (from cultures grown at 30°C, 220 r.p.m. to exponential phase) were lysed, proteins were precipitated with TCA and resuspended in 1 × sample loading buffer (0.05 M Tris–HCl pH 6.8, 2% SDS, 10% glycerol, 0.1 M DTT, 0.025% bromophenol blue) and boiled for 10 min at 95°C. In all, 0.5 OD600 per lane was loaded on a 4–20% SDS–PAGE (precast, Bio-Rad). Gels were blotted onto nitrocellulose membranes (Whatman). Membranes were blocked for 1 h in 3% BSA in PBST20 (1 × PBS supplemented with 0.1% Tween-20), followed by incubation with 1/1000 α-HA 16B12 mouse antibody (Covance) ON at 4°C. After three 15 min washes with PBST20, anti-mouse horseradish peroxidase (HRP) coupled antibody (1/3000 in 3% BSA in PBST20, Promega) was incubated for 1 h at RT and washed as above. Western blots were developed using ChemiGlow West (Cell Biosciences) and signals were detected with a FluorChem 8900 (Alpha Innotec).
DNA immunoprecipitation
DIP was performed as described (Mischo et al, 2011). In brief, strains were grown in rich medium at 30°C to an OD600 of 1–1.5. Cells were washed once with 1 × TE (10 mM Tris–HCl pH 7.5, 1 mM EDTA pH 8). The pellet was resuspended in an EDTA/zymolyase solution (600 μl 0.5 M EDTA pH 8, 10 μl zymolyase 20 mg/ml) and incubated for 1 h at 37°C. Cells were centrifuged (15 900 g, 30 s) and the pellet was resuspended with a syringe (1 ml needle 27G¾ 0.4 mm) in 500 μl RLT buffer (RNeasy kit, QIAGEN) and 5 μl β-mercaptoethanol. After adding 250 μl of water and 750 μl of phenol:chloroform:isoamyl alcohol (25:24:1, pH 7.8–8.2), the solution was transferred into 2 ml Phase Lock Gel Eppendorf tubes (5 Prime GmbH) and centrifuged (15 900 g, 5 min). Nucleic acids were precipitated in the presence of 100 mM NaCl and 50% isopropanol for 10 min at RT and spun down for 10 min at 15 900 g at 4°C. The pellet was washed with 70% ethanol, resuspended in water and sonicated for 25 min (30 s ON, 60 s OFF; high; Diagenode Bioruptor) to yield DNA fragments below 500 bp. In all, 100 μg of each sample was incubated with 10 U RNase H (1 U/μl, Roche) or H2O at 37°C for 90 min, after which the reaction was stopped by adding 2 μl 0.5 M EDTA. For immunoprecipitation, 50 μl of DNA was diluted 10-fold with FA1 buffer (0.1% SDS, 1% Triton X-100, 10 mM HEPES pH 7.7, 0.1% sodium deoxycholate, 275 mM NaCl), 1 μg S9.6 antibody (ATCC HB-8730, kindly provided by Stephen Leppla) and 20 μl (bed volume) Protein G Sepharose (GE Healthcare) were added and incubated for 90 min at 4°C on a rotating wheel. An IP with 1 μg of mouse IgG antibody (Santa Cruz Biotechnology) served as a control. Immunoprecipitates were washed and eluted as described (Luke et al, 2008) and purified using the QIAquick PCR purification kit. The eluted samples (50 μl H2O) were quantified by real-time PCR as described (Pfeiffer and Lingner, 2012). Primer concentrations, sequences and amplified products are listed in Supplementary Table S2.
RNA isolation and qRT-PCR
For RNA isolation, cells were grown to an OD600 of 0.6–1.0 as described and RNA was extracted including three DNase I treatments (Pfeiffer and Lingner, 2012). Reverse transcription of TERRA and qPCR was performed as described (Iglesias et al, 2011; Pfeiffer and Lingner, 2012). Primer concentrations, sequences and amplified products are listed in Supplementary Table S2. Primers for PMA1 3′ and PMA1 DS amplification were taken from publications (Zenklusen et al, 2002; Mischo et al, 2011). The specificity of the primers was tested by TOPO cloning and sequencing (Iglesias et al, 2011).
