Abstract
Objective
To investigate the effect of 0.2% and 2% chlorhexidine (CHX) used as a therapeutic primer on the long-term bond strengths of two etch-and-rinse adhesive systems.
Methods
Adper Scotchbond 1XT (SB1) and XP-Bond (XPB) were evaluated. Etched dentin substrates were assigned to six treatment groups: (1) 0.2% CHX + SB1; (2) 2% CHX + SB1; (3) SB1 (control); (4) 0.2% CHX + XPB; (5) 2% CHX + XPB; (6) XPB (control). Composite build-ups were made and beams prepared for microtensile bond strength test. Beams were divided in three subgroups and either immediately pulled to failure or stored in artificial saliva for 6 or 12 months prior to testing. Data were evaluated by three-way ANOVA. Additional adhesive interfaces were prepared to investigate nanoleakage expression by TEM.
Results
SB1 and XPB showed similar immediate bond strength values with or without CHX pre-treatment (p>0.05). After 12 months, bonds fell from 43.9 ± 9.5 MPa to 20.1 ± 5.4 MPa and from 39.6 ± 9.4 MPa to 14.2 ± 5.0 MPa in control specimens for SB1 and XPB respectively, while bond fell only from 41.9±9.6MPa to 33.2 ± 8.3 MPa and 38.3 ± 8.9 MPa to 26.5 ± 10.9 (for SB1 and XPB respectively) when 0.2% CHX was previously used. CHX concentration did not affect bond strength values (0.2% vs 2%, p>.05). Nanoleakage increased during aging in controls, but reduced silver deposits were found in CHX-treated specimens.
Conclusions
Chlorhexidine significantly reduced the loss of bond strength seen in control bonds. Since no bacterial growth was present in the aging conditions, the results of this study suggest that endogenous factors thought to degrade the adhesive interface can be inhibited by CHX. Further in vivo trials should confirm the role of CHX in bond durability.
Keywords: chlorhexidine, dental bonding systems, hybrid layer, aging, dentin
INTRODUCTION
Dental adhesives have changed restorative dentistry. However, despite excellent immediate bond strength, limited durability of resin-dentin bonds has been revealed both in in vivo1,2,20,22,23,31and in simulated in vitro aging conditions.12,13 Different factors are shown to affect, synergistically, the integrity of each compartment of the hybrid layer, thereby contributing with a rapid and catastrophic failure of resin-dentin bonds.
Water sorption, elution of unreacted monomers, plasticization of polymer chains and water-mediated hydrolysis are some of the aging phenomena that can severely affect the mechanical and morphological integrity of the resinous component of hybrid layers. Increasing incorporation of high concentrations of ionic and polar resin monomers to current simplified etch-and-rinse adhesives seems to be the keystone in lack of stability of these materials.1,2,6,19,28,38,42-46 Nevertheless, degradation may also affect the other component of the hybrid layer, that is, its collagen matrix. If collagen fibrils are unprotected by hydrophobic resin coating, they may be vulnerable to degradation due to the activity of collagenolytic enzymes either secreted by bacteria of the plaque or by activation of endogenous dentin matrix metalloproteinases (MMPs).30,33,34
MMPs are zinc- and calcium-dependent proteases 39,48 that are responsible for degrading practically all extracellular matrix components of connective tissues. Recent studies revealed that human dentin contains endogenous MMPs, such as collagenases (MMP-8), gelatinases (MMP-2 and MMP-9) and enamelysin (MMP-20).29,40,41 Within the oral environment, MMPs have shown to have a relevant implication in caries progression mechanism47 and periodontal disease.39 Recently, it was also demonstrated that human dentin may exhibit significant collagenolytic and gelatinolytic activities after the application of acidic bonding agents.30,33 Accordingly, this dentinal MMP activity is thought to play a role in the breakdown of sub-optimally infiltrated dentin collagen matrices within the hybrid layers.34
Dentin collagenolytic and gelatinolytic activities30,33,34 can be reduced or suppressed by protease inhibitors34, indicating that MMP inhibition could be beneficial in the preservation of hybrid layers. Based on this findings, the classical 2% CHX cleaning solution, normally used as a cavity disinfectant,11,15,37 has been also employed to preserve the hybrid layer integrity.9,10,24. The rationale behind this new use of CHX relies on its broad inhibitory effect on numerous MMPs.18
While CHX has indicated its beneficial effects on the preservation of dentin bond strength, when applied prior to bonding with Scotchbond 1 (3M ESPE, St Paul, MN, USA)9,10, it would be of great interest to evaluate whether such benefits are dependent on CHX concentration and/or on adhesives composition. The aim of this in vitro study was to investigate the long-term effect of 0.2% and 2% CHX on the mechanical durability of resin-bonded dentin treated with two simplified etch-and-rinse adhesives. Interfacial nanoleakage expression of the dentin-bonded interfaces was also investigated using transmission electron microscope (TEM). The null hypotheses tested were that (1) neither 0.2 or 2% CHX would prevent reductions in dentin bond strength over time, and that (2) CHX would not affect interfacial nanoleakage expression during aging.
