Abstract
Nature 501: 380–384
Somatic stem cell activity is critical for tissue homeostasis. Defects in stem cells are thought to be involved in many diseases, including inherited disorders and aging (He et al, 2009). In a recent paper published in Nature, Adorno et al (2013) demonstrate that there is a general somatic stem cell defect in Down syndrome (DS), a congenital disorder with triplication of human chromosome 21 (HSA21; Roper and Reeves, 2006; Mégarbané et al, 2009). They report that the deubiquitinase Usp16 gene located on HSA21 is a key epigenetic switch that regulates stem cell self-renewal and senescence in DS, and suggest that inhibiting or reducing HSA21 may be beneficial in treating the sequelae of DS.
DS is the most common human chromosomal abnormality with triplication of all or part of HSA21 in humans. Individuals with DS uniformly exhibit varying degrees of developmental delays, mental retardation and premature ageing (Roper and Reeves, 2006; Mégarbané et al, 2009). While the underlying mechanisms of these multifactorial alterations in different systems remain to be defined, one possibility is that the functional decline of adult stem cells may partially lead to DS-associated pathologies.
Self-renewal of stem cells is essential for the maintenance and regeneration of tissues that exhibit high rates of cell turnover, such as the haematopoietic system (He et al, 2009). Adorno et al (2013) first examined the self-renewal of haematopoietic stem cells derived from two DS mouse models, Ts65Dn and Ts1Cje, in which Ts1Cje mice have 25% less trisomic genes triplicated than in the Ts65Dn mice (Roper and Reeves, 2006). Consistent with prior studies (Lorenzo et al, 2011), haematopoietic stem cells from Ts65Dn mice, trisomic for two-thirds of HSA21 genes, exhibit a significant reduction of self-renewal and failure in haematopoietic homeostasis. In contrast, Ts1Cje mice have no defect in stem cell self-renewal.
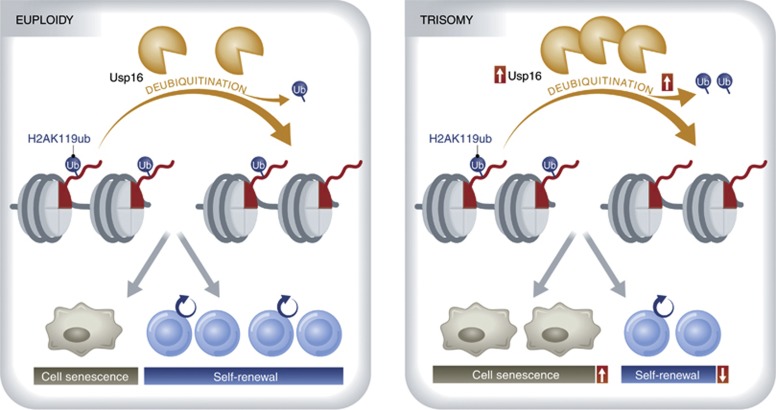
Based on the presumption that some distinctively triplicated genes in the Ts65Dn mouse lead to the haematopoietic stem cell defect, the authors compared triplicated genes in the Ts65Dn versus the Ts1Cje mouse models of DS. One of the genes they identified is the ubiquitin-specific peptidase 16 (Usp16), a deubiquitinating enzyme, that regulates cell cycle progression and gene expression through specific deubiquitination of histone, H2A, by removing ubiquitin from lysine 119 (K119) of H2A (Joo et al, 2007; Figure 1). Self-renewal of haematopoietic stem cell defects are fundamentally rescued in Ts65Dn mice by reducing Usp16 in Ts65Dn mice to a similar expression level to control’s via short hairpin RNA (shRNA) to Usp16. Thus, triplication of Usp16 contributes to the reduction of self-renewal of haematopoietic stem cells. Most DS patients have an extra copy of HSA21 in every cell from the point of conception (Mégarbané et al, 2009). To determine the consequences of Usp16 triplication in the other somatic stem cells, Adorno et al (2013) generated genetically engineered Ts65Dn/Usp16het mice, in which a diploid dosage of normal Usp16 is present with one mutant Usp16 allele and the other triplicated DS genes. These studies reveal that depletion of an extra allele of Usp16 greatly rescues the expansion defects across multiple tissues examined, including mammary epithelial cells, fibroblasts and neural progenitors in Ts65Dn mice. The authors find that overexpression of Usp16 in normal human fibroblasts and neural progenitors lead to reduced expansion, similar to the strong proliferation defect of human DS fibroblasts (Carmeliet et al, 1991; Kimura et al, 2005). Notably, shRNA knockdown of Usp16 rescue the self-renewal defect in human DS fibroblasts.