DNA isolation, terminal transferase-mediated tailing and telomere PCR
DNA isolation of single colonies grown as described above, terminal transferase reactions and telomere PCR were performed as described (Pfeiffer and Lingner, 2012) using 750 nM of oligonucleotides oG18-BamHI and oBL1180 (1L telomere PCR). PCR products were analysed on a 2.5% agarose gel. Telomere PCR products were TOPO cloned (Invitrogen) and sequenced using M13rev. For each telomere product, at least 10 different TOPO clones were sequenced and each experiment was performed in triplicate.
Supplementary Material
Acknowledgments
We thank Dr R Crouch (NIH) for the RNase H1 overexpression plasmid pYGW-RNH1. We thank Dr C Leysath in Dr S Leppla’s laboratory (NIH) for generously providing antibody S9.6. We thank P Magliano and TF Lunardi for technical assistance. VP was supported by an EMBO postdoctoral fellowship and LG by an ISREC doctoral fellowship. Research in JL’s laboratory was supported by the Swiss National Science Foundation, an European Research Council advanced investigator grant (grant agreement number 232812), an Initial Training Network (ITN) grant (CodeAge) from the European Commission’s Seventh Framework Programme (grant agreement number 316354), the Swiss Cancer League and EPFL.
Author contributions: LG discovered THO at human telomeres. JC, VP and JL designed the research. JC and VP performed all experiments. JL, JC and VP wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Askree SH, Yehuda T, Smolikov S, Gurevich R, Hawk J, Coker C, Krauskopf A, Kupiec M, McEachern MJ (2004) A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc Natl Acad Sci USA 101: 8658–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky A, Dunaway S, Torres JZ, Bessler JB, Zakian VA (2006) The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev 20: 3104–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J (2007) Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318: 798–801 [DOI] [PubMed] [Google Scholar]
- Bermejo R, Lai MS, Foiani M (2012) Preventing replication stress to maintain genome stability: resolving conflicts between replication and transcription. Mol Cell 45: 710–718 [DOI] [PubMed] [Google Scholar]
- Boguslawski SJ, Smith DE, Michalak MA, Mickelson KE, Yehle CO, Patterson WL, Carrico RJ (1986) Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods 89: 123–130 [DOI] [PubMed] [Google Scholar]
- Casteel DE, Zhuang S, Zeng Y, Perrino FW, Boss GR, Goulian M, Pilz RB (2009) A DNA polymerase-{alpha}{middle dot}primase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J Biol Chem 284: 5807–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R, Redon S, Raftopoulou C, Wischnewski H, Gagos S, Azzalin CM (2011) Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. EMBO J 30: 4047–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe L, Verdun RE, Haggblom CI, Karlseder J (2004) Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306: 1951–1953 [DOI] [PubMed] [Google Scholar]
- Crouch RJ, Arudchandran A, Cerritelli SM (2001) RNase H1 of Saccharomyces cerevisiae: methods and nomenclature. Methods Enzymol 341: 395–413 [DOI] [PubMed] [Google Scholar]
- El Hage A, French SL, Beyer AL, Tollervey D (2010) Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev 24: 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstemann K, Hoss M, Lingner J (2000) Telomerase-dependent repeat divergence at the 3′ ends of yeast telomeres. Nucleic Acids Res 28: 2690–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Lott PL, Christensen HC, Korf I, Chedin F (2012) R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol Cell 45: 814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Felipe-Abrio I, Aguilera A (2009) The S-phase checkpoint is required to respond to R-loops accumulated in THO mutants. Mol Cell Biol 29: 5203–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Garcia-Rubio M, Bermejo R, Gaillard H, Shirahige K, Marin A, Foiani M, Aguilera A (2011) Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. EMBO J 30: 3106–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi S, Puglisi A, Dmitriev PV, Lopes M, Shore D (2004) Pol12, the B subunit of DNA polymerase alpha, functions in both telomere capping and length regulation. Genes Dev 18: 992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Min JN, Wang Y, Huang C, Peng T, Chai W, Chang S (2012) CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J 31: 2309–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang A, Storz G, Gottesman S, Leppla SH (2006) An antibody-based microarray assay for small RNA detection. Nucleic Acids Res 34: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Dai X, Chai W (2012) Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res 22: 1681–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas P, Aguilera A (2003) Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell 12: 711–721 [DOI] [PubMed] [Google Scholar]