MATERIALS AND METHODS
Specimens preparation
One hundred-eight extracted non-carious human third molars, stored for 1 month or less in 0.5% chloramine T solution at 4°C, were selected for the study. These teeth were collected after the patients’ informed consent was obtained for research purposes under a protocol approved by the institutional review board of the University of Trieste. Occlusal enamel and superficial dentin were removed perpendicular to the long axis of each tooth by a low speed diamond saw under water irrigation (Micromet, Remet, Bologna, Italy) and a standardized smear layer was created on the exposed middle/deep coronal dentin with 180-grit wet silicon carbide paper. The exposed dentin surfaces were etched with 35% phosphoric acid for 15 s (etching gel, 3M ESPE, St Paul, MN, USA), rinsed with water, gently air-dried for 2 s and kept moist until adhesive was applied following the wet bonding technique. The teeth were equally and randomly assigned to six treatment groups (N=18), in which acid-dentin surfaces were: Group 1 - treated with an aqueous solution of 0.2% CHX digluconate, then bonded with Adper Scotchbond 1XT (SB1, 3M ESPE); Group 2: treated with an aqueous solution of 2% CHX digluconate, then bonded with SB1; Group 3: directly bonded with SB1 without any pre-treatment (control); Group 4: treated with an aqueous solution of 0.2% CHX digluconate, then bonded with XP-Bond (XPB, Dentsply De Trey, Konstanz, Germany); Group 5: treated with an aqueous solution of 2% CHX digluconate, then bonded with XPB or Group 6: directly bonded with XPB without any pre-treatment (control). Adhesive composition and group treatment are listed in Table 1.
Table 1.
Composition of dental bonding systems tested in the study
| Adhesive | Composition |
|---|---|
| Adper Scotchbond 1XT |
Etchant:
|
| XP-Bond |
Etchant:
|
Regardless of the CHX concentration, the water solution of CHX was generously applied on the etched surface as a therapeutic primer and gently blot-dried after a dwell time of 30 seconds. After gentle air drying, SB1 or XPB were applied in accordance with manufacturers’ instructions. Each adhesive system was light-cured for 20 s with a quartz-tungsten-halogen light-curing unit (Curing Light 2500, 3M EPSE) delivering 600 mW/cm2. Four 1-mm thick layers of microhybrid resin composite (Filtek Z250, 3M ESPE) were incrementally placed over the bonded dentin surface and individually polymerized for 40 s.
Microtensile bond strength evaluation
Resin-dentin sticks with cross-sectional area of approximately 0.9 mm2 were created from each bonded teeth using a low speed saw under water irrigation, in accordance with the protocol to prepare non-trimming specimens for microtensile technique.10 The dimensions of each stick were individually measured with a digital caliper to the nearest 0.01 mm and was recorded for subsequent bond strength calculation. Sticks from each tooth were divided and randomly assigned to three storage groups: T0:: sticks were stored in artificial saliva (the solution was prepared in accordance with Pashley et al., 2004 34) for 24 h at 37°C; T6: sticks were stored for 6 months in artificial saliva at 37°C; T12: sticks were stored for 12 months in artificial saliva at 37°C. After storage, each stick was attached to a modified jig for microtensile testing and stressed under tension until failure with a simplified universal testing machine at a crosshead speed of 1 mm/min (Bisco Inc., Schaumburg, IL, USA).
Failure modes were evaluated at 50X (Stemi 2000-C, Carl Zeiss Jena GmbH, Germany) and classified as cohesive (C), adhesive (A), or mixed (M) failures. The number of prematurely debonded sticks per each tested group was also recorded, but not included in the statistical analysis. All premature failures occurred during the cutting procedure, which was performed at time zero, when all specimens were prepared. Thus, inclusion of these data in the statistical analysis was not consider since they could end up masking the bond strength value that was obtained at time zero (T0), making it to appear deliberately lower than the values obtained at the other periods of evaluation (T6 and T12), wherein premature failures were not indeed observed.
Statistical analysis
As values were normally distributed (Kolmogorov-Smirnof test), data were analyzed with a three-way ANOVA (tested variables were: adhesive, CHX concentration, time of storage), adjusting the estimates of the standard errors for multiple observations from each tooth, using the correlation within teeth, via the Huber-White sandwich estimate. Statistical significance was set at p<.05.