Figure 1.
Usp16 deubiquitinates uH2A (ubiquitinated H2A) by removing ubiquitin from H2AK119. In the case of triplication of Usp16, H1AK119 ubiquitination is decreased leading to a reduction of self-renewal and accelerated senescence.
Dynamic ubiquitination and deubiquitination of H2A modulates multiple cellular processes. Usp16 deubiquitinates H2A, the most abundant ubiquitinated mammalian chromatin protein, whose monoubiquitinated form has been linked to transcriptional control by Bmi1 (a component of the Polycomb group complex/PRC1; Vissers et al, 2008). Given the role of Bmi1 in regulating cell proliferation and senescence through targeting CDKN2a, the authors compare the senescence of fibroblasts in Ts65Dn and Ts65Dn/Usp16het mice. The authors find that fibroblasts from Ts65Dn mice show accelerated senescence and a concomitant increase in the expression of p16Ink4a and p19Arf, two ageing biomarkers encoded by CDKN2a (Jacobs et al, 1999). shRNA-mediated downregulation of either CDKN2a or Usp16 significantly decreases p16Ink4a and p19Arf levels and rescues the cell proliferation and senescence defects. Given the remarkable effect of the increased dosage of Usp16 in stem cell self-renewal and senescence, these data strongly indicate that there is a common mechanism governing the functional decline of stem cells in DS through an Usp16 gene dosage-dependent deubiquitinating mechanism in the H2A-Bmi/PRC1-CDKN2a pathway (Figure 1).
In summary, Adorno et al (2013) have taken an important step in illuminating the mechanism for DS and provide new insights into the pathophysiological impact of Usp16 on DS stem cells that occurs in a gene-dosage-dependent manner. The full extent of the role of deubiquitinases in human disease remains to be elucidated. The identification of Usp16, which is involved in wide range of key regulatory processes, may provide a new therapeutic target for DS. In addition, these discoveries raise important questions to be addressed in future work. Can inhibition or reduction of Usp16 levels improve cognitive function in patients with DS or can the natural history and clinical outcome of DS be changed by manipulating the levels or activity of Usp16 at an early age?
Footnotes
The authors declare that they have no conflict of interest.
References
- Adorno M, Sikandar S, Mitra SS, Kuo A, Di Robilant BN, Haro-Acosta V, Ouadah Y, Quarta M, Rodriguez J, Qian D, Reddy VM, Cheshier S, Garner CC, Clarke MF (2013) Usp16 contributes to somatic stem-cell defects in Down's syndrome. Nature 501: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet G, David G, Cassiman JJ (1991) Cellular ageing of Alzheimer's disease and Down syndrome cells in culture. Mut Res 256: 221–231 [DOI] [PubMed] [Google Scholar]
- He S, Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25: 377–406 [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M (1999) The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397: 164–168 [DOI] [PubMed] [Google Scholar]
- Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, Chang C, Wang H (2007) Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature 449: 1068–1072 [DOI] [PubMed] [Google Scholar]
- Kimura M, Cao X, Skurnick J, Cody M, Soteropoulos P, Aviv A (2005) Proliferation dynamics in cultured skin fibroblasts from Down syndrome subjects. Free Radic Biol Med 39: 374–380 [DOI] [PubMed] [Google Scholar]
- Lorenzo LP, Chen H, Shatynski KE, Clark S, Yuan R, Harrison DE, Yarowsky PJ, Williams MS (2011) Defective hematopoietic stem cell and lymphoid progenitor development in the Ts65Dn mouse model of Down syndrome: potential role of oxidative stress. Antioxid Redox Signal 15: 2083–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethore MO, Delabar JM, Mobley WC (2009) The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med 11: 611–616 [DOI] [PubMed] [Google Scholar]
- Roper RJ, Reeves RH (2006) Understanding the basis for Down syndrome phenotypes. PLoS Genet 2: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers JH, Nicassio F, van Lohuizen M, Di Fiore PP, Citterio E (2008) The many faces of ubiquitinated histone H2A: insights from the DUBs. Cell Div 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]