- Iglesias N, Redon S, Pfeiffer V, Dees M, Lingner J, Luke B (2011) Subtelomeric repetitive elements determine TERRA regulation by Rap1/Rif and Rap1/Sir complexes in yeast. EMBO Rep 12: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153: 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa AS, Zhou JQ, Schulz VP, Monson EK, Zakian VA (2002) Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev 16: 1383–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Luke B, Panza A, Redon S, Iglesias N, Li Z, Lingner J (2008) The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol Cell 32: 465–477 [DOI] [PubMed] [Google Scholar]
- Makovets S, Herskowitz I, Blackburn EH (2004) Anatomy and dynamics of DNA replication fork movement in yeast telomeric regions. Mol Cell Biol 24: 4019–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Rog O, Cooper JP (2006) Semi-conservative DNA replication through telomeres requires Taz1. Nature 440: 824–828 [DOI] [PubMed] [Google Scholar]
- Mischo HE, Gomez-Gonzalez B, Grzechnik P, Rondon AG, Wei W, Steinmetz L, Aguilera A, Proudfoot NJ (2011) Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol Cell 41: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeschke K, Capra JA, Zakian VA (2011) DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell 145: 678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschini M, Toro TB, Lubin JW, Braunstein-Ballew B, Morris DK, Lundblad V (2012) A naturally thermolabile activity compromises genetic analysis of telomere function in Saccharomyces cerevisiae. Genetics 191: 79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer V, Lingner J (2012) TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet 8: e1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Zakian VA (2000) The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev 14: 1777–1788 [PMC free article] [PubMed] [Google Scholar]
- Redon S, Reichenbach P, Lingner J (2010) The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res 38: 5797–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon AG, Jimeno S, Aguilera A (2010) The interface between transcription and mRNP export: from THO to THSC/TREX-2. Biochim Biophys Acta 1799: 533–538 [DOI] [PubMed] [Google Scholar]
- Rougemaille M, Dieppois G, Kisseleva-Romanova E, Gudipati RK, Lemoine S, Blugeon C, Boulay J, Jensen TH, Stutz F, Devaux F, Libri D (2008) THO/Sub2p functions to coordinate 3′-end processing with gene-nuclear pore association. Cell 135: 308–321 [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA (2008) Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol 10: 228–236 [DOI] [PubMed] [Google Scholar]
- Sfeir A, De Lange T (2012) Removal of shelterin reveals the telomere end-protection problem. Science 336: 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, De Lange T (2009) Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ, Gromak N (2011) Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell 42: 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JA, Wang F, Chaiken MF, Kasbek C, Chastain PD 2nd, Wright WE, Price CM (2012) Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J 31: 3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling PC, Chan YA, Minaker SW, Aristizabal MJ, Barrett I, Sipahimalani P, Kobor MS, Hieter P (2012) R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev 26: 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekvolgyi L, Rakosy Z, Balint BL, Kokai E, Imre L, Vereb G, Bacso Z, Goda K, Varga S, Balazs M, Dombradi V, Nagy L, Szabo G (2007) Ribonucleoprotein-masked nicks at 50-kbp intervals in the eukaryotic genomic DNA. Proc Natl Acad Sci USA 104: 14964–14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MT, Arneric M, Sperisen P, Lingner J (2004) Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell 117: 323–335 [DOI] [PubMed] [Google Scholar]
- Vannier JB, Pavicic-Kaltenbrunner V, Petalcorin MI, Ding H, Boulton SJ (2012) RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 149: 795–806 [DOI] [PubMed] [Google Scholar]
- Wahba L, Amon JD, Koshland D, Vuica-Ross M (2011) RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell 44: 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Takai H, De Lange T (2012) Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and Fill-In by POT1b-associated CST. Cell 150: 39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Vinciguerra P, Wyss JC, Stutz F (2002) Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol 22: 8241–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.