Nanoleakage evaluation
Additional 12 teeth were selected for evaluation of interfacial nanoleakage. Dentin disks from middle/deep dentin were prepared and bonded as previously described, i.e. either with SB1 or XPB and with or without 0.2% or 2% CHX as additional primer. Specimens were then vertically cut into 1-mm thick slabs to expose the bonded surfaces and submitted to the three storage times in artificial saliva at 37°C: T0 24 h, T66 months or T12 12 months.42,44
In brief, slab specimens were covered with nail varnish, leaving 1 mm free at the interface, then immersed in a 50 wt% ammoniacal AgNO3 solution42,44 for 24 h. Specimens were then photodeveloped to reduce the diamine silver ions ([Ag(NH3)2]+) into metallic silver grains. The silver-impregnated specimens were fixed, dehydrated in an ascending ethanol series and embedded in epoxy resin (Epon 812, Fluka, Switzerland) and processed for TEM analysis. Ultrathin sections containing the bonded interfaces were collected on 100-meshes carbon and formvar coated grids and examined by an independent observer under transmission electron microscope (Philips CM-10) operating at 70 kV, without further staining.
RESULTS
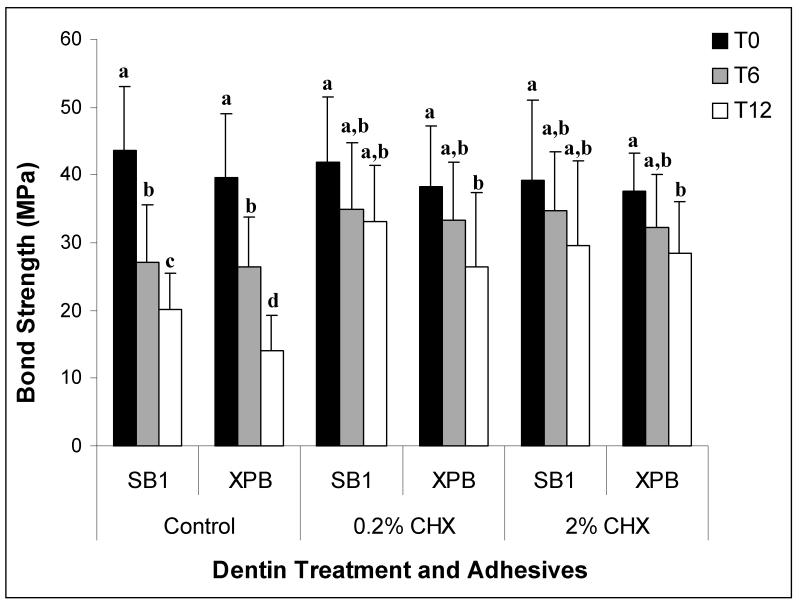
Mean bond strength and failure mode obtained at T0, T6 and T12 (i.e. after 24 h, 6 months and 12 months of storage, respectively) are summarized in Table 2 and Graph 1. No difference was found (p>.05) in immediate bond strength values (T0) exhibited by experimental and control conditions, regardless of the tested adhesive or the concentration of CHX (SB1+0.2%CHX= 41.9 ± 9.6 MPa; SB1+2%CHX= 39.1 ± 11.9MPa; SB1 control= 43.9 ± 9.5 MPa, XPB+0.2%CHX= 38.3 ± 8.9 MPa; XPB+2%CHX= 37.6±5.6 MPa; XPB control= 39.6 ± 9.4 MPa) (p>0.05). At T6, the control, no CHX-treated specimens composed of either SB-1 or XPB exhibited significant decreases (p<0.05) in microtensile bond strength (SB1 control= 27.2 ± 8.4 MPa; XPB control= 26.5 ± 7.3 MPa), while CHX-treated specimens bonded with both adhesives showed a lack of significant decrease (p>0.05) in bond strength when compared to their correspondent T0 specimens (SB1+0.2%CHX= 35.0 ± 9.7 MPa; SB1+2%CHX= 34.8 ± 8.6 MPa; XPB+0.2%CHX= 33.3 ± 8.5 MPa; XPB+2%CHX= 32.2 ± 7.9 MPa). Following this same trend, after 12 months of storage in artificial saliva (T12), a significant decrease of bond strength values (p<0.05) was observed for control specimens irrespectively of the adhesive. In these control groups, reductions in microtensile bond strengths for SB1 was 54%, and for XPB fells 65 %, compared to T0 control values (SB1 control= 20.1 ± 5.4 MPa; XPB control=14.2 ± 5.0 MPa). In contrast, T12 specimens pre-treated with either 0.2% or 2% CHX showed higher bond strength (p<.05) comparable than the untreated controls. The bond strengths of 0.2 or 2% CHX-treated specimens only fell 21% and 32%, respectively (SB1+0.2%CHX= 33.2 ± 8.3 MPa; SB1+2%CHX= 29.5 ± 12.7 MPa; XPB+0.2%CHX= 26.5 ± 10.9 MPa; XPB+2%CHX= 28.5 ± 7.5 MPa, Fig.1).
Table 2.
Percentages of failure modes after microtensile test. T0, T6 and T12 indicate specimens that were tested after storage for 24 h, 6 months or 12 months, respectively. Fractures were classified as: A, adhesive; CD, cohesive failure in dentin; CC, cohesive failure in resin composite; M, mixed failure.
| Adhesive system | Adper Scotchbond 1XT | XP Bond | ||||
|---|---|---|---|---|---|---|
| Storage time | T0 | T6 | T12 | T0 | T6 | T12 |
| 0.2% chlorhexidine | 30% A 70% M [5/163] |
30% A 5% CC 65% M [5/154] |
30% A 70% M [6/161] |
40% A 5% CD 55% M [7/158] |
35% A 65% M [6/154] |
30% A 10% CD 60% M [4/159] |
| 2% chlorhexidine | 35% A 10% CD 55% M [8/167] |
35% A 5% CD 60% M [5/162] |
50% A 5% CD 45% M [7/149] |
45% A 55% M [4/163] |
30% A 10% CC 60% M [5/162] |
40% A 15% CD 45% M [7/164] |
| Control group | 35% A 65% M [6/159] |
30% A 10% CC 60% M [9/172] |
35% A 10% CD 55% M [5/167] |
35% A 65% M [6/154] |
35% A 5% CD 60% M [7/165] |
35% A 15% CC 50% M [6/158] |
[number of premature failed sticks/number of intact sticks tested].
Figure 1.
Bond strengths (MPa) to control (no CHX-treated), 0.2% CHX-treated and 2% CHX-treated specimens bonded with either Adper Scotchbond 1XT (SB1) or XP-Bond (XPB) that were tested immediately (T0- black bars) or after six months (T6- gray bars) or after 12 months (T12- white bars) of aging in artificial saliva. The height of the bars is the bond strength mean; half-brackets indicate plus one standard deviation. Groups identified with different letters were statistically different (p<0.05).
No differences were found between the two tested CHX concentrations, regardless of storage time or adhesive system (0.2% CHX vs 2% CHX p>0.05). Similarly, no differences were found between the adhesives regardless of storage and CHX treatment (SB1 vs XPB p>0.05).
TEM analysis of the interfacial nanoleakage expression revealed the presence of silver uptake within all observed adhesive interface. Increasing concentration of silver uptake, particularly for control specimens, was patent over time of aging. At T0, silver nanoleakage within the hybrid layer of SB1 specimens was characterized by minor crystal-like silver deposits and some globular aggregates (Fig 2a). Nanoleakage was also found along resin tags, as silver grains between resin tags and the peritubular dentin walls. XPB showed lower nanoleakage expression (at T0) compared to SB1, which was characterized by minor globule-like silver deposits within the hybrid layer (Fig. 2a). Both adhesives showed no differences in the nanoleakage expression between CHX-treated specimens (using either 2% or 0.2%) and controls (data not shown). That is, they all showed some silver uptake. After six months of storage (T6), nanoleakage expression of control specimens was increased when compared to CHX-treated specimens (data not shown). However, the largest differences in interfacial nanoleakage expression were found at T12 between control specimens and adhesive interfaces pre-treated with CHX. In fact, controls showed so much silver uptake throughout the hybrid layers that they obscured the collagen fibrils in both SB1 (Fig. 2b) and XPB bonded specimens (Fig. 3b). Conversely specimens pre-treated with CHX revealed small nanoleakage expression, regardless of the adhesive or CHX concentration (Fig. 2c, 2d, 3c, 3d), that were comparable to the nanoleakage seen in T0 controls.
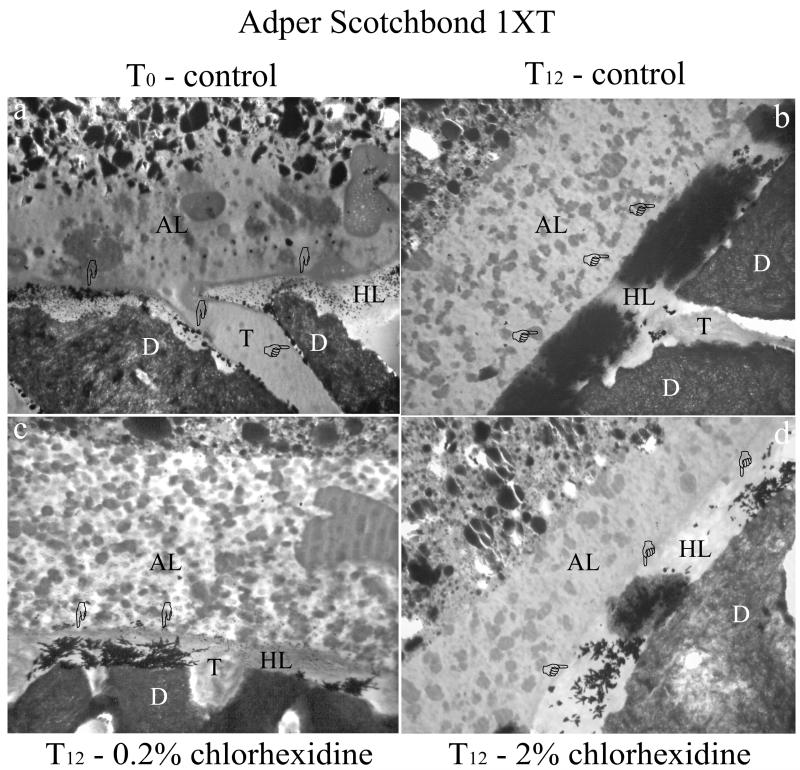
Figure 2.
TEM micrographs of unstained undemineralized adhesive interface created with Adper Scotchbond 1XT on middle/deep human dentin. All images were chosen as representative for the treatment group. Fig. 2a: TEM micrograph of the resin-dentin interface, showing the presence of a 1-2 μm thick, hybrid layer (HL). Nanoleakage expression is visible at the top of the hybrid layer, with fine, isolated silver grains (pointers) and larger spherical silver deposits (pointers), that are probably affiliated with the partially demineralized, unstained collagen fibrils. The adhesive layer (AL) contains nanofiller clusters and polyalkenoic acid copolymer within the polymerized adhesive. Very fine silver grains could be seen within the adhesive layer at higher magnification (not shown). D: intertubular dentin; T: dentinal tubule. Fig. 2b: After aging (T12), control specimen shows large nanoleakage throughout the entire thickness of the hybrid layer (HL) revealing intense collagen destruction. The silver deposits appear as homogenously distributed over the mineralized dentin (D). Minimal nanoleakage can be found within the adhesive layer (AL). dentinal tubule (T). Fig. 2c: Adhesive interface created after pre-treatment of the etched dentin surface with 0.2 % chlorhexidine for 30 s and aged for 12 months in artificial saliva (T12). Nanoleakage expression is slightly higher than T0 specimens as only a minor increment of silver deposits can be observed. Water-tree like silver aggregates are sometimes evident (pointer). Adhesive layer (AL); dentinal tubule (T); dentin (D). Fig. 2d: similar findings can be found in the adhesive interface created after the use of 2% chlorhexidine for 30 s as additional primer on acid etched dentin and after 12 months aging (T12). Nanoleakage (pointers) is higher than T0 specimens, but lower than T12 control specimens. Adhesive layer (AL); hybrid layer (HL); dentin (D).
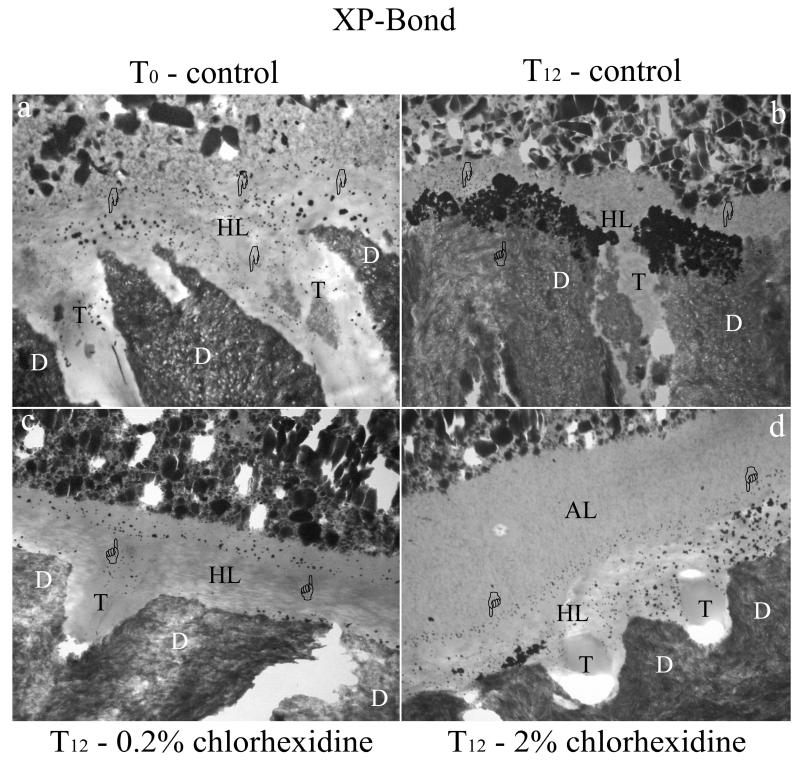
Figure 3.
TEM micrographs of unstained undemineralized adhesive interface created with XP-Bond on middle/deep human dentin. All images were chosen as representative for the treatment group. Fig. 3a: XP-Bond shows the presence of a 1-2 μm thick hybrid layer (HL). Nanoleakage expression is found within the hybrid layer, with fine, isolated silver grains (pointers). The overall nanoleakage expression is lower than Adper Scotchbond 1XT under all tested conditions. The adhesive layer shows variable thickness from few microns (as represented in the image) to 5-6 μm. In all cases nanofiller clusters with different size are visible. D: dentin; T: dentinal tubule. Fig. 3b: After aging (T12), control specimen shows large and homogenous nanoleakage expression (pointers) within the hybrid layer (HL), especially at the peritubular dentin level. dentinal tubule (T); dentin (D). Fig. 3c: Adhesive interface created by XP-Bond when applied on .2 % chlorhexidine pre-treated dentin and aged for 12 months in artificial saliva (T12). Similar to Adper Scotchbond 1XT nanoleakage was higher than T0 specimens, but lower than chlorhexidine untreated specimens. Nanoleakage (pointer) was scattered in the higher part of the hybrid layer (HL) and within resin tags infiltrating the dentinal tubules (T); dentin (D). Fig. 3d: similar findings can be found within the adhesive interface created by XP-Bond o 2% chlorhexidine treated dentin at T12. Adhesive layer (AL); hybrid layer (HL); dentin (D), dentinal tubule (T).
DISCUSSION
The results of this study showed that CHX used as a therapeutic primer on acid-etched dentin does not interfere with immediate bond strength of SB1 and XPB, and that its application significantly helps to decrease the rate of bond strength reduction over time (Fig. 1). After either 6 (T6) or 12 months of storage (T12), CHX-treated specimens showed higher bond strengths and reduced hybrid layer degradation (i.e. lower nanoleakage expression; Fig.2c, 2d, 3c, 3d) compared to control specimens (i.e. not treated with CHX; Fig. 2b, 3b) which, in turn, exhibited a significant reduction in bond strength (Fig. 1) and a higher nanoleakage expression (Fig.?). These results support the rejection of the two null hypotheses, because application of CHX in both tested concentrations (0.2% and 2%) significantly prevented the rapid loss of bond strength and reduced the nanoleakage expression of acid-etched dentin bonded with the simplified etch-and-rinse adhesives, Scotchbond 1XT and XP-Bond.
The first evidence that acid-etched dentin can be degraded by endogenous proteolytic enzymes was reported by Pashley et al.34, revealing that demineralized collagen matrices stored in artificial saliva, free from bacterial contamination, were almost completely destroyed after 250 days in vitro. Conversely, at that same study, dentin specimens showed no evident morphological changes after aging when they were stored in the presence of a cocktail of various proteolytic enzyme inhibitors or in pure mineral oil. The conclusions of that study supported, for the first time, the hypothesis that endogenous dentin MMPs are responsible for morphological changes in dentin collagen matrix, which is evidently shown in in vitro studies, as a progressive thinning of collagen fibrils. This is thought to be responsible for the slow disappearance of collagen matrix that may be observed in incompletely infiltrated hybrid layers in bonded-dentin after middle to long-term aging.2,12,17,21 Using fluorescein-labelled type I collagen from bovine skin, Pashley et al. 34 also revealed that this collagenolytic activity could be inhibited by 0.2% CHX.
An in vivo study on primary teeth showed that after only 6 months of clinical service, extensive nanoleakage and degradation of hybrid layers were detectable in TEM undemineralized sections even in teeth that had clinically intact, acid-etched enamel cavosurface margins.24 Conversely, when CHX was applied on etched-dentin, the hybrid layer deterioration was significantly reduced.24 This expedited degradation was probably related to the fact that the etch- and-rinse adhesive (Adper Scotchbond 1, 3M ESPE) was applied to caries-affected dentin, in which MMPs may have been increased or over-activated by the carious process.24
We speculate that the massive silver uptake seen in the control specimens (Figs. 2b and 3b) was due to large water-filled voids in the hybrid layers that, in turn, would be resultant from degradation of the matrix and/or resin. This severe destruction of the hybrid layer was visible in control specimens of both SB1 and XPB bonded specimens at 12 months of aging in aqueous solution (Fig. 2b, 3b). Conversely, similar findings were not evident in CHX-treated specimens which showed consistently reduced silver uptake after 12 months of storage (T12; Fig 2c,2d,3c,3d). These observations suggest that the breakdown of resin-infiltrated dentin may be governed by host-derived factors, such as the action of endogenous collagenolytic enzymes on partially-exposed collagen fibrils.
Despite its antibacterial capability, CHX was shown to inhibit the activity of MMP-2, -8 and -9 through a chelating mechanism.18 We speculate that the application of CHX on the exposed collagen of the acid-etched dentin for the dwell period of at least 30 seconds may keep the organic matrix of dentin saturated with CHX. Accordingly, CHX may remain entrapped between collagen fibrils when the interfibrillar spaces become filled with resin during adhesive impregnation. The prolonged protective effect of CHX on bond strength over 12 months compared to untreated specimens that were tested after the same storage time (T12) suggests that CHX may remain effective for a long time, even though the resin-bonded dentin beams represent a form of accelerated aging. In beams that are only 0.9 × 0.9 mm in cross-section water need only diffuse 450 μm from any surface to reach the center of the beam.12,13 Such water diffusion would have been expected to dilute or displace CHX from dentin, however, even after 12 months of aging the preventive role of CHX on bond strength preservation and nanoleakage reduction were clearly confirmed in our study. Nevertheless, future studies need to quantify the substantivity of CHX to resin-bonded dentin.
Since most of the simplified adhesives have been shown to behave as semi-permeable membranes after polymerization, we hypothesized that water can permeate the adhesive interface and further activate MMPs that can degrade sub-optimally infiltrated collagen fibrils. As collagenases are hydrolases, they require water to be activated.48 In addition water may remove the hydrophilic monomers contained within the adhesive blends through a mechanism of water sorption, swelling and leaching that contribute to increase the number of exposed collagen fibrils susceptible to enzymatic cleavage.28 Finally, since simplified adhesive were shown to have a reduced extent of polymerization which is correlated to their permeability,3,7 elution of un-cured monomers might contribute to increasing resin permeability and to expose additional collagen fibrils to activated MMPs.
Extensive degradation of hybrid layers has been reported in other studies8,12,14,20,25,27,28,49 supporting the hypothesis that aging of the adhesive interface is a multifactor degradation process and that different approaches may contribute to stabilize the hybrid layer over time. Despite the use of CHX to prevent degradation, it has been recently shown that the use of an hydrophobic coating can reduce water sorption25 and improve bond stability.32 Recently it has also been suggested that the use of electric impulses may improve dentin impregnation of simplified adhesives through a superimposed iontophoretic force that may drive polar monomers into the demineralized dentin network, improving impregnation and the final quality of the hybrid layer.4,35
In conclusion, the results of this study indicate that microtensile bond strength of beams exposed for 12 months to a buffer solution can be prevented from degradation by the use of CHX as a primer on acid etched dentin (for 30 s dwell time) even at very low concentration (0.2%) which was not previously assayed (Fig. 1). As it is well known that CHX is a MMPs inhibitor,3 it is reasonable to speculate that CHX may have inhibited the activity of MMPs resulting in preventing collagen degradation over time.
The findings suggest that CHX is effective in reducing the rapid degradation of hybrid layers that are composed of simplified etc-and-rinse adhesives, encouraging further clinical investigation in the prevention of degradation of hybrid layers over time by the use of MMPs inhibitors prior to bonding. Moreover adhesive build-in enzyme inhibitors may be even more promising in terms of simplification of the bonding procedure and higher substantivity over time.
ACKNOWLEDGEMENTS
The adhesive systems were generously donated by 3M ESPE and Dentsply De Trey. The authors wish to thank Mr. Claudio Gamboz for extensive technical assistance and Mr. Aurelio Valmori for photographical assistance. The investigation was supported with MIUR grants (Italy) and by R01 DE 015306 from the National Institute of Dental and Craniofacial Research (NIDCR) to D.H.P. (P.I.).
REFERENCES
- 1.Armstrong SR, Keller JC, Boyer DB. The influence of water storage and C-factor on the dentin-resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent Mater. 2001;17:268–276. doi: 10.1016/s0109-5641(00)00081-6. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, et al. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent. 2004;29:705–712. [PubMed] [Google Scholar]
- 3.Breschi L, Cadenaro M, Antoniolli F, Sauro S, Biasotto M, Prati C, et al. Polymerization kinetics of dental adhesives cured with LED: correlation between extent of conversion and permeability. Dent Mater. 2007 doi: 10.1016/j.dental.2006.06.040. in press. [DOI] [PubMed] [Google Scholar]
- 4.Breschi L, Mazzoni A, Pashley DH, Pasquantonio G, Ruggeri A, Jr, Suppa P, et al. Electric impulse-assisted application of self-etch adhesives to dentin. J Dent Res. 2006;85:1092–1096. doi: 10.1177/154405910608501205. [DOI] [PubMed] [Google Scholar]
- 5.Breschi L, Mazzoni A, Ruggeri A, Jr, Cadenaro M, Di Lenarda R, Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Burrow MF, Satoh M, Tagami J. Dentin bond durability after three years using a dentin bonding agent with and without priming. Dent Mater. 1996;12:302–307. doi: 10.1016/s0109-5641(96)80038-8. [DOI] [PubMed] [Google Scholar]
- 7.Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Lenarda R, Prati C, et al. Degree of conversion and permeability of dental adhesives. Eur J Oral Sci. 2005;113:525–530. doi: 10.1111/j.1600-0722.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 8.Carrilho MRO, Carvalho RM, Tay FR, Yiu CK, Pashley DH. Durability of resin-dentin bonds related to water and oil storage. Am J Dent. 2005;18:315–319. [PubMed] [Google Scholar]
- 9.Carrilho MRO, Carvalho RM, Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrilho MRO, Geraldeli S, Tay FR, de Goes M, Carvalho RM, Tjäderhane L, et al. In Vivo Preservation of hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MP, Meiers JC. The effect of dentin disinfectants on shear bond strength of resin-modified glass-ionomer materials. Quintessence Int. 1997;28:545–51. [PubMed] [Google Scholar]
- 12.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 13.De Munck J, Van Meerbeek B, Van Landuyt K, Lambrechts P. Influence of a shock absorbing layer on the fatigue resistance of a dentin-biomaterial interface. Eur J Oral Sci. 2005;113:1–6. doi: 10.1111/j.1600-0722.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 14.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res. 2005;84:355–359. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 15.el-Housseiny AA, Jamjoum H. The effect of caries detector dyes and a cavity cleansing agent on composite resin bonding to enamel and dentin. J Clin Pediatr Dent. 2000;25:57–63. doi: 10.17796/jcpd.25.1.781012816642v3t5. [DOI] [PubMed] [Google Scholar]
- 16.Finer Y, Santerre JP. Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res. 2004;83:22–26. doi: 10.1177/154405910408300105. [DOI] [PubMed] [Google Scholar]
- 17.García-Godoy F, Tay FR, Pashley DH, Tjäderhane L, Pashley EL, King NM. In vivo degradation of resin-bonded dentin after 3 years of storage. Am J Dent. 2007;20:109–113. [PubMed] [Google Scholar]
- 18.Gendron R, Greiner D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwinnett AJ, Yu S. Effect of long-term water storage on dentin bonding. Am J Dent. 1995;8:109–111. [PubMed] [Google Scholar]
- 20.Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79:1385–1391. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Ohno H, Sano H, Tay FR, Kaga M, Kudou Y, et al. Micromorphological changes in resin-dentin bonds after 1 year of water storage. J Biomed Mater Res. 2002;63:306–311. doi: 10.1002/jbm.10208. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Tay FR, Ohno H, Sano H, Kaga M, Yiu C, et al. SEM and TEM analysis of water degradation of human dentin collagen. J Biomed Mater Res. 2003;66:287–298. doi: 10.1002/jbm.b.10560. [DOI] [PubMed] [Google Scholar]
- 24.Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 25.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 26.Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–1719. doi: 10.1016/s0142-9612(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 27.Koshiro K, Inoue S, Tanaka T, Koase K, Fujita M, Hashimoto M, et al. In vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. Eur J Oral Sci. 2004;112:368–375. doi: 10.1111/j.1600-0722.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 28.Malacarne J, Carvalho RM, de Goes MF, Svizerd V, Pashley DH, Tay FR, et al. Water sorption/solubility of dentinal adhesives resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Mazzoni A, Mannello F, Tay FR, Tonti G, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res. 2007;86:436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjaderhane L, et al. Reactivation of quenched endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Mjör IA, Gordan VV. Failure, repair, refurbishing and longevity of restorations. Oper Dent. 2002;27:528–534. [PubMed] [Google Scholar]
- 32.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–21. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 34.Pashley DH, Tay FR, Yiu CKY, Hashimoto M, Breschi L, Carvalho R, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 35.Pasquantonio G, Tay FR, Mazzoni A, Suppa P, Ruggeri A, Jr, Falconi M, et al. Electric device improves bonds of simplified etch-and-rinse adhesives. Dent Mater. 2007;23:513–518. doi: 10.1016/j.dental.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 37.Say EC, Koray F, Tarim B, Soyman M, Gulmez T. In vitro effect of cavity disinfectants on the bond strength of dentin bonding systems. Quintessence Int. 2004;35:56–60. [PubMed] [Google Scholar]
- 38.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–218. [PubMed] [Google Scholar]
- 39.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 40.Sulkala M, Larmas M, Sorsa T, Salo T, Tjaderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002;81:603–607. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 41.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Tay FR, Hashimoto M, Pashley DH, Peters MC, Lai SC, Yiu CK, Cheong C. Aging affects two modes of nanoleakage expression in bonded dentin. J Dent Res. 2003;82:537–541. doi: 10.1177/154405910308200710. [DOI] [PubMed] [Google Scholar]
- 43.Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent Res. 2002;30:371–382. doi: 10.1016/s0300-5712(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 44.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81:472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 45.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726–31. [PubMed] [Google Scholar]
- 46.Tay FR, Pashley DH. Water treeing—a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16:6–12. [PubMed] [Google Scholar]
- 47.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinase in dentin matrix during breakdown in carious lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 48.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function, and biochemistry. Cir Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 49.Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, et al. